Out of all the proposed beneficial effects on health of long chain omega-3 fatty acids, those affecting cardiovascular disease are, nowadays, those that receive more attention in clinical medicine. Evidence from epidemiological, observational and clinical trial studies have led the American Heart Association (AHA) to recommend their consumption, and thus omega-3 fatty acids have emerged as real players in the prevention of cardiovascular (mainly coronary) events(Reference Kris-Etherton, Harris and Appel1, Reference Lichtenstein, Appel and Brands2). These recommendations include two servings of blue fish a week for the general population (to achieve a mean of 500 mg/d), and 1 g/d of marine omega-3 (EPA and DHA) in patients with coronary disease. As the contribution of omega-3 in the diet of many Western countries is far below the recommended figures, there is a clear need to increase their consumption. Although the inclusion of novel agents in the therapeutic guidelines of the AHA scientific committee is reserved for those interventions whose effectiveness and safety is beyond all doubt, controversial results have been published in recent reviews and meta-analysis reporting both positive and negative findings on the effectiveness of omega-3 fatty acids(Reference Lavie, Milani and Mehra3–Reference Filion, El Khoury and Bielinski6).
Research into omega-3 fatty acids has evolved from studies where their activity was tested, to a more complex scenario, where authors look for the potential underlying mechanisms that may be causing these effects. In other words, authors have switched from a perspective in which they treated to show the effects of omega-3 to another in which they aim to show how they work. Furthermore, this may lead to a bias when trying to evaluate the efficacy of the intervention, which is theoretically yet to be proven. Scientists may well think that journals are not very eager to include articles that show ‘again’ that omega-3 fatty acids are effective, instead of why they are so. Actually, research in this field is extremely active in this moment. A prior examination of the studies reported in PubMed regarding omega-3 fatty acids and cardiovascular disease or cardiovascular risk factors accounted for more than seventy thousand items. Over the last four years, the production of such studies has doubled (Fig. 1). In this extremely active context, is therefore not surprising that those studies that summarize the overall scientific information available at a given moment tend to modify their opinion depending on the articles included.
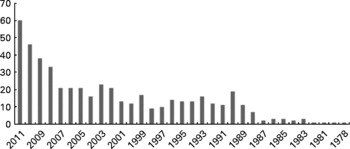
Fig. 1 Published items: Clinical trials, reviews and meta-analysis, involving studies on omega-3 fatty acids and cardiovascular events or cardiovascular risk factors, based on the 494 items (452 clinical trials and 42 reviews and meta-analysis) found pertinent for this review (pre-selected).
In the present review, we will summarize the information available as of January 20th, 2012, on the effects of omega-3 fatty acids on cardiovascular events, evaluating the evidence from clinical trials and randomized controlled trials. With this purpose in mind, we will conduct a search for recent reviews and meta-analysis, and a primary search for original clinical trials and randomized controlled trials.
Material and methods
The research question applied to the systematic review was ‘Are marine omega-3 fatty acids useful for reducing the incidence of total death, cardiovascular death coronary events or cardiovascular events?’ This article is limited to long chain (also called marine) omega-3. We did not review the effects of other omega-3 fatty acids. We focused only on clinical trials and randomized controlled trials. We limited our search to trials with a follow-up of 6 months or more.
Types of outcome measurements
Primary outcome
Odds ratio of cardiovascular events (defined as stroke, coronary events, myocardial infarction or angina, peripheral limb disease event or death from cardiovascular causes).
Secondary outcomes
Odds ratio for total mortality, cardiac death or total coronary events (both fatal and non-fatal).
Search methods for identification of studies
The search strategy for the identification of the studies is summarized in Fig. 2. The review included articles written in English, and published before January 20th, 2012. We used the two most commonly used electronic databases (Medline via PubMed and EMBASE), and combined search terms for omega-3 fatty acids (Omega-3 OR omega 3 OR polyunsaturated fatty acid OR pufa OR eicosapentanoic acid OR EPA OR ethyl eicosapentaenoic acid OR eepa OR docosahexanoic acid OR DHA OR docosapentaenoic acid OR DPA OR fish oil OR n-3 fatty acids OR long chain fatty acids OR oily fish OR fish oil) with those for cardiovascular disease (Cardiovascular OR coronary OR stroke OR peripheral limb disease OR peripheral artery disease OR heart OR heart failure OR prevention). We set a time limit for reviews and meta-analysis articles (published after January 1st, 2000).

Fig. 2 Schematic representation of selection of the articles included in this review.
Study selection
When analyzing reviews and meta-analyses, the authors decided whether the item was pertinent or not, based on the title and abstract reading. If pertinent, the referenced articles included in the item (review or meta-analysis) were passed to the list of potential articles to include in this review. When evaluating the clinical trials and randomized controlled trials, the authors made an initial decision on the pertinence of the article and whether it should remain on the list based on the title and abstract reading, with the following inclusion and exclusion criteria:
(1) Inclusion criteria
Randomized controlled trials and clinical trials which directly assessed the impact of the intake of measured quantities of marine omega-3 fatty acids (either in the form of foods or supplements or capsules) on any of the MeSH terms used for defining clinical outcomes, for at least 6 months.
Studies which reported total deaths and, at least, one of the following: Cardiovascular mortality, acute (fatal and/or non-fatal) myocardial infarction (AMI), angina, revascularization, stroke (fatal and/or non-fatal), left ventricle function.
(2) Exclusion criteria
Articles written in languages other than English.
Sub-studies of other studies that qualify for inclusion.
Studies in which α-linolenic acid (ALA) was present as an active means of treatment.
Studies in which the methodology described in the title/abstract showed critical concerns.
Studies in which the omega-3 fatty acids were part of another concomitant treatment, not applied to the group not receiving omega-3.
Studies in which another active treatment was tested in synergy with omega-3.
Studies in which the intake of omega-3 was not measured or estimated in g/d.
Studies limited to populations with ophthalmologic, obstetric, oncological, gynecologic, renal, neurological or psychiatric disorders.
(3) Eligibility
Eligible patients were men and women aged 18 years or older who were involved in any of the studies which met the inclusion criteria and did not fulfill any exclusion criteria.
The two authors who validated the studies that appeared in the first search (J. D-L and P. P-M) rated all the articles as pertinent or non-pertinent. Pertinent articles were measured against the Jadad Scale for quality score(Reference Jadad, Moore and Carroll7). A score equal or lower to 2/5 in the Jadad score led to the article's withdrawal from the list. Where only one of the authors rated the article as pertinent, a third author (F. P-J) rated the article, and decided on the pertinence.
Study analysis
Statistical analysis was performed using Review Manager 5·017 (Cochrane Collaboration, Oxford, UK). We assessed heterogeneity between studies using Chi2 indicating significant heterogeneity and I2 with suggested thresholds for low (25 %–49 %), moderate (50 %–75 %), and high (>75 %) values. We used the fixed effects model as default, and the random effects model when heterogeneity between studies was high for the end point of interest. For all analyses, we considered p ≤ 0·05 (2-sided) as significant. Summary effect estimates are presented as an odds ratio (OR) with 95 % confidence intervals (CI). Overall effect was assessed by the Z test. We used the Mantel-Haenszel statistical methods for analysis. For cardiovascular events, we considered all components of coronary events, stroke, cardiac death, or peripheral vascular disease events, when available from the articles, irrespective of whether the article included all or some of them.
Self-quality assessment
We used the Quality of Reporting of Meta-Analyses (QUOROM) guidelines to perform the self-quality assessment of the present work. This file can be located in the manuscript (Supplementary Table 1).
Results
The search for clinical trials and randomized controlled trials resulted in 3246 articles, of which 332 were found pertinent. The search for reviews and meta-analysis in omega-3 resulted in 691 articles, of which 42 were found pertinent. The evaluation of the references of these articles led to the inclusion of 120 additional references. A total of 452 articles passed the first evaluation stage on pertinence. Further evaluation of these articles, submission to inclusion and exclusion criteria and to the Jadad Scale led to the final number of 21 articles that are included in the analysis(Reference Dehmer, Popma and van den Berg8–Reference Nodari, Triggiani and Campia28). This is illustrated as a flow-chart in Fig. 2.
(A1) Effects on cardiovascular events
Cardiovascular events were reported in 14 of the selected studies, involving 45 285 participants. Cardiovascular events were lower in the omega-3 group, with 3902 events in 22 669 participants in the omega-3 arm and 4102 events in 22 616 participants in the control group (OR 0·90; [0·85–0·96]); Z = 3·28, p = 0·001 (Fig. 3). We found medium heterogeneity in this group (Chi2 = 27·77; I2 = 53 %). Two studies, Burr 2003 and Nodari 2011, caused the largest part of the heterogeneity, accounting for 29 % and 14 % of the I2 index, respectively. Furthermore, excluding these 2 studies resulted in an I2 = 0 %.
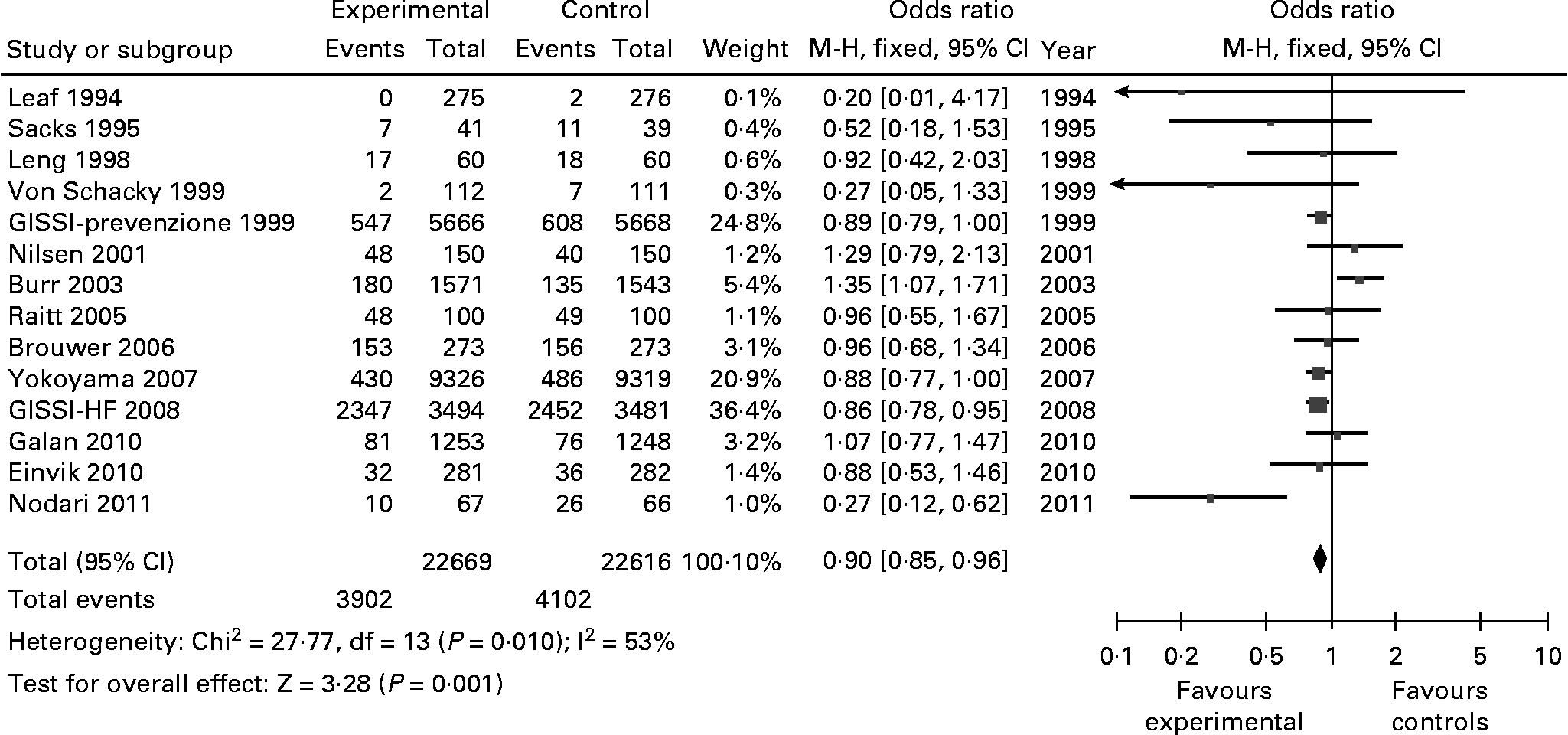
Fig. 3 Cardiovascular Events (fatal and non-fatal, involving coronary, cardiac, stroke, peripheral artery disease events). M-H: Mantel-Haenszel. Fixed: Fixed effects. CI: Confidence Interval.
(A2) Effects on total mortality
Seventeen studies described at least one death and reported total mortality (50 468 participants). Pooled total mortality of all studies reported 2205 deaths in 25 288 participants in the omega-3 group and 2283 deaths in 25 180 participants in the control group, resulting in an OR of 0·95 (0·89–1·02) with a trend towards lower mortality for the Omega-3 group (Z for overall effect 1·45, p = 0·15) (Fig. 4). There was no evidence of significant heterogeneity of results (Chi2 = 22·17; p = 0·14; I2 = 28 %). Again, the Burr 2003 study showed a divergence of results, accounting for 18 % of the 28 % of the I2 index.
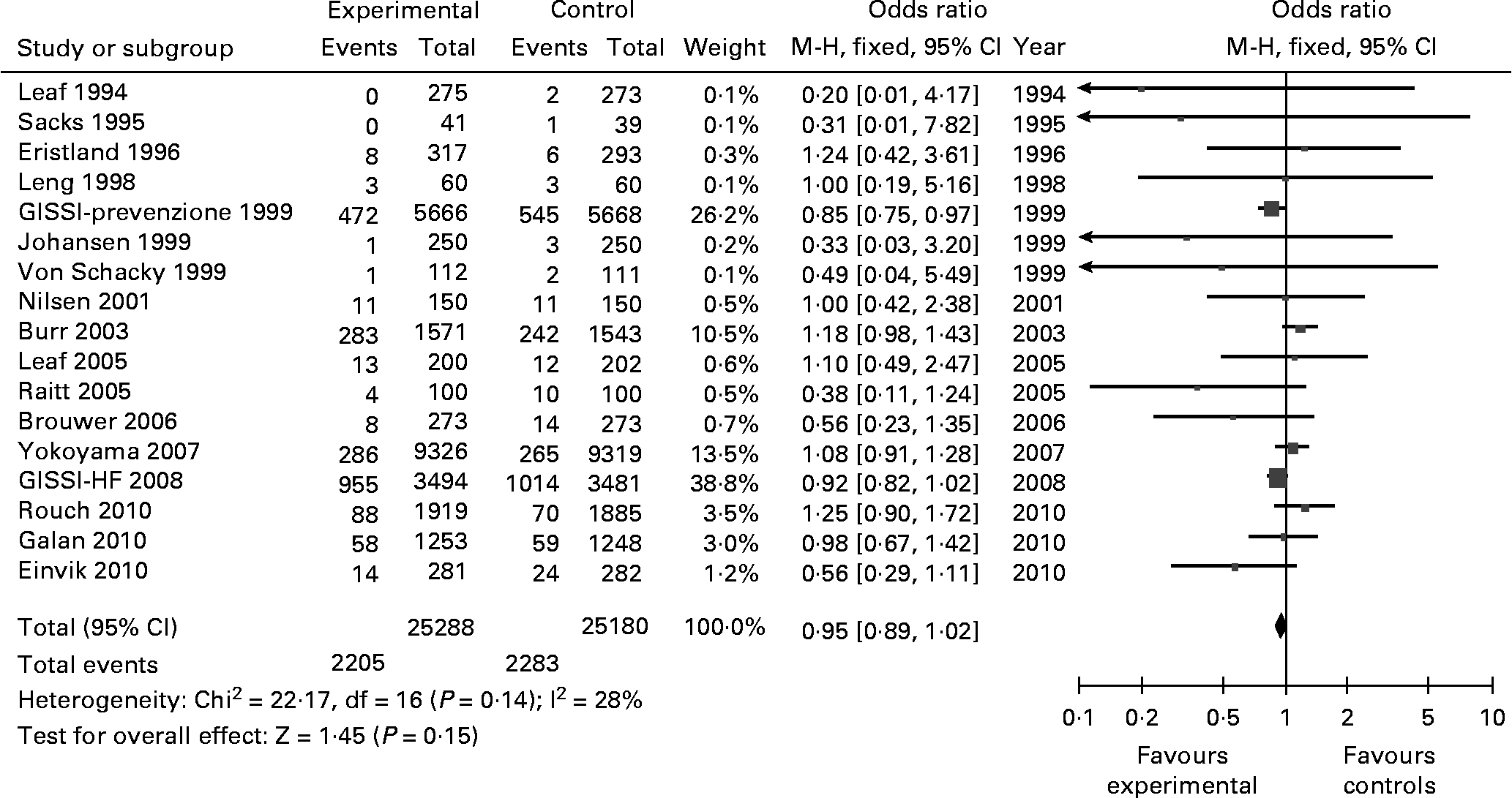
Fig. 4 Total mortality in the revised articles. M-H: Mantel-Haenszel. Fixed: Fixed effects. CI: Confidence Interval.
(A3) Effects on cardiac death
Of the 21 articles included in the present review, 13 reported cardiac deaths, of a total of 46 737 participants. The number of cardiac deaths reported was 1108 in 23 409 persons in the omega-3 group and 1198 in 23 328 in the control group. This yielded an OR of 0·91 indicating a lower cardiac death in the omega-3 group (CI 0·83–0·99; Z for overall effect 2·13; p = 0·03) (Fig. 5). Evidence of heterogeneity was not found (Chi2 = 17·62; p = 0·13). I2 index (32 %) was mainly influenced by the GISSI-Prevenzione 1999 (15 %), and the Burr 2003 (32 %) studies.
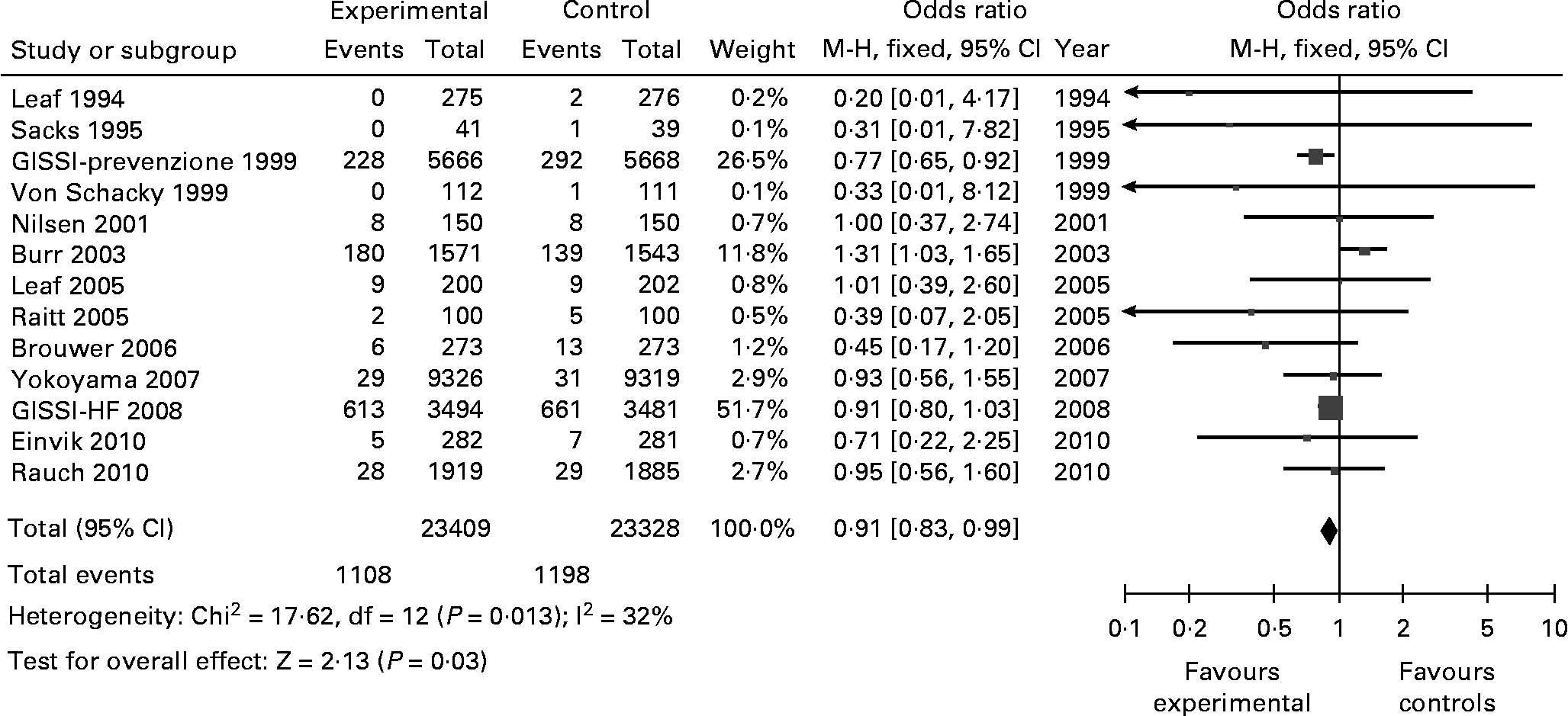
Fig. 5 Cardiac Deaths reported. M-H: Mantel-Haenszel. Fixed: Fixed effects. CI: Confidence Interval.
(A4) Coronary events
Coronary events were reported in 12 studies, from a total of 41 560 patients. These studies reported a total of 889 coronary events (in 20 792 persons) in the omega-3 group and 1066 events in the control group (in 20 768). These numbers resulted in an OR of 0·82 (0·75–0·90), Z = 4·17; p < 1 × 10− 4 (Fig. 6). There was no heterogeneity in the results of these 11 studies (Chi2 = 8·71; p = 0·65; I2 = 0 %).
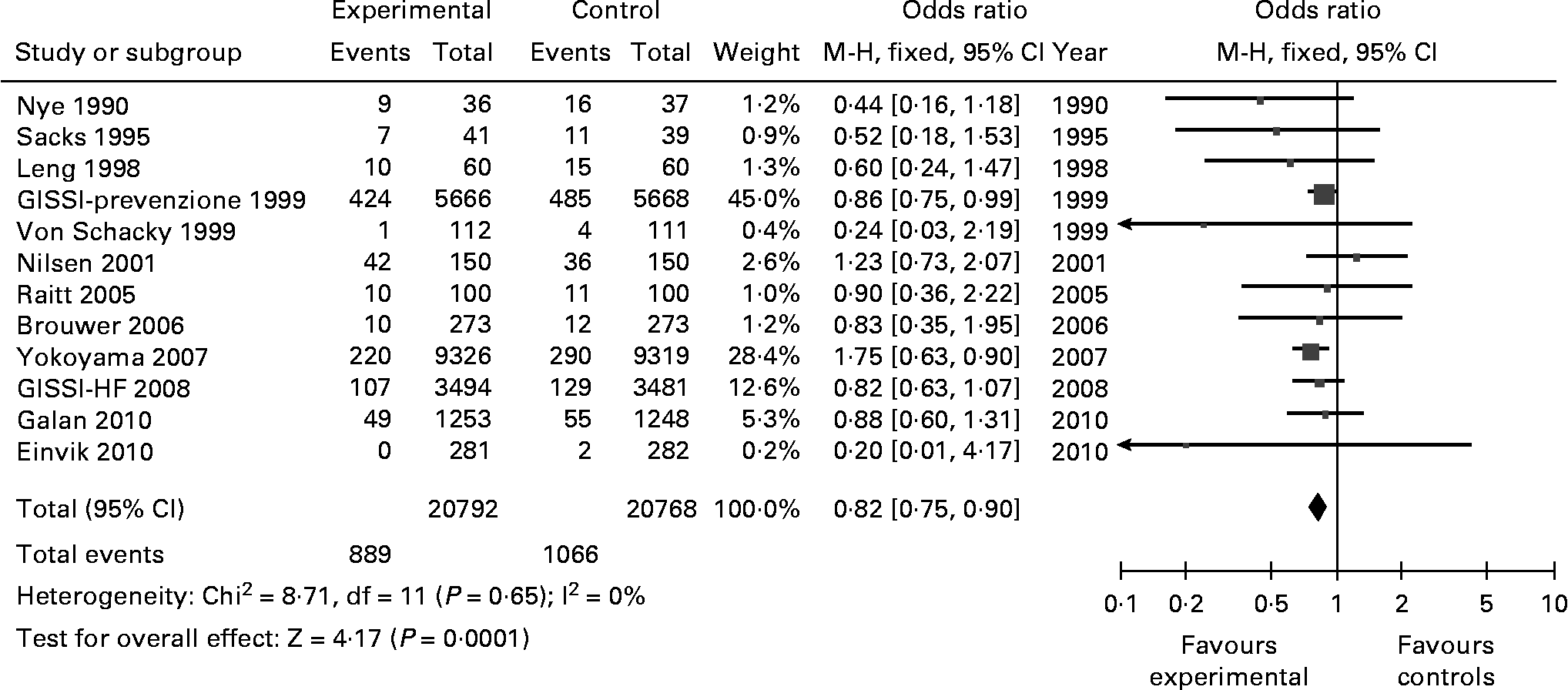
Fig. 6 Coronary events (fatal and non-fatal). M-H: Mantel-Haenszel. Fixed: Fixed effects. CI: Confidence Interval.
Discussion
In the present article we reviewed the evidence from randomized controlled trials and clinical trials involving the use of omega-3 fatty acids for at least six months until January 20th, 2012. In total, of 452 initial potential items, 21 studies matched the inclusion and exclusion criteria and ranked 3/5 or higher on the Jadad Scale(Reference Dehmer, Popma and van den Berg8–Reference Nodari, Triggiani and Campia28) (Table 1). As a primary outcome, we observed that the use of omega-3 results in a fall of approximately 10 % in cardiovascular events (OR 0·90; [0·85–0·96]); Z = 3·28, p = 0·001). We also observed lower frequency of cardiac death (OR 0·91; [0·83–0·99]; Z for overall effect 2·13; p = 0·03), and coronary events (OR of 0·82 (0·75–0·90), Z = 4·17; p < 1 × 10− 4). Although we did not find differences in total mortality, there was a trend towards a lower mortality in omega-3 users (OR of 0·95 [0·89–1·02], Z = 1·45, p = 0·15). We did not stratify the populations according to risk, and included all participants in the same equation, which implies that, in fact, a greater effect of omega-3 could have been obtained if the population is reduced to secondary prevention only. However, we wanted to include the as much information available from the current data as possible.
Table 1 Summary of the articles included in this review

Table1: Main Outcomes, doses, follow-up and main results of the articles included in this review.
* Approx content in EPA/DHA is 0·80 g for each 1 g of capsule content. Where there are notable differences to this fact in the present list, the number of capsules and/or total omega-3 amount is shown.
† Total months, or average mean follow-up when available. Drugs appearing in last column are grouped as they appear in the original reports.
‡ All patients were advised to follow a National Cholesterol Education Program Step I type diet.
§ Diet counseling was aimed at increasing the use of vegetable oils and margarines, vegetables, fruit and fish, and to decrease the use of meat and fat from animal sources. Special oil and margarines containing rapeseed were provided for these participants. 1) To eat at least two portions of oily fish each week, or to take up to 3 g of fish oil (‘Maxepa’) as a partial or total substitute. 2) To eat four to five portions of fruit and vegetables (apart from potatoes) and drink at least one glass of natural orange juice daily, and also increase the intake of oats, so as to obtain a higher intake of vitamin C and at least 8 g of soluble fibre from all sources every day. 3) A combination of both these forms of advice. 4) ‘Sensible eating’- non-specific advice that did not include either of the above interventions. AAS: acetylsalicylic acid. ACE-I: Angiotensin-converting enzyme inhibitor. ARB: Angiotensin II receptor blocker. ALD: aldosterone receptor blocker. AMI: Acute myocardial infarction. BB: beta-blocker. CCB: Calcium channel blockers ICD: implantable cardioverter-defibrillator. OAC: Oral Anticoagulants. T1AA : Type 1 antiarrythmic agents; VT: ventricular tachycardia, VF: ventricular fibrillation.
We excluded from the present study a recent study by Kromhout et al, which evaluated the effect of marine omega-3, ALA or placebo on the incidence of cardiovascular events after AMI(Reference Kromhout, Giltay and Geleijnse29). The study was well designed and conducted, but used ALA as an active arm, which we excluded from this study. Furthermore, the methodology of the statistical approximation made it impossible to compare directly the effects of marine omega-3 versus placebo, when ALA is excluded from the analysis.
Most of the studies analyzed in the present work showed an intriguing uniformity in the direction of their results. The divergent results of one study (Burr et al, 2003)(Reference Burr, Ashfield-Watt and Dunstan19) are particularly interesting, as they served as a mirror image for nearly all the outcomes. In fact, all the outcomes of all the studies showed a low rate of inter-assay heterogeneity I2 (below 25 %), when this study was excluded. Actually, we performed an alternative examination of the results excluding this study, and as well as increasing all the significant findings on cardiovascular events, cardiac death and coronary events, we found a significant change in the total mortality outcome, by lowering the OR to 0·93, 0·87–0·99, Z = 2·18; p = 0·03). It is not easy to work out the explanation for this fact. Some authors have recently reported methodological issues during this study(Reference Lavie, Milani and Mehra3, Reference von Schacky and Harris30–Reference Saravanan, Davidson and Schmidt33), such as a transient cessation of the study for a year due to lack of funds, or the fact that participants were not uniformly randomized to omega-3 supplements, and that these were only given to the participants who entered the study after a one-year break, which could have created a bias. Some of the authors who recently conducted meta-analyses even excluded the Burr study(Reference Lavie, Milani and Mehra3, Reference von Schacky and Harris30, Reference Marik and Varon32). We agree that such issues place limitations on the article, but do not go as far as to discredit it: hence, we have included it. We excluded just one study on methodological grounds, on the light of previous reports(Reference Singh, Niaz and Sharma34–Reference White36).
All the results coming from reviews and meta-analyses (and so the ones coming from this work) have to be accepted with caution, due to the fact that the number of studies included are hardly ever designed with the same type of patients, under the same conditions, or with the same intervention. The present study offers results from studies coming from different sets of patients (primary, secondary prevention, spanning over a large range of ages, some of them only accepting men, others only involving persons about to undergo revascularization…), and with remarkable differences in the treatment doses (ranging from 0·3 to 6·9 g) (Table 1). Thus, pooling all the participants together may make it difficult to discriminate effects in subgroups. Furthermore, the size of the study is a critical factor when evaluating the results of a systematic review or meta-analysis. The fact that the two GISSI studies are the largest ever conducted (comprising about 20 000 patients) mean that the present article is clearly influenced by the great difference in size, as are most of the other studies included. Another fact to be noted is that the differences in the medication given to the patients in the different groups may have conditioned the results of the meta-analysis. For example, the GISSI prevenzione study was performed in 1999, when some of the current protocols for the treatment of coronary disease had not been initiated. The Rauch study, on the other hand, used state-of-the-art treatment (in 2010) alongside the omega-3 allocation, and failed to find differences in the outcomes depending on omega-3. This may suggest that the improvements in the clinical outcomes provided by current medication could minimize the positive effects of omega-3 (Table 1). Nevertheless, the other recent studies (from 2007 on) showed a similar trend to the GISSI-prevenzione study, despite including most of the medication currently used.
Another important hypothetical point to be considered when dealing with the effects of a certain nutrient (or a substance that may be ingested, such as omega-3 fatty acids) in health or disease is the possibility that the effects of the ‘controlled’ intake of the nutrient (in this case, omega-3) may be influenced by the background diet, and the ‘uncontrolled’ additional intake made by the participants. Moreover, the average intake of the given nutrient in the different populations studied may also influence the results. The effects of omega-3 in populations where its mean consumption is low may be higher than in those populations where the dietary habits include the use of blue fish as a habitual food. For example, even ‘control’ subjects, randomized to not receive supplements of omega-3 in populations like Japan (where usual diet is up to 15 times higher than in western countries(Reference Iso, Kobayashi and Ishihara37)), probably have good average omega-3 intake, when compared to populations with western dietary models, and, hence, there may be an underestimation of the effects of omega-3 due to the fact that even ‘control’ subjects are receiving a good dose of omega-3 via diet. In this sense, omega-3 supplements for people who have a high dietary omega-3 intake fail to reduce triglycerides or modify other plasma lipids(Reference Watanabe, Watanabe and Kumagai38). In contrast, populations with low average omega-3 consumption would be theoretically better able to study the effects of the ‘regulated’ intake of omega-3, given that the consumption in normal subjects would be more sporadic. However, these other models could, on the other hand, influence the results of the quantification of the effects of omega-3. In populations with a very low omega-3 intake, it could occur that even with the supplementation of omega-3 (either by dietary or pharmacological means), omega-3 concentration in plasma would not reach a certain level, or, furthermore, that the effects of a simple intervention with a certain amount of omega-3 would not be enough to counteract the deleterious effects of the global diet. Although the information provided in many of the studies included in the present review do not allow us to estimate the omega-3 intake, we included the dietary advice given in these studies in Table 1. The dietary advice given (when available) does not seem to influence the results of the intervention with omega-3.
Another possible concern when evaluating this particular topic is the fact that the generalized opinion that omega-3 is good for health (and in particular cardiovascular disease) may be influencing the intake of omega-3 (in the form of fish) in the general population, and, more accurately, in those persons that suffer from coronary or other cardiovascular disease. This way, even ‘control’ subjects in randomized control studies may have a higher fish consumption than matched healthy persons in their respective populations, and subsequently, a lower difference between the ‘active’ and the ‘placebo’ group would show that even the ‘placebo’ group are receiving (at least partially) the beneficial effects of their intake of fish.
Adherence may be an important factor when analyzing the results, and, in modern studies, persons at secondary prevention receive a large number of medications, especially in the immediate period following the cardiac event. For example, in the OMEGA trial(Reference Rauch, Schiele and Schneider27), which failed to show any effect of 1 g/d of omega-3 in clinical outcomes, more than the 80 % of the patients were taking over 5 different drugs a day, apart from the omega capsules. In a recent sub-study from the JELIS study, adherence was an important determinant of outcomes(Reference Origasa, Yokoyama and Matsuzaki39). The rate of adherence was in itself a determinant of the clinical outcomes, even within the treatment arm, and the poor responders had an 80 % possibility of receiving the omega-3 pills. Nevertheless, the OMEGA investigators reported a compliance of 70 % or more in 93 % of the omega-3 group. Regarding this last study, it is also worth noting also that data from the randomization table shown in the article allow us to infer an OR for the combined risk of stroke, previous AMI, previous revascularization, previous by-pass, or moderate (35 %–44 %) and severe ( < 35 %) reduction of the ejection fraction of 1·12 (1·04–1·20) for the active treatment (omega-3) versus control. In other words, the patients allocated to omega-3 were persons at higher risk (12 %) for the combined items of these conditions(Reference Rauch, Schiele and Schneider27), which may have influenced the ‘negative’ results.
When evaluating the effects of omega-3 fatty acids on clinical outcomes, we are very limited by the time of exposure, the statistical power and the dosage used in the studies. In fact, most of the studies ‘designed’ to discriminate clinical endpoints in a short time (less than six months) fail to find differences - probably because the effects would not become evident until much later. In addition, some of the studies used doses lower than 0·5 g EPA/DHA, which may not allow the trigger plasma concentration required for omega-3 effects to be reached. In other words, some of the beneficial effects of omega-3 may require a prolonged period (up to years) at a certain dose. It has been suggested that although antithrombosis effects may appear with low doses and a low time of exposure (weeks), others, like blood pressure regulation, lowering of triglycerides or lowering of heart rate, could take anything up to a few years(Reference Mozaffarian40). Another important factor to be pointed is that some of recent studies calculated their sample size according to expected event rates that were not achieved. For example, in the recent OMEGA trial investigators had a statistical power of 19 % to detect differences of risk reduction of 25 %. As the risk reduction shown in the omega-3 intake for the clinical outcomes covered in the present study ranges from 5–18 %, the probability of this study discriminating such differences was extremely low(Reference Rauch, Schiele and Schneider27). As an example of the possible infra-treatment and/or low follow-up, in the GISSI-HF trial, allocation to marine omega-3 (1 g/d) required a follow-up of at least 3·9 years to find differences in total mortality or cardiovascular events(Reference Gissi, Tavazzi and Maggioni24). In our opinion, only the evaluation of clinical events from long-term exposure to omega-3 fatty acids (up to ten years) with amounts of at least 1 g EPA/DHA would answer this question.
Another issue to take into account when evaluating the apparent disparity of results between studies is population genetics. It is true that genetic background plays an important role in many cardiovascular risk factors, like lipid metabolism, both in fasting and postprandial states(Reference Perez-Martinez, Delgado-Lista and Perez-Jimenez41, Reference Teslovich, Musunuru and Smith42). It is precisely in the modulation of the lipid profile caused by the intake of omega-3 that leads some authors to justify the favorable effects of these products. Recently, Pishva et al showed how a mutation in the FABP gene may account for up to 70 % of the effect of the omega-3 fatty acids on triglyceride levels(Reference Pishva, Mahboob and Mehdipour43). Furthermore, Olano-Martin found that the carriers of another gene mutation at the ApoE locus show an increase in LDL levels when exposed to a high DHA intake(Reference Olano-Martin, Anil and Caslake44). This gene-diet interaction was also previously published by Caslake(Reference Caslake, Miles and Kofler45). Thus, a different prevalence of gene variations in the populations submitted to each study may lead to different results when evaluating outcomes related to the lipid effects of omega-3.
Although this review is limited to cardiovascular events in randomized controlled trials and clinical trials, we also wanted to remind the reader that many reviews and meta-analyses have included evidence on the efficacy of omega-3 coming from observational and cohort studies, and most of them report a much closer inverse relationship between omega-3 consumption (whether from dietary intake or in the form of supplements) and cardiovascular disease(Reference Mente, de Koning and Shannon4, Reference Saravanan, Davidson and Schmidt33, Reference Mozaffarian and Rimm46).
The exact mechanisms by which omega-3 performs its functions are still into debate, but the main mechanisms proposed are plaque stabilization(Reference Thies, Garry and Yaqoob47), lipid profile(Reference Harris, Miller and Tighe48), anti-inflammatory(Reference Serhan, Chiang and Van Dyke49), blood pressure(Reference Morris, Sacks and Rosner50), heart failure(Reference Gissi, Tavazzi and Maggioni24, Reference Yamagishi, Nettleton and Folsom51), or anti- arrhythmic properties(Reference Leaf, Albert and Josephson20, Reference Mozaffarian, Psaty and Rimm52, Reference Gillet, Roger and Bougnoux53). It is precisely over this last effect where major controversy exists today. Initial data from the GISSI-Prevenzione found that most of the decrease in mortality associated with the intake of omega-3 could be due to a decrease in the frequency of sudden death, which leads to the theory that these fatty acids could have anti-arrhythmic properties(15). Nevertheless, the evolution of the study on this topic has shed more controversy than light on the issue. Although it seems, in the view of some observational and cohort studies, that omega-3 may have the effect of lowering the appearance of atrial fibrillation, especially in the type linked to atrial remodelling associated with age(Reference Mozaffarian, Psaty and Rimm52) (but not in the type associated with cardiac surgery)(Reference Saravanan, Bridgewater and West54), the impact of omega-3 in ventricular arrhythmias is much more debatable. Both pro- and anti-arrhytmic effects of these fatty acids have been reported(Reference Leaf, Albert and Josephson20, Reference Raitt, Connor and Morris21, Reference Brouwer, Zock and Camm22, Reference Brouwer, Raitt and Dullemeijer55) and, perhaps, both really exist. From the different in vitro and in vivo studies, it has been established that omega-3 shortens the action's potential duration and slows down impulse conduction(Reference Verkerk, van Ginneken and Berecki56, Reference Coronel, Wilms-Schopman and Den Ruijter57). These actions may reduce the appearance of a triggered activity and subsequent ventricular fibrillation to those usually present in the recent post-infarct state, and, on the other hand, facilitate the appearance of re-entry induced ventricular fibrillation, such as those provoked by other clinical or anatomical causes(Reference Saravanan, Davidson and Schmidt33).
Regarding the safety of using omega-3, a recent work revised the incidence of side effects. The only adverse effect that reached statistical significance were gastrointestinal side effects (mainly nausea)(Reference Filion, El Khoury and Bielinski6), which occur more frequently at 4 g/d or higher doses, in up to 20 % of the patients (or 4 % of the patients below 3 g/d)(Reference Wang, Harris and Chung58), which may be accompanied by a fishy taste when belching and may be minimized by taking the supplements with meals or freezing the capsule(Reference Chalupka59). The most severe potential adverse effect studied regarding omega-3 is the potential risk of bleeding, by their effects on platelet metabolism(Reference Knapp, Reilly and Alessandrini60, Reference Kristensen, Iversen and Schmidt61). However, studies which evaluated the safety of using omega-3 up to 4 g/d in takers of anti-platelets and anticoagulants, reported no increased risk on minor or major bleeding events(Reference Eritsland, Arnesen and Seljeflot62, Reference Watson, Joy and Nkonde63), which has been also confirmed in a recent meta-analysis(Reference Filion, El Khoury and Bielinski6). Another potential side effect of the consumption of omega-3 from a dietary source (fish) is the mercury intake that may be involved, especially in big fishes (blue fin tuna, shark, tilefish, swordfish, or king mackerel). Although mercury intake has been related to cardiovascular disease, this relationship is still not yet clearly proven, especially in the amounts that can be derived from eating fish, and to date, the net health benefits of overall fish consumption in adults are clear (for a recent review, please see(Reference Park and Mozaffarian64)).
In vitro and animal studies suggest that PUFA are more prone to become oxidized than other sources of fatty acids, such as MUFA(Reference Fremont, Gozzelino and Franchi65). Furthermore, it has been shown that diets rich in omega-3 fatty acids cause an increased susceptibility of lipids to oxidation(Reference Hau, Smelt and Bindels66). Nevertheless, other studies have not found such results(Reference Bonanome, Biasia and De Luca68–Reference Higdon, Du and Lee70), and a recent study did not find any differences in the different markers of oxidative stress in over 400 patients with metabolic syndrome who consumed four dietary models for 12 weeks, one of which enriched in 1·24 g/d of long chain omega-3 fatty acids(Reference Petersson, Riserus and McMonagle71). In conclusion, although omega-3 fatty acids have been shown to increase the susceptibility to oxidation of circulating lipids, studies have not proved that they are linked to an increase in markers of oxidative stress. Further studies are needed to clarify this question.
In conclusion, we have reviewed the effects of marine omega-3 fatty acids on cardiovascular events from randomized controlled trials and clinical trials. The accumulated evidence, as of January, 2012, indicates that marine omega-3, when administered as food or in supplements for at least 6 months, reduces cardiovascular events by 10 %, cardiac death by 9 % and coronary events by 18 %, while showing a trend for a lower total mortality (5 %, p = 0·13). These results are based in the evaluation of studies that included mainly persons with high cardiovascular risk, and in studies which are highly heterogenic in the dose administered, although there is no evidence of dose-dependent protection. Our results, along with the existing evidence on the myriad of physiological effects of omega-3 on human health (coagulation, heart rate, heart rhythm, blood lipids, etc), reinforce the AHA recommendations for the intake of omega-3 in the prevention of cardiovascular disease, especially in persons with high cardiovascular risk and secondary prevention.
Acknowledgements
The authors' responsibilities were as follows: J. D.-L. and P. P.-M. searched the literature, were involved in the conception and design of the study, performed the statistical analysis and drafted the manuscript. J. L.-M. and F. P.-J. supervised the study and provided valuable advice; F. P.-J. also helped search the literature, providing opinions on the pertinence of the articles when there were discrepancies between J. D.-L. and P. P.-M.; all the authors contributed to the interpretation of data, critical review and revision of the manuscript. This work was supported partly by public funds: research grants from the Spanish Ministry of Science and Innovation (AGL 2004-07907, AGL2006-01979, and AGL2009-12270 to J. L.-M., SAF07-62005 to F. P.-J. and FIS PI10/01041 to P P-M, PI10/02412 to F. P.-J.); Consejería de Economía, Innovación y Ciencia, Proyectos de Investigación de Excelencia, Junta de Andalucía (P06-CTS-01425 to J. L.-M., CTS5015 and AGR922 to F. P.-J.); Consejería de Salud, Junta de Andalucía (06/128, 07/43, and PI0193/09 to J L-M, 06/129 to F. P.-J., 0118/08 to F F-J, PI-0252/09 to J. D.-L., and PI-0058/10 to P. P.-M.); Fondo Europeo de Desarrollo Regional (FEDER). The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain. The authors report no conflicts of interest.









