The beneficial roles of n-3 long-chain PUFA (n-3 LC-PUFA, >C20) in particular of EPA (20 : 5n-3) and DHA (22 : 6n-3) for retinal and brain development during infancy, prevention of autoimmune disorders and some types of cancers and cardiovascular diseases are well recognised(Reference Connor1, Reference Simopoulos2). EPA and DHA are primarily provided by seafood, whereas these fatty acids are mostly absent from terrestrial foods(Reference Mozaffarian and Wu3). Thus, health organisations worldwide recommend the consumption of fish or food supplements rich in EPA and DHA such as fish oil (FO)(4). However, FO are mostly obtained from capture-derived oily fish, a limited resource whose production has remained steady during the last decade(5). Besides, fish have a limited ability to synthesise EPA and DHA from the precursor α-linolenic acid (ALA; 18 : 3n-3). Bioconversion of 18-carbon PUFA to EPA and DHA depends on the elongation and desaturation capacity of different fish species(Reference Sargent, Tocher, Bell and Halver6, Reference Castro, Tocher and Monroig7). Generally speaking, freshwater fish possess a higher ability to convert ALA and linoleic acid (LA) into LC-PUFA(Reference Leaver, Villeneuve and Obach8, Reference Sargent, Bell and McEvoy9) than marine fish(Reference Tocher10). Moreover, LC-PUFA synthesis capacity also appears to differ among different marine species(Reference Monroig, Tocher and Navarro11–Reference Xu, Dong and Ai14). For instance, gilthead seabream (Sparus aurata) shows very low desaturation and elongation activity to produce EPA and DHA from ALA(Reference Tocher and Ghioni15). A fatty acyl desaturase 2 gene (fads2) has been cloned from gilthead seabream(Reference Seiliez, Panserat and Corraze16), encoding Δ6 desaturase (rate-limiting in LC-PUFA synthesis) and being regulated by the diet(Reference Izquierdo, Robaina and Juárez-Carrillo17). Since some terrestrial vegetable oils (VO) are rich in ALA and constitute a more sustainable feedstuff than FO, recent studies have tried to enhance the ability of gilthead seabream to synthesise EPA and DHA, namely, to up-regulate fads2 expression and improve dietary lipid utilisation(Reference Izquierdo, Turkmen and Montero18–Reference Turkmen, Zamorano and Fernández-Palacios20).
In mammals, changes in dietary fat levels and quality during early developmental stages modulate metabolism in later life stages(Reference Ainge, Thompson and Ozanne21–Reference Lillycrop and Burdge23) through a process that is known as nutritional programming. For instance, feeding rats a high-saturated-fat diet during pregnancy and lactation reduces DHA synthesis capacity and decreases mRNA expression of Fads2 and induced persistent changes in the LC-PUFA profiles of the offspring fed ALA or LA (18 : 2n-6)(Reference Kelsall, Hoile and Irvine24). Moreover, Fads2 mRNA expression is negatively correlated with CpG methylation in the promoter of this gene, denoting that epigenetic regulation of Fads2 may contribute to the long-term regulation of LC-PUFA synthesis in the offspring(Reference Hoile, Irvine and Kelsall25). Additional studies in rodents showed that dietary PUFA exert epigenetic changes that may lead to differential expression and phenotypes(Reference Lillycrop and Burdge23). Similarly, in gilthead seabream, parental feeding with progressively increased levels of ALA and LA and reduced EPA and DHA significantly up-regulated fads2 expression in the offspring(Reference Izquierdo, Turkmen and Montero18). Besides, offspring were better able to utilise dietary fats with low EPA and DHA and high ALA and LA, as it was denoted by their improved growth when fish were fed VO(Reference Izquierdo, Turkmen and Montero18). Moreover, this improved growth was persistent along fish life almost until reproduction, being related to the improved utilisation of dietary lipids as denoted by the regulation of lipoprotein lipase, carnitine palmitoyltransferase 1 and PPARα (ppara)(Reference Turkmen, Zamorano and Fernández-Palacios20). Besides, recent studies suggest the epigenetic basis of these changes in the offspring induced by parental diets(Reference Hoile, Irvine and Kelsall25).
However, little is known on the effect of fat quality of parental feeding on offspring health aspects such as immune system functioning or stress resistance. Dietary fatty acids play an important role in human(Reference Simopoulos2, Reference Calder26, Reference Yaqoob and Calder27) and fish health(Reference Montero, Izquierdo, Turchini, Ng and Tocher28). For instance, dietary fat quantity and quality have a profound effect on immune system functioning(Reference Calder26, Reference Yaqoob and Calder27, Reference Calder29–Reference Yaqoob31). Thus, dietary n-6 fatty acids, high in many VO, stimulate the production of pro-inflammatory eicosanoids and cytokines in terrestrial animals(Reference Calder26) and fish(Reference Montero, Izquierdo, Turchini, Ng and Tocher28, Reference Secombes, Hardie and Daniels32), whereas dietary DHA and EPA suppress the production of these pro-inflammatory substances(Reference Calder33). Among other physiological mechanisms, inflammation processes are mediated by the activation of phospholipases A2 that releases LC-PUFA from membrane phospholipids and cyclo-oxygenases (COX) that produce eicosanoids from these fatty acids(Reference Izquierdo, Koven and Holt34). Although COX have a great affinity for arachidonic acid (AA) yielding prostaglandins from 2-series, EPA is also a good substrate for COX producing 3-series prostaglandins and other eicosanoids that are less potent than AA derivatives and play an important anti-inflammatory role, through mediators termed E-series resolvins(Reference Serhan35). DHA-derived D-resolvins also have anti-inflammatory properties(Reference Marcheselli, Hong and Lukiw36). Thus, AA-derived eicosanoids enhance the production of inflammatory cytokines such as TNF-α and IL-1(Reference Calder26, Reference Montero, Terova and Rimoldi37) that are the first cytokines up-regulated after injuries or infection, whereas EPA and DHA reduce chronic inflammation by indirectly down-regulating both cytokines(Reference Farooqui, Horrocks and Farooqui38). Effects of the LC-PUFA on the recognition of specific antigens or pathogens have been less studied. For instance, the cell surface proteins, major histocompatibility complex I (MHCI) and II (MHCII) are up-regulated by AA-derived prostaglandins in mammals and fish(Reference Torrecillas, Román and Rivero-Ramírez39, Reference Hwang, Cho and Kwak40).
Dietary LC-PUFA markedly improve stress resistance in fish(Reference Montero, Izquierdo, Turchini, Ng and Tocher28). Thus, the increase of DHA and EPA in fish larvae improves resistance to handling or acute thermal stress(Reference Liu, Caballero and Izquierdo41) and, in juveniles, prevents elevation of cortisol levels during chronic stress(Reference Montero, Tort and Izquierdo42). Also, cortisol release by head kidney cells after stimulation with adrenal corticotrophin hormone is modulated by PUFA through lipoxygenase products(Reference Ganga, Tort and Acerete43). Moreover, these lipids may bind to glucocorticoid receptors, modulating the action of cortisol and its gene expression(Reference Montero, Terova and Rimoldi37). Besides, LC-PUFA also regulate the expression of mitochondrial and cytoplasmic chaperons such as heat shock protein 70 (HSP70) and HSP90, which are necessary for the assembly and maintenance of glucocorticoid receptors, increasing the binding capacity of this steroid receptor(Reference Benedito-Palos, Ballester-Lozano and Simó44). To our knowledge, there is no published information on the effect of parental feeding with different types of oils on the expression of these genes in the offspring.
Previous studies showed that broodstock nutrition in gilthead seabream can induce a nutritional programming effect on the offspring and alter the expression of lipid metabolism-related genes such as fads2, elovl6 (elongation of long-chain fatty acids family member 6) and cpt1 (carnitine palmitoyltransferase I) which can improve the utilisation of VO sources during the early(Reference Izquierdo, Turkmen and Montero18) and later juvenile stages(Reference Turkmen, Zamorano and Fernández-Palacios20). However, health and stress-related responses to high VO diets in the larval stage, larval growth and utilisation of the similar diets as a nutritional programming tool were not yet studied. The objective of the present study was to determine the potential persistence effect of parental feeding on offspring performance along the life span and expression of selected genes related to health and stress resistance. For that purpose, gilthead seabream broodstock were fed diets containing various FO/VO ratios to determine their effects on egg and offspring liver fatty acid profiles and performance along embryogenesis, larval development and juvenile on-growing periods. Afterwards, juveniles were submitted to a nutritional challenge feeding them diets containing different FO/VO ratios.
Materials and methods
All the experiments mentioned below were conducted according to the European Union Directive (2010/63/EU) on the protection of animals for scientific purposes at Fundación Canaria Parque Científico Tecnológico (FCPCT), University of Las Palmas de Gran Canaria (Canary Islands, Spain).
Parental feeding
A total of thirty-six gilthead seabream brood fish, ranging between 2 and 4 years of age, were randomly selected from another broodstock group of 120 fish in total. The selected individuals were randomly allocated to twelve 1000-litre capacity round fibreglass tanks with a ratio of two males and one female per tank. At the beginning of the experiment, the average weight and furcal length of the females were 1·35 ± 0·19 kg and 39·7 ± 2·4 cm, while males were 1·02 ± 0·08 kg and 35·8 ± 0·7 cm, respectively. Broodstock feeding was conducted in tanks with a flow-through system filled with filtered seawater (37·0 ± 0·5‰ salinity) at a renewal of 100 % per h and with strong aeration. Water temperature was ambient and ranged between 19·6 ± 0·2 and 21·3 ± 0·3°C. Tanks received indirect sunlight and the light period ranged between 10 and 12 h between January and May. Since gilthead seabream continues spawning for over 90 d, broodstock fish were firstly acclimated to the experimental tanks and fed with commercial diets (Skretting) from 7 January to 24 February and spawning was monitored. Since spawning quality parameters of broodstock kept in different tanks did not differ, tanks were randomly assigned to the different diet groups and fish were fed with one of the four experimental diets (Table 1) from 25 February to 3 May. Fish were fed twice per d (08.00 and 15.00 hours) with a daily ration of 1 % initial biomass of each corresponding experimental tank. Egg samples of all spawns per tank were collected for biochemical analysis during the initial period fed commercial diet and the second period of feeding the broodstock with experimental diets. Offspring from all the experimental groups were fed the same type of diet (commercially available) during the whole period from mouth opening until the nutritional challenge test. A schematic view of this nutritional programming history is shown in Fig. 1.
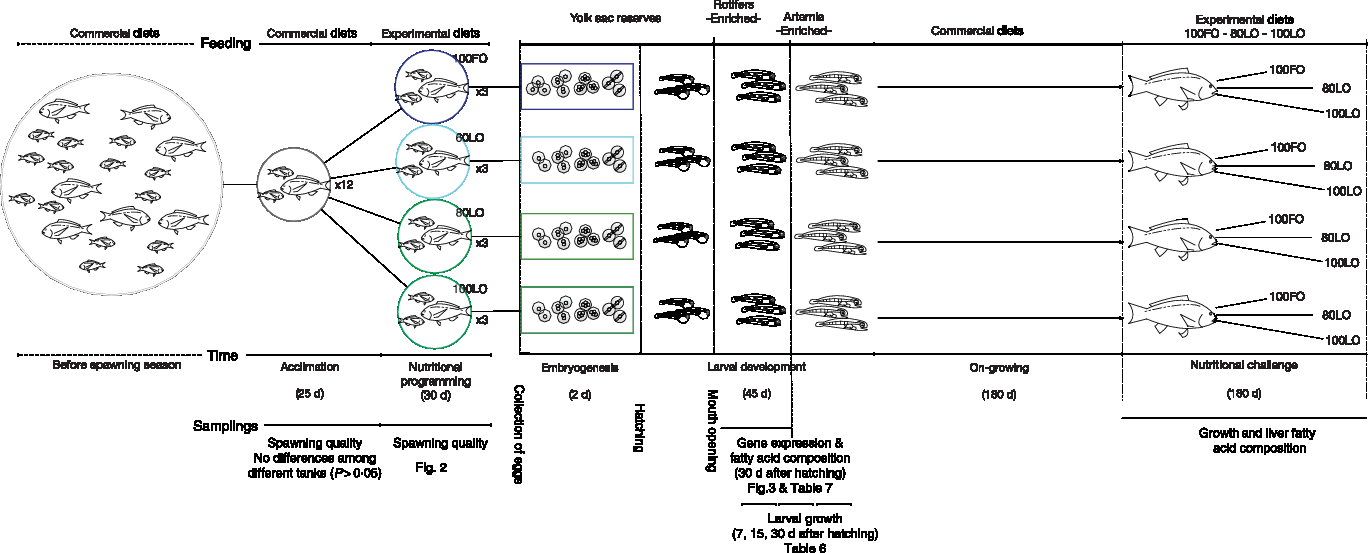
Fig. 1. Schematic view of the experimental design on nutritional programming in gilthead seabream. FO, fish oil; LO, linseed oil.
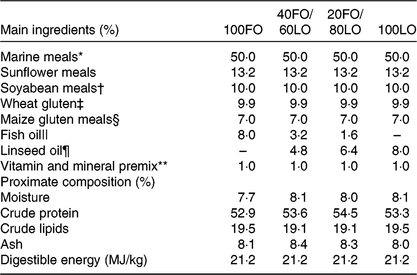
Table 1. Main ingredients, proximate composition and energy contents in the practical diets used for the nutritional programming of gilthead seabream broodstock fed different substitution levels of fish oil (FO) by vegetable oils
LO, linseed oil.
* Contains fish meal NA LT 70 and fish meal SA 68 (Feed Service Bremen).
† 48 Hi Pro Solvent Extra (Svane Shipping).
‡ Hedegaard.
§ Cargill.
|| South American fish oil, LDN Fish Oil.
¶ Ch. Daudruy.
** Supplied the following vitamins (mg/kg): A 3·8, D 0·05, E 102·4, K3 9·8, B1 2·7, B2 8·3, B6 4·8, B12 0·25, B3 24·8, B5 17·2, folic acid 2·8, H 0·14, C 80; minerals (mg/kg): Co 0·94, iodine 0·7, Se 0·2, Fe 32·6, Mn 12, Cu 3·2, Zn 67; other (g/kg): taurine 2·45, methionine 0·5, histidine 1·36, cholesterol 1·13. DSM, Evonik Industries and Deutsche Lanolin Gesellschaft.
Broodstock diets were formulated to have different proportions of FO and linseed oil (LO): 100FO, 40FO/60LO, 20FO/80LO and 100LO (Table 1). Substitution of FO in broodstock diets by LO raised the 18C fatty acids that are precursors of fads2, such as ALA and LA (18 : 2n-6), and reduced the 20 and 22C end products of fatty acid desaturation, such as AA, EPA and DHA (Table 2).
Table 2. Main fatty acids (FA) of practical diets used for the nutritional programming of gilthead seabream broodstock fed different substitution levels of fish oil (FO) by vegetable oils (% total FA)
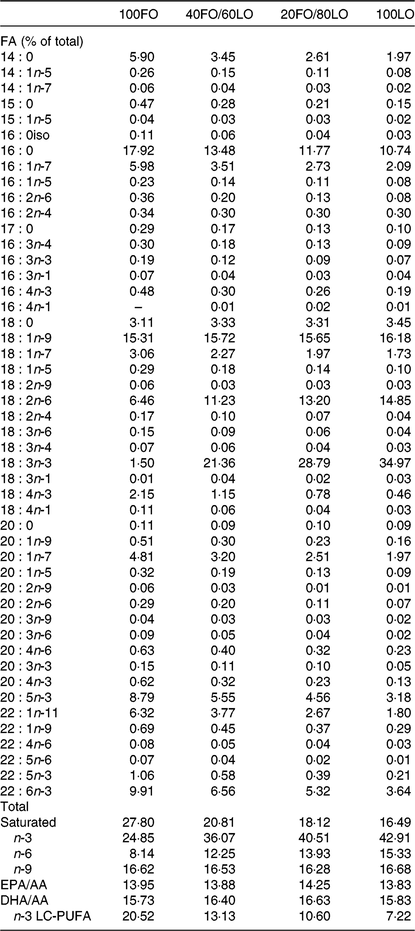
LO, linseed oil; AA, arachidonic acid; LC, long-chain.
Offspring production and quality during embryogenic stages
The amount and quality of the eggs and embryos produced were daily determined before and after feeding the experimental diets as explained above. For that, spontaneously spawned eggs obtained from each experimental broodstock were collected during the whole experimental period. Spawning occurred naturally in separate tanks with two males and one female as explained above. Eggs were collected from each tank in 5-litre beakers every day at 08.00 hours and transferred to the laboratory with aeration. Five samples of 5 ml were taken by graduated glass pipette and were counted in a Bogorov chamber under a binocular microscope (Leica Microsystems) to calculate the total number of eggs, the percentage of fertilised eggs and to determine the morphological characteristics. Egg viability rates were defined as the percentage of morphologically normal eggs at the morula stage and described as transparent, perfectly spherical with clear, symmetrical early cleavages(Reference Fernández-Palacios, Norberg, Izquierdo and Holt45). After that, fertilised eggs were individually incubated in ninety-six-well ELISA microplates using a micropipette. These eggs were kept at a constant 23°C temperature. After 24 and 72 h, rates of hatching and survival after 3 d were counted under a binocular microscope. With these percentage values, the total number of fertilised, viable and hatched eggs and larvae survived 3 d after hatching (dah) were calculated per female per kg basis.
Offspring performance during larval stages
After 1 month of feeding, the broodstock with the experimental diets and fertilised eggs from individual tanks were stocked into separate mass production tanks (2000 litres) at a density of 100 eggs/ml, and larvae produced under the same rearing protocol; each parental diet group was produced in triplicates. In brief, sand-filtered and UV-sterilised seawater flow was progressively increased from 10 to 40 % per h until 46 dah. Water was continuously aerated (125 ml/min) attaining 6·1 ± 0·4 parts per million (ppm) dissolved O2; average water temperature and pH along the trial were 20·8 ± 1·3°C and 7·89 ± 0·6, respectively; a 1500–3500 lx 12 h single central light (Mod TLD 36 W/54) photoperiod was provided and living phytoplankton (Nannochloropsis sp.) (250 ± 100 × 103 cells/ml) was added to the rearing tanks. Regarding feeding, from 3 to 32 dah, larvae were fed twice per d with rotifers (Brachionus plicatilis) at 5–10 rot/ml enriched those with commercially available emulsions (ORI-GREEN); from 15 to 17 dah, Artemia sp. enriched using the same emulsions as rotifers were added three times per d and from 20 dah, larvae were fed commercial diets (Gemma Micro). At 5, 15 and 30 dah, growth was determined by measuring the total length and body weight of thirty larvae anaesthetised with clove oil.
The nutritional challenge of juveniles
To challenge the ability of the progeny to utilise low FO diets, 4-month-old juveniles obtained from broodstock groups fed the different FO/LO ratios and reared until days as explained above and were fed either a positive control diet 100FO or the two most challenging diets 80LO and 100LO (Table 1). Diet 60LO was not tested since a marginal reduction in n-3 LC-PUFA (about 2·3 % n-3 LC-PUFA in dry diet) would be more than sufficient to fulfil the essential fatty acid requirements of gilthead seabream juveniles(Reference Ibeas, Izquierdo and Lorenzo46). Juveniles with a mean wet weight of 44·1 ± 0·5, 47·5 ± 0·2, 47·3 ± 0·3 and 38·4 ± 1·0 g from the progeny of broodstock fed with 100FO, 60LO, 80LO and 100LO, respectively, were randomly distributed into thirty-six tanks (thirty fish/treatment). All tanks (200-litre fibreglass cylinder tanks with a conical bottom and painted a light grey colour) were supplied with filtered seawater (37 ppm salinity) at a rate of 100 litres/h to assure good water quality during the entire trial. Water entered from the tank surface and drained from the bottom to maintain high water quality, which was tested daily, and no deterioration was observed. Water was continuously aerated (125 ml/min) attaining 6·1 ± 0·3 ppm dissolved O2. Average water temperature and pH along the trial were 21·6 ± 0·8°C and 7·82, respectively. Photoperiod was kept at 12 h light–12 h dark by fluorescent daylight, and the light intensity was kept at 1700 lx (digital Lux Tester YF-1065; Powertech Rentals). Juveniles from each broodstock were daily fed until apparent satiation with one of the three different diets in triplicates for 60 d. The growth was determined by measuring the wet body weight after 24 h starvation. Before measurements, all fish were anaesthetised with 10 ppm clove oil–methanol (50:50) in seawater. The whole body weight of all fish from each tank was determined at the beginning and the end of the feeding trial.
Proximate and fatty acid analysis
Experimental diets, spawned eggs, whole larvae and liver of juveniles were analysed for proximate and fatty acid composition. Moisture, protein(47) and crude lipid(Reference Folch, Lees and Sloane-Stanley48) contents of the eggs, larvae, juveniles and diets were analysed. Fatty acid methyl esters were obtained by trans-methylation of crude lipids as previously described(Reference Christie49). Fatty acid methyl esters were separated using gas chromatography (GC-14A; Shimadzu) following the conditions described previously(Reference Izquierdo, Watanabe and Takeuchi50) and identified by comparison to previously characterised standards and GLC-MS (Polaris QTRACETM Ultra; Thermo Fisher Scientific).
Molecular studies
At 30 dah, about 100 mg of unfed seabream larvae obtained from broodstock groups fed the different FO/LO ratios were collected from each tank and preserved in 500 μl of RNA Later (Sigma–Aldrich) overnight at 4°C. Then, RNA Later was removed, and samples were kept at –80°C until RNA extraction and analyses. RNA extractions were done using the TRI reagent (Sigma–Aldrich). For that purpose, approximately 75 mg of larvae were weighed, 1 ml of TRI reagent and four pieces of 1 mm diameter zirconium glass beads were added to 2 ml volume of Eppendorf tubes. Samples were homogenised using TissueLyzer-II (Qiagen) for 60 s with a frequency of 30/s until tissue thoroughly dissolved. A volume of 250 µl chloroform was added to homogenised samples and then centrifuged at 12 000 g for 15 min at 4°C for phase separation. The transparent upper aqueous phase containing RNA was mixed with 75 % ethanol and transferred into an RNeasy spin column where total RNA bonded to a membrane. After that RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen) with the protocol supplied by the manufacturer. Before real-time PCR analysis, two different potential housekeeping genes, β-actin (acbt) and ribosomal protein L27 (rpl27), were tested, and the stability across experimental groups was confirmed. Real-time quantitative PCR was performed in an iQ5 Multi-colour Real-Time PCR detection system (Bio-Rad) using acbt and rpl27 as the housekeeping genes in a final volume of 15 μl/reaction well and with 100 ng of total RNA reverse-transcribed to complementary DNA (cDNA). The primer concentration was 0·4 pmol/μl. Samples, housekeeping genes, cDNA template and reaction blanks were analysed in duplicate (Table 3). Primers for pla2 (thermostable phospholipase A2) and hsp90 were designed using Primer3web (http://primer3.ut.ee) version 4.1.0 from the sequence deposited in Genbank (https://www.ncbi.nlm.nih.gov/genbank/); access numbers are given in Table 3. Primer efficiency was tested with serial dilutions of a cDNA pool (1:5, 1:10, 1:100 and 1:1000) and was between 98 and 109·2 % for genes tested. A ninety-six-well PCR plate was used to analyse each gene, primer efficiency and blank samples (Multiplate; Bio-Rad). Melting-curve analysis was performed, and the amplification of a single product was confirmed after each run. Fold expression of each gene was determined by the ΔΔ CT method (2ΔΔCT)(Reference Livak and Schmittgen59). PCR efficiencies were similar, and no efficiency correction was required(Reference Livak and Schmittgen59, Reference Schmittgen and Livak60). Fold expression was related to that of offspring obtained from FO diet-fed broodstock (100FO).
Table 3. Primers, GeneBank accession numbers and reference articles for sequences of target and housekeeping genes
Tm, melting temperature; fads2, fatty acid desaturase 2; pla2, thermostable phospholipase A2; cox2, cyclo-oxygenase-2; tnf-α, TNF-α; ilb1, IL-1; mhc1, major histocompatibility complex class I; mhc2, major histocompatibility complex class II; gr, glucocorticoid receptor; hsp70, heat shock protein 70; hsp90, heat shock protein 90; actb, cytoplasmic actin 1; rpl27, ribosomal protein L27a.
Statistical analysis
The results are expressed as means and standard deviations (n 3) if not otherwise stated in the tables and figures. The data were compared statistically using ANOVA, at a significance level of 5 %. For the juvenile nutritional challenge test, due to the differences in the initial weights, this parameter was taken as a covariate in the statistical analysis. All variables were checked for normality and homogeneity of variance using the Kolmogorov–Smirnoff and the Levene test, respectively(Reference Sokal and Rohlf61). If significant differences were detected with ANOVA, means were compared by a Tukey test. All data were analysed using IBM SPSS v23.0.0.2 for Mac (IBM SPSS Inc.).
Results
Parental feeding and offspring production and performance during embryonic stages
Before the nutritional stimulus through the broodstock diet when all fish were fed with the same commercial diet, no significant (P > 0·05) differences were found in the total number of eggs produced by kg female per spawn (59 996·10 (sd 31 209·00)) or any spawning quality parameters (96·7 (sd 5·0) % fertilisation, 62·9 (sd 17·6) % viable eggs, 95·9 (sd 5·9) % hatching and 54·3 (sd 17·8) % 3-d-old larvae). However, during the broodstock nutritional stimulus, the increased substitution of dietary FO by LO up to 80–100 % LO significantly reduced the total number of eggs produced by kg female per spawn, whereas 60 % substitution did not affect this quality parameter (Fig. 2).
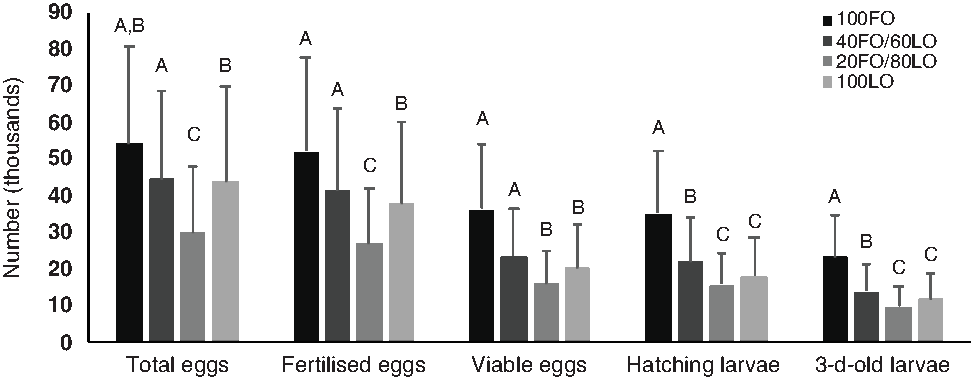
Fig. 2. Spawning quality in broodstock fed diets with the progressive substitution of fish oil (FO) by linseed oil (LO) during 4 months of spawning (n 105). Values are means, with standard deviations represented by vertical bars. A,B,C Mean values with unlike letters are significantly different (P < 0·05).
The total number of fertilised eggs followed the same tendency as the total number of eggs produced (Fig. 2) since fertilisation rates were not affected by the broodstock diet (P > 0·05). The inclusion of LO up to 80–100 % markedly reduced the percentage of viable eggs, and, consequently, the total number of viable eggs and hatched larvae were significantly lower when broodstock were fed with these diets than with the 100FO or 40FO/60LO diets (Fig. 2). Finally, survival of 3 dah larvae was also markedly reduced when broodstock were fed with 20FO/80LO and 100LO (Fig. 2).
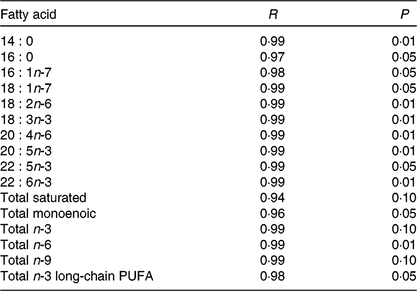
Before the nutritional stimulus through the broodstock diet, when all groups were fed with the same commercial diet, eggs proximate composition did not significantly differ among the different broodstock (average values of four groups (%), protein: 58·8 (sd 4·5), lipid: 24·1 (sd 1·1), moisture: 88·5 (sd 0·2), ash: 17·0 (sd 0·7) and P > 0·05). Besides, there were no significant differences in the fatty acid composition of the eggs obtained from the broodstock during this adaptation period before feeding the experimental diets (LA: 7·7 (sd 1·4), ALA: 6·8 (sd 2·0), AA: 1·0 (sd 0·2), EPA: 7·3 (sd 1·8), DHA: 15·0 (sd 1·9), % of total fatty acids, average values of all experimental groups and n 4). On the contrary, feeding broodstock the different FO/LO diets, although did not affect egg proximate composition (% of the total, protein: 58·8 (sd 4·5), lipid: 24·2 (sd 1·1), moisture: 88·5 (sd 0·2), ash: 17·1 (sd 0·7), average values of all experimental groups and n 4) significantly modified fatty acid profiles (Table 4). Thus, dietary FO substitution by LO significantly (P < 0·05) reduced the egg contents in SFA, particularly 14 : 0, 15 : 0 and 16 : 0 monoenoic, particularly 14 : 1 and 16 : 1, as well as n-3 LC-PUFA, including EPA and DHA (Table 4). On the contrary, FO substitution by LO increased the egg contents in ALA, raising the n-3 fatty acid levels and LA and n-6, particularly in fish fed high-LO diets: 20FO/80LO and 100LO (Table 4). From all the fatty acids identified in seabream eggs, the levels of ten of them were highly correlated with fatty acid profiles of broodstock diet (Table 5).
Table 4. Fatty acid (FA) composition (% total FA) of eggs obtained from gilthead seabream broodstock after feeding broodstock diets with the progressive substitution of fish oil (FO)*
(Mean values and standard deviations)
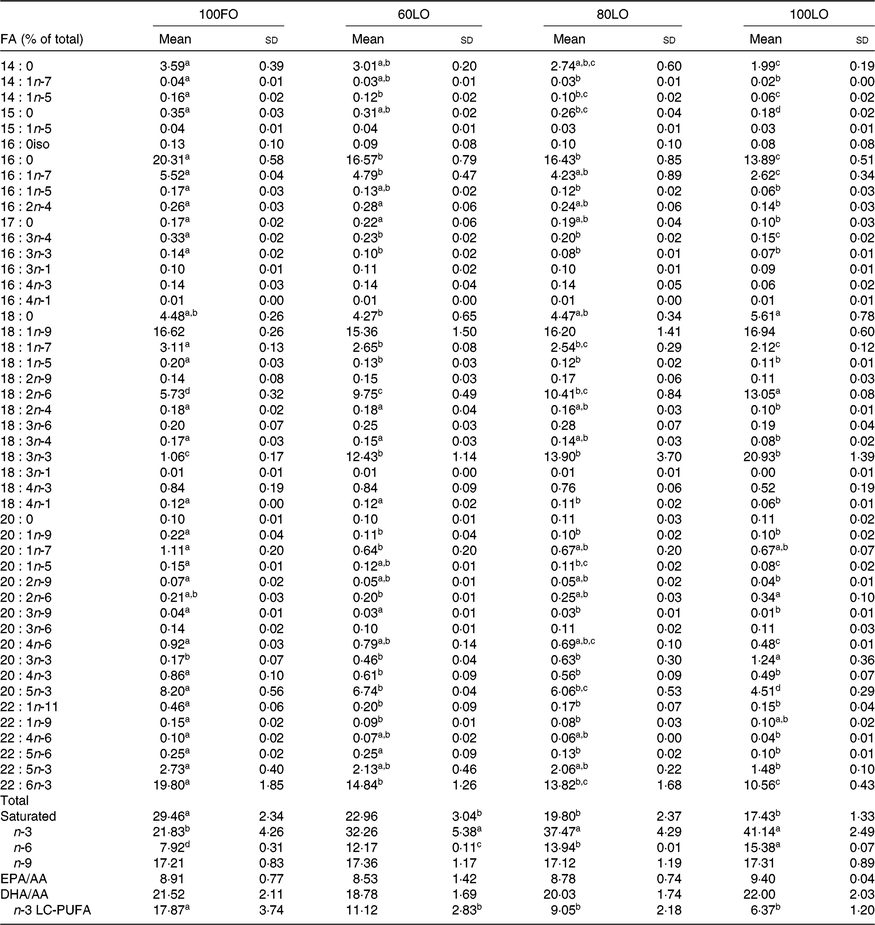
LO, linseed oil; AA, arachidonic acid; LC, long-chain.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* n 4 (one pool of eggs from all the spawns per broodstock tank).
Table 5. Relation between dietary and egg fatty acid content for each given fatty acid or groups of fatty acids (n 12)
Offspring performance during larval stages
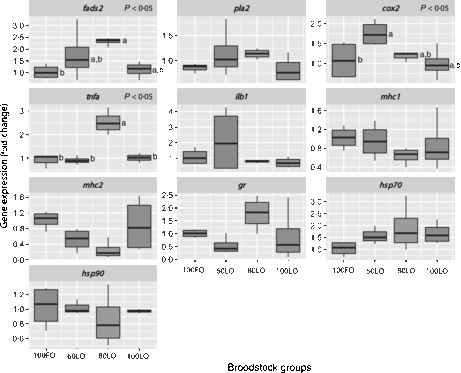
Even though all larvae underwent the same common rearing protocols with rotifers, Artemia and microbound weaning diets, at 30 dah, larval growth in terms of total length (P < 0·05) was lower in offspring from parents fed 100LO (Table 6). Parental feeding with 80 % FO substitution by LO (80LO group) increased the relative expression of the fads2 gene in the 30 dah larvae in comparison to 100FO (P > 0·05) (Fig. 3), whereas higher levels of inclusion of LO (100LO) in the broodstock diets led to a reduction in fads2 expression that showed intermediate values. Expression of pla2 followed a similar trend but did not show significant differences. The cox2 gene expression level was also up-regulated by the increase in LO in parental diets up to 40FO/60LO (P < 0·05, Fig. 3) and did not differ with the expression in larvae from parents fed higher LO levels. Besides, TNF-α gene (tnf-α) expression was highest in 20FO/80LO larvae when compared with the other ones (P < 0·05, Fig. 3). There were no significant differences in the expression of the IL-1 gene (ilb1). Although we did not find any significant differences among mean values of mhc1 andmhc2 (P > 0·05), expression of mhc2 was negatively related to the increase in dietary LO levels in broodstock diets up to 20FO/80LO (R −0·82, P = 0·001), in contrast to what was observed in fads2 expression levels. Finally, regarding the stress-related genes, no clear differences were found in the expression of gr (glucocorticoid receptor) or hsp90 (P < 0·05), whereas hsp70 expression was higher, although not significant, in LO substitution groups than in the other groups (R 0·48, P = 0·07) (Fig. 3).
Table 6. Growth of larvae obtained from broodstock fed diets with the progressive substitution of fish oil (FO) by linseed oil (LO) and undergoing the same commercial feeding protocol from first feeding*
(Mean values and standard deviations)

dah, Days after hatching.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* n 60.

Fig. 3. Box and whisker plots of relative fold expression of ten different genes at 30 d posthatch gilthead seabream larvae. fads2, Fatty acid desaturase 2; pla2, thermostable phospholipase A2; cox2, cyclo-oxygenase-2; tnfa, TNF-α; ilb1, IL-1; mhc1, major histocompatibility complex class I; mhc2, major histocompatibility complex class II; gr, glucocorticoid receptor; hsp70, heat shock protein 70; hsp90, heat shock protein 90; FO, fish oil; LO, linseed oil. Indications are as follows: error bars = maximum and minimum fold expression, boxes = upper and lower quartiles, and vertical lines inside the box areas = median. n 4 for all the groups and genes. a,b Values with unlike letters are significantly different (P < 0·05). No indications mean no significant difference (P > 0·05).
Analyses of the fatty acid composition of the 30 dah larvae showed that there were no significant differences in the individual fatty acids among different fish groups (P > 0·05) (online Supplementary Table 1). However, conversion rates of fatty acids from 18 : 3n-3 to 18 : 4n-3 and from 20 : 4n-3 to 20 : 5n-3 showed a strong correlation with fads2 gene expression (0·93 and 0·92, respectively) (Table 7). Besides, correlations with fads2 expression were lower for 18 : 2n-6 to 18 : 3n-6 and 20 : 3n-6 to 20 : 4n-6 conversion (Table 7). Moreover, end products of LC-PUFA synthesis such as 22 : 5n-6 (DPA) and DHA were highly correlated with fads2 expression in 30 dah larvae (Table 7).
Table 7. Principal substrates and products of Δ-6-desaturase enzyme and correlation with fatty acid desaturase 2 (fads2) gene expression at 30 days after hatching larvae obtained from broodstock fed with different diets
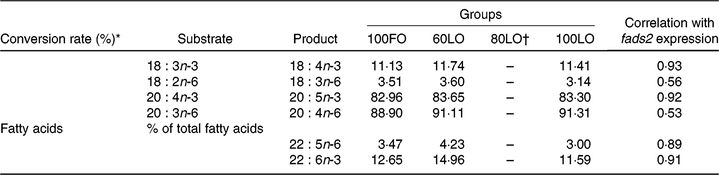
* Conversion rates were calculated as 100×(product area/(product area + substrate area)).
† No data available.
The nutritional challenge of juveniles
Before the beginning of the nutritional challenge, initial weights of juveniles coming from 100LO-fed broodstock were smaller than the rest (P < 0·05), followed by those coming from broodstock fed 100FO diet (Table 8). Therefore, there was a significant effect of the broodstock diet (family effect) on the initial weight denoted by two-way ANOVA (P < 0·001) (Table 8). During the nutritional challenge, the lowest growth was obtained in fish coming from broodstock fed FO diet, whereas FO replacement by LO in broodstock diet enhanced the growth of juveniles (Table 8). As a consequence, after 60 d of feeding the nutritional challenge diets, there was a significant effect of the broodstock diet (family effect) on the final body weight, weight gain and specific growth rate, as denoted by two-way ANOVA (P < 0·001) (Table 8).
Table 8. Growth performance parameters of 4-month-old juveniles fed with three different diets for 60 d
(Mean values and standard deviations; n 3)

FO, fish oil; LO, linseed oil; SGR, specific growth rate.
* Two-way ANOVA: broodstock origin defined as family, diets indicate different diets fed during the juvenile stage. Due to differences in initial weights, two-way ANOVA, initial weights are taken as covariate. * P < 0·001 and – P > 0·05 (no difference).
† Weight gain = final average weight − initial average weight.
‡ SGR (%/d) = (Ln (final weight (g)) − Ln (initial weight (g)))/(number of days) × 100.
Broodstock diets and juvenile challenge diets did not significantly (P > 0·05) affect the lipid content of liver tissue (Table 9). Fatty acid profiles of liver from juveniles after the nutritional challenge were significantly affected by both the juvenile’s diet and the broodstock diet (Table 9). In particular, an increase in LO in the juvenile diet significantly (P < 0·001) raised 18 : 2n-6, 18 : 3n-6 and 18 : 3n-3, reflecting the diet contents, whereas the LO increase in the parental diet leads to a general reduction (P < 0·001) in these fatty acids that are precursors of LC-PUFA (Table 9). Regarding LC-PUFA, there was a reduction in EPA, AA or DHA levels with the increase in LO in the diet of juveniles (P < 0·001) (Table 9), whereas an increase in LO levels in the broodstock diet up to 60LO tends to increase AA and DHA (P < 0·01) and EPA (P < 0·001). However, 20 : 3n-6 was found to be similar across juvenile dietary treatments (P > 0·05) (Table 9).
Table 9. Lipid (% of DM) and fatty acid composition (% total fatty acids) of livers of juveniles from gilthead seabream broodstock fed diets with the progressive substitution of fish oil (FO) by linseed oil (LO)†
(Mean values and standard deviations)

A,B Differences between fish fed with different diets during the nutritional challenge coming from the same parental feeding.
x,y,z Differences between fish coming from different parental feeding and fed the same diet during the nutritional challenge (P < 0·05).
** P < 0·001, * P < 0·01 and – P > 0·05 (no difference).
† n 3 (pool of three livers from each tank).
Discussion
Given the importance of EPA and DHA for human health(Reference Connor1), and given the limited availability of feedstuffs high in these essential fatty acids(5), it is necessary to maximise the ability of farmed animals, particularly fish, and to synthesise EPA and DHA from their 18C atom precursors. Among different strategies, nutritional programming of farmed animals to better utilise dietary fatty acids is considered very promising(Reference Izquierdo, Turkmen and Montero18, Reference Turkmen, Castro and Caballero19). In mammals, nutritional interventions during the early developmental stages through dietary changes along pregnancy and lactation may induce differentiations in phenotypes that are induced by conditions during these stages(Reference Gluckman, Hanson and Spencer62). Besides, PUFA are among the nutritional factors known to regulate metabolic decisions of the offspring and adaptation to the environment later in life(Reference Lillycrop and Burdge23).
The results of the present study confirmed that it is possible to induce positive and persistent changes in the LC-PUFA profiles in offspring through parental feeding. Thus, the increased replacement of FO by LO in broodstock diets was directly reflected in egg fatty acid profiles that allowed supplying lower LC-PUFA and higher 18C fatty acid precursors as a nutritional stimulus in an early stage of the development. These changes in egg fatty acid profiles would be expected, being gilthead seabream a multibatch spawner whose oligolectic eggs largely depend on the continuous intake of nutrients during reproduction(Reference Fernández-Palacios, Norberg, Izquierdo and Holt45). The results are in agreement with the reduced LC-PUFA and increased LA and linolenic acid contents in eggs of gilthead seabream broodstock fed low FO diets found in previous studies(Reference Izquierdo, Turkmen and Montero18, Reference Fernández-Palacios, Norberg, Izquierdo and Holt45, Reference Mourente and Odriozola63) and induced the nutritional programming of the offspring during early embryogenesis. Consequently, during later developmental stages (30 dah larvae), the increased FO replacement by LO in the broodstock diets enhanced the offspring ability to synthesise LC-PUFA, as denoted by the increased fatty acids conversion rates and production of end products of fatty acids biosynthesis, DPA and DHA. These adaptations in the offspring fatty acid profiles were similar when juveniles coming from broodstock fed up to 60 % replacement of FO by LO showed increased AA, DHA and, mostly, EPA in the liver when they were fed diets with low LC-PUFA and high 18C fatty acid precursors. These results confirm the long-term effect of feeding broodstock with different FO/LO ratios on the fatty acid profiles of the offspring along the life span(Reference Izquierdo, Turkmen and Montero18, Reference Turkmen, Castro and Caballero19, Reference Adam, Skjærven and Whatmore64, Reference Morais, Mendes and Castanheira65). However, it is important to note that the long-term effects seem to be related to the ratios between different 18C fatty acids (especially LA and ALA) and n-3 LC-PUFA (especially EPA and DHA) in the broodstock diets(Reference Izquierdo, Turkmen and Montero18, Reference Turkmen, Zamorano and Fernández-Palacios20). Studies regarding the effects of 18C fatty acid-rich diets are being conducted to give a better understanding of individual fatty acids on nutritional programming of gilthead seabream (Turkmen et al., unpublished results).
Changes in larval fatty acid profiles were highly correlated with the up-regulation of fads2 in seabream larvae coming from parents fed increased FO replacement by LO up to 60 %. These results agree well with studies conducted in mammals where maternal diets affect the fatty acid composition of offspring liver, inducing long-term changes in PUFA metabolism with Fads2 expression(Reference Hoile, Irvine and Kelsall25). The first step of n-3 LC-PUFA synthesis in vertebrates is achieved by fatty acyl Δ-6-desaturase (Δ-6) enzyme, encoded by fads2 gene, by introducing a double bond in a specific position of LC fatty acids. In the first step of this pathway, LA (18 : 2n-6) and ALA (18 : 3n-3) can be converted to 18 : 3n-6 and 18 : 4n-3, respectively. After an elongation step, these fatty acids can be converted to 20 : 3n-6 and 20 : 4n-3. From these precursors, there is another desaturation step catalysed by a fatty acyl Δ-5-desaturase (Δ-5) enzyme involved in the conversion to 20 : 5n-3 (EPA) and 20 : 4n-6 (AA). Up to date, there is only one desaturase gene (fads2) isolated from gilthead seabream(Reference Seiliez, Panserat and Corraze16), and low Δ-5 activity has been found during in vitro studies(Reference Tocher and Ghioni15). Fish species have evolved differently, and fatty acid desaturase can vary among different species(Reference Castro, Tocher and Monroig7). For instance, in zebrafish (Danio rerio), the fads2 gene gives rise to both Δ-5 and Δ-6 activities(Reference Hastings, Agaba and Tocher66). Indeed, in the present study, a strong correlation was found between fads2 expression levels and conversion rates for products of Δ-6 activity, ALA (18 : 3n-3) to 18 : 4n-3 and Δ-5 activity, 20 : 4n-3 to EPA, from n-3 series. However, such correlations were lower for fatty acids from n-6 series, namely, 18 : 2n-6 (LA) to 18 : 3n-6 conversion and 20 : 3n-6 to 20 : 4n-6. This fact could be related to a higher affinity of fads2-derived enzymes to 18 : 3n-3 and 20 : 4n-3 substrates rather than 18 : 2n-6 and 20 : 3n-6, as proposed for different fish species(Reference Bell, Tocher and Farndale67–Reference Thanuthong, Francis and Senadheera70) including gilthead seabream(Reference Zheng, Seiliez and Hastings68), or to the higher content in ALA in diets and fish tissues.
In addition, increase replacement of FO by LO in broodstock diets increased growth rates in juveniles during the nutritional challenge test. It is also possible that the higher growth rate of juveniles from 100LO broodstock, in comparison to those from 100FO-fed broodstock, was related to a potential compensatory growth caused by their lower size at the beginning of the challenge test. However, the initial size of juveniles from 80LO broodstock was not lower than that of juveniles from 100FO broodstock, whereas their weight gain when fed either 100FO or 80LO was 32–40 % higher, confirming the positive effect of FO replacement by LO in broodstock diets. These results are in agreement with the positive effect of FO replacement by LO in broodstock diets on growth of juveniles challenged with low FO and low fishmeal(Reference Izquierdo, Turkmen and Montero18, Reference Turkmen, Zamorano and Fernández-Palacios20). Overall, the present study showed that by modifying the fatty acid profiles of parental diets, namely, replacing FO by LO, it is possible to nutritionally programme gilthead seabream offspring to be better adapted to utilise low FO/high LO diets during juvenile stages.
However, too large substitution of FO by LO in broodstock diets may have negative consequences for the offspring. For instance, FO replacement of 80 and 100 % by LO in broodstock diets leads to reduced fecundity in gilthead seabream, denoted by a reduction in the number of eggs produced, and lower viability of the embryos produced, reflected in a lower survival in 3-dah larvae. These results are in agreement with previous studies(Reference Izquierdo, Turkmen and Montero18) and are related to the essential role of n-3 LC-PUFA in the embryonic and larval development and the high requirements for these fatty acids in broodstock diets(Reference Fernández-Palacios, Izquierdo and Robaina71, Reference Izquierdo, Fernandez-Palacios and Tacon72). Moreover, these negative effects of too high FO replacement in broodstock diets were persistent along offspring life. Thus, although all larvae were reared following the same feeding protocol and conditions, there was a lower growth in 30-dah offspring obtained from 100 % LO-fed broodstock, compared to those from other broodstock groups. Besides, fads2 expression showed the intermediate level in these larvae although fed with the same commercial diets. It is known that very high replacement of FO by LO in the diets can down-regulate fads2 expression in gilthead seabream(Reference Izquierdo, Robaina and Juárez-Carrillo17, Reference Izquierdo, Turkmen and Montero18, Reference Turkmen, Castro and Caballero19). The mechanisms behind down-regulation of fads2 by n-3 LC-PUFA are not clear yet(Reference Tocher73), whereas it is well known that high levels of n-3 LC-PUFA, especially DHA, can inhibit fads2 expression in Atlantic salmon(Reference Betancor, Sprague and Sayanova74–Reference Thomassen, Rein and Berge76).
Regarding the expression of health-related genes, in 30-dah larvae, the increased substitution of FO by LO in broodstock diets up to 60 or 80 %, respectively, leads to the up-regulation of cox2 or tnf-α. COX2 is one of the enzymes that locally produces in the cells eicosanoids, oxygenated derivatives of LC-PUFA(Reference Rowley, Knight and Lloyd-Evans77), and has a great affinity for AA producing 2-series prostanoids, among them PGE2 induces production and release of inflammatory cytokines such as TNF-α, IL-1 and IL-6(Reference Calder26). In the present study, up-regulation of cox2 in 30-dah offspring of broodstock fed 60 % LO, although all larvae were fed the same diet, may be related to the fatty acid profiles in early stages, since eggs from these parents showed the lowest EPA/AA (8·5 in 60 % LO v. 9·4 in 100 % LO eggs) and, particularly, DHA/AA ratios (18·8 in 60 % LO v. 22·0 in 100 % LO eggs). Indeed, EPA and DHA reduce gene expression of Cox2 in mammals(Reference Farooqui, Horrocks and Farooqui38, Reference Song, Li and Leonard78) and fish(Reference Torrecillas, Román and Rivero-Ramírez39, Reference Ganga79) and, therefore, lower proportional contents of these fatty acids in developing embryo may have induced the up-regulation of cox2, which persisted even in 30-dah larvae. It is possible that, even if cox2 was up-regulated in offspring from broodstock fed 60 % LO, there was a higher EPA and DHA content in eggs that would inhibit the production of PGE2 avoiding the pro-inflammatory effect of these prostanoids. However, further replacement of FO by LO up to 80 % in the diets, led to reduced EPA and DHA contents in the eggs, which could be partly related to the up-regulation of tnf-α. These results would agree with the up-regulation of cox2 and tnf-α found in the posterior gut of seabass juveniles fed reduced dietary levels of n-3 LC-PUFA(Reference Torrecillas, Román and Rivero-Ramírez39). However, expression of other cytokines, such as ilb1, or histocompatibility complex mhc1 and mhc2 was not significantly affected. Still, the expression of mhc2 follows the same trend to be reduced in the offspring with the FO replacement by LO in broodstock diets up to 80 % (R = −0·82, P = 0·001). This tendency agrees well with the down-regulation of mhc2 found in the anterior gut of European sea bass juveniles fed low-fishmeal and -FO diets(Reference Torrecillas, Román and Rivero-Ramírez39). Nevertheless, the effect of n-3 LC-PUFA on cytokine production may be different depending on the cellular type, as found in mammals(Reference Petursdottir and Hardardottir80), and it should be considered that in the present study we measured the gene expression levels in the whole body. Overall, these results indicate a significant effect of parental feeding on certain pro-inflammatory genes in the offspring, in agreement with the up-regulation of tnf-α found also in mammals when parents were fed micronutrient-deficient diets(Reference Kumar, Lalitha and Pavithra81). In juveniles of several fish species, FO replacement by VO, namely, unbalanced n-3/n-6 ratios, markedly affects stress resistance and plasma cortisol levels(Reference Montero, Izquierdo, Turchini, Ng and Tocher28, Reference Ganga79, Reference Bell, McVicar and Park82). The heat shock proteins, in turn, facilitate stress response by increasing the binding capacity of the steroid receptor(Reference Basu, Srivastava and van Eden83). In the present trial, fatty acid profiles of the broodstock diet did not affect gr or hsp90 expression in 30 dah larvae(Reference Izquierdo, Koven and Holt34). There was a trend towards up-regulation of hsp70 with the increased LO up to 80 % that would be in agreement with the up-regulation of hsp70 in European seabass larvae fed increased LA(Reference Izquierdo, Koven and Holt34). Further studies must be conducted to clearly determine the potential effects of nutritional programming through broodstock diets on stress resistance of gilthead seabream offspring.
In summary, the present study confirms our previous studies showing that it is possible to nutritionally programme gilthead seabream offspring through the modification of the fatty acid profiles of parental diets to improve the growth performance of juveniles fed low FO diets, inducing long-term changes in PUFA metabolism and up-regulation of fads2 expression during the larval stage. However, high replacement of FO by LO in broodstock diets may negatively affect embryo and larvae development, reducing growth and survival. Finally, the study also provides the first pieces of evidence of the up-regulation of pro-inflammatory process-related genes in the offspring of seabream fed increased FO replacement by LO. Further studies are being conducted to determine the effects of nutritional programming in stress and disease resistance of seabream offspring and to find the optimal ratios of FO/LO ratio in the parental diets. Other indicators of good performance would also be included in the evaluation.
Acknowledgements
The authors thank Dr Sadasivam Kaushik for his suggestions on the final version of the manuscript. The authors also thank anonymous referees who gave very valuable suggestions in the revision of this article. The views expressed in this work are the sole responsibility of the authors and do not necessary reflect the views of the European Commission.
This work has been (partly) funded under the EU seventh Framework Program by the ARRAINA project no. 288925: Advanced Research Initiatives for Nutrition & Aquaculture.
M. I. and S. T. designed the study, analysed the data and wrote the manuscript. H. F.-P., C. M. H. and D. M. conducted broodstock, larvae and juvenile trials, respectively. Molecular biology samples were analysed by S. T. under the supervision of M. J. Z. All authors read and approved the final manuscript. S. T. had primary responsibility for final content. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000977