Excessive systolic blood pressure (SBP) reactivity to the cold pressor test (CPT), through increased sympathetic activity, may predict the development of hypertension in young, healthy adults( Reference Menkes, Matthews and Krantz 1 , Reference Park, Middlekauff and Campese 2 ). A greater sympathetic reactivity to the CPT has been demonstrated in overweight than in lean adults despite similar brachial blood pressure (BP) responses( Reference Park, Middlekauff and Campese 2 ). However, brachial BP has lower sensitivity to the CPT than aortic BP( Reference Edwards, Gauthier and Hayman 3 ). In young, normotensive adults, CPT increases aortic BP, wave reflection and arterial stiffness (pulse wave velocity (PWV))( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 , Reference Geleris, Stavrati and Boudoulas 5 ). An increased BP reactivity to CPT may predict future increases in PWV( Reference Bellinazzi, Sposito and Schreiber 6 ), which precedes the development of hypertension in obesity( Reference Weisbrod, Shiang and Al Sayah 7 ).
Isometric handgrip exercise (IHG) also elicits a sympathetic-mediated increase in BP( Reference Delaney, Greaney and Edwards 8 ) known as the exercise pressor response. The BP response to IHG is determined by neural signals from the brain (central command) and active muscles (mechanoreceptors and metaboreceptors)( Reference Delaney, Greaney and Edwards 8 , Reference McNulty, Moody and Wagenmakers 9 ). Vascular responses to muscle metaboreflex activation can be isolated from central command and mechanoreceptor influences by trapping metabolites in the previously exercised muscle via arterial occlusion (post-exercise muscle ischaemia (PEMI))( Reference Delaney, Greaney and Edwards 8 – Reference Davies, Frenneaux and Campbell 10 ). Exaggerated BP and vasoconstrictor responses to PEMI have been shown in young, obese adults with pre-hypertension( Reference da Silva, Martinez and Faria 11 ).
Acute increases in aortic haemodynamics and PWV are exaggerated during concurrent IHG and CPT (cold-pain stimulus) in young, healthy adults( Reference Geleris, Stavrati and Boudoulas 5 , Reference Koutnik, Figueroa and Wong 12 ). Similarly, we recently reported that PEMI with concurrent CPT (PEMI+CPT) caused greater increases in aortic haemodynamics and PWV than PEMI alone in young, healthy, overweight/obese men( Reference Kalfon, Campbell and Alvarez-Alvarado 13 ). As the combined stimuli of exercise and cold may increase the risk for adverse cardiovascular events in susceptible individuals by elevating myocardial work( Reference Manou-Stathopoulou, Goodwin and Patterson 14 ), therapies targeted to attenuate cardiac afterload during stress would be cardioprotective.
l-Arginine is the substrate for endothelial nitric oxide (NO) production, a potent vasodilator. l-Citrulline supplementation (l-CIT) increases plasma l-arginine and NO levels more efficiently than l-arginine supplementation( Reference Bailey, Blackwell and Lord 15 , Reference Morita, Hayashi and Ochiai 16 ). At rest, l-CIT has reduced BP, wave reflection and PWV in middle-aged adults with increased BP and/or arterial stiffness( Reference Ochiai, Hayashi and Morita 17 , Reference Figueroa, Wong and Hooshmand 18 ), but not in young, healthy men( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 ). l-CIT, either synthetic or from watermelon, has attenuated aortic haemodynamic responses to CPT in young, normotensive( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 ) and older, hypertensive adults( Reference Figueroa, Wong and Kalfon 19 ). Yet, the possible benefits of l-CIT on vascular reactivity during metaboreflex activation remain unknown.
The aim of this study was to determine the effects of l-CIT on aortic haemodynamics and PWV responses to stress in healthy, young men. We hypothesised that l-CIT would attenuate vascular reactivity to PEMI with and without CPT, although the benefits would be more apparent during combined stress.
Methods
Study population
In total, sixteen overweight/obese (BMI>25 and <40 kg/m2) healthy males (18–35 years) participated in this study. Participants were non-smokers, sedentary (<120 min/week of exercise) and free of overt chronic diseases. Exclusion criteria included brachial systolic BP (SBP)≥140 mmHg and use of medications and/or any supplements that may affect outcome variables. Participants were asked to maintain their ordinary diet and exercise habits. The study was approved by the Institutional Human Subjects Committee, and participants gave their written informed consent before participation.
Study design
This was a randomised, double-blind, placebo-controlled study. Participants were familiarised with the study protocols. Cardiovascular parameters were evaluated in a quiet, temperature-controlled room (22–24°C) after an overnight fast and abstinence from beverages containing caffeine or alcohol for 12 h and from intense or prolonged physical activity 24 h before testing.
After 15 min of supine rest, cardiovascular parameters were measured in duplicate and averaged at rest and during the last minute of IHG and PEMI without CPT. Following 20 min of recovery, cardiovascular parameter measurements were repeated at rest, IHG and PEMI+CPT. The trial order was randomised. After baseline measurements, participants were randomly assigned to placebo (maltodextrin) or l-CIT for 14 d, followed by a 14-d washout period, and were then crossed over to the other supplementation. Measurements were repeated 14 d after both periods.
Measurements
Anthropometrics
Body weight and height were measured using a Seca Scale and stadiometer (Sunbeam Products) to the nearest 0·1 kg and 0·01 m, respectively. Waist circumference was measured using a tape measure to the nearest 0·5 cm at the superior border of the iliac crest.
Haemodynamics
An automated oscillometric device (HEM-705CP; Omron Healthcare) was used to record brachial BP. These values were used to calibrate radial pressure waveforms obtained from a 10 s epoch with a high-fidelity tonometer (SPT-301B; Millar Instruments). Aortic waveforms were derived from a generalised transfer function (SphygmoCor, AtCor Medical) and composed of forward (P1) and reflected (P2) waves. Augmented pressure (AP=P2–P1) expressed as a percentage of the aortic pulse pressure gives the augmentation index (AIx). Transit time of the reflected wave (Tr) indicates the round-trip travel of the forward wave to the peripheral reflecting sites and return to the aorta. Tr and AIx have been used as markers of aortic stiffness and wave reflection, respectively. The intraclass correlation coefficient for aortic haemodynamics in our laboratory calculated on two separate days was ≥0·94.
Arterial stiffness
Following a 15-min rest period, PWV was evaluated using a device (VP-2000; Omron Healthcare) composed of BP cuffs positioned around the arms (brachial artery) and ankles (posterior tibial artery). Waveforms were measured simultaneously by cuff sensors, whereas the transient time was calculated automatically by relating the foot of the forward wave to the R-wave of the electrocardiogram. The distance between sampling points was calculated automatically according to the participant’s height. Brachial-ankle pulse wave velocity (baPWV) was measured as the time it takes for the pulse wave to travel from the arm sensor to the ankle sensor, divided by the distance between the sensors (m/s). Heart rate (HR) was measured from the electrocardiogram. baPWV was not measured during IHG because of the difficulty in collecting pulse waveforms in the exercising arm.
Exercise and metaboreflex activation
The highest of three maximal compressions using a handgrip dynamometer (Lafayette Instrument Co.) was considered the maximal voluntary contraction (MVC). IHG was performed with the dominant arm at 30 % of MVC for 2 min using a handgrip dynamometer. Visual feedback and verbal encouragement were provided to maintain the targeted grip force; 10 s before completion of the IHG, a cuff positioned proximally on the exercising forearm was inflated to suprasystolic levels (200 mmHg or at least 50 mmHg above the brachial SBP during IHG) for 3 min with an automated pneumatic device (Hokanson E20; Hokanson, Inc.).
Cold pressor test
The left foot of participants was passively immersed into ice-cold water (4°C) for 3 min during the PEMI+CPT trial. Participants were instructed to maintain consistent breathing rate/depth throughout the test.
l-Citrulline supplementation
Participants were instructed to ingest four capsules of 750 mg l-citrulline or placebo (NOW Foods) twice a day (before breakfast and sleep) for 14 d. To avoid acute vascular effects of l-CIT, the last capsule was ingested 48 h before the 2-week testing. Participants returned unused capsules and records to assess their compliance.
Statistical analyses
On the basis of previous data( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 ), it was calculated that sixteen participants would provide 85 % power (two-sided α=0·05) to detect a 7·5 % decrease in brachial SBP during PEMI+CPT after l-CIT. Statistical analyses were conducted using SPSS 21.0. Normality was confirmed by the Shapiro–Wilk test for all measurements. An independent t test was used to assess possible between-group differences at baseline. Separate two-way repeated-measures ANOVA was performed to determine differences within and between interventions in cardiovascular parameters in the four conditions: rest, IHG, PEMI and PEMI+CPT. When a significant group-by-time interaction was identified, paired t tests were used to assess within-group differences between baseline and 2 weeks. Data are presented as mean values with their standard errors. Statistical significance was set at P<0·05.
Results
Participant characteristics are presented in Table 1. Weight, BMI and waist circumference did not change significantly after both supplementations. Cardiovascular parameters before and after the supplementations are presented in Tables 2 and 3. There were no differences between supplementations at baseline and no significant changes were detected after 2 weeks.
Table 1 Study participant characteristics (Mean values with their standard errors; n 16)
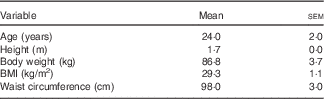
Table 2 Haemodynamic parameters before and after supplementation (Mean values with their standard errors; n 16)

L-CIT, L-citrulline; IHG, isometric handgrip; PEMI, post-exercise muscle ischaemia; PEMI+CPT, PEMI with concurrent cold pressor test; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
* P<0·05, ** P<0·01, *** P<0·001 v. before.
† Group×time interaction from ANOVA.
Table 3 Wave reflection parameters before and after supplementation (Mean values with their standard errors; n 16)

L-CIT, L-citrulline; AP, augmentation pressure; IHG, isometric handgrip; PEMI, post-exercise muscle ischaemia; PEMI+CPT, PEMI with concurrent cold pressor test; AIx, augmentation index; Tr, transit time of the reflected wave.
* P<0·05, *** P<0·001 v. before.
† Group×time interaction from ANOVA.
During IHG, l-CIT decreased brachial SBP (P<0·05), diastolic blood pressure (DBP) (P<0·05) and mean arterial pressure (MAP) (P<0·05) as well as aortic SBP (P<0·01), AP (P<0·001) and AIx (P<0·001) compared with the placebo group (group-by-time interactions P<0·05). l-CIT had no effect on HR, aortic DBP, aortic MAP and Tr.
During isolated PEMI, l-CIT reduced brachial DBP (P<0·05) and aortic DBP (P<0·01), MAP (P<0·05) and AIx (P<0·001) compared with placebo (group-by-time interaction P<0·05). HR, brachial SBP and MAP, aortic SBP, AP, Tr and baPWV (Fig. 1) were not affected by l-CIT.

Fig. 1 Brachial-ankle pulse wave velocity (baPWV) at rest (a), during post-exercise muscle ischaemia (PEMI) (b) and PEMI+cold pressor test (c) before and after placebo (![]() ) and l-citrulline (
) and l-citrulline (![]() ) supplementation. Values are means with their standard errors. * P<0·05 v. before; † P<0·05 v. placebo.
) supplementation. Values are means with their standard errors. * P<0·05 v. before; † P<0·05 v. placebo.
During PEMI+CPT, l-CIT decreased brachial SBP (P<0·01), DBP (P<0·001) and MAP (P<0·001) as well as aortic SBP (P<0·001), DBP (P<0·001), AP (P<0·001), AIx (P<0·001) and baPWV (Fig. 1, P<0·05) compared with placebo (group-by-time interaction P<0·05). l-CIT did not affect HR and Tr.
Discussion
The main findings of our study are that l-CIT attenuated (1) aortic BP and AIx responses to IHG, PEMI and PEMI+CPT and (2) baPWV response to PEMI+CPT but not to PEMI alone in young, healthy, overweight/obese men. These findings suggest that l-CIT effectively buffered exercise- and cold-induced vascular reactivity.
l-CIT failed to reduce resting cardiovascular parameters in healthy, overweight/obese, young men. These findings are consistent with previous data showing unapparent effects of l-CIT on resting BP and wave reflection in young, normotensive, lean men due to normal BP and arterial function( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 ). In contrast, Bailey et al. ( Reference Bailey, Blackwell and Lord 15 ) recently reported a decrease in resting brachial BP in young, normotensive men after 1 week of l-CIT. It is possible that these results could reflect an acute vascular effect as l-citrulline was ingested 90 min before the BP measurement. Cardiovascular parameters were measured 48 h after the last l-citrulline dose to assess the chronic rather than the acute vascular effects. We have reported unapparent vascular effects of l-CIT in normotensive or pre-hypertensive individuals( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 , Reference Figueroa, Sanchez-Gonzalez and Perkins-Veazie 20 ). In contrast, l-CIT and watermelon supplementation have reduced resting BP in pre-hypertensive( Reference Figueroa, Sanchez-Gonzalez and Perkins-Veazie 20 ) and hypertensive adults( Reference Figueroa, Wong and Hooshmand 18 , Reference Figueroa, Wong and Kalfon 19 ) as well as resting baPWV in middle-aged and older adults( Reference Ochiai, Hayashi and Morita 17 , Reference Figueroa, Wong and Hooshmand 18 ).
l-CIT attenuated brachial BP and wave reflection responses to IHG. Previous studies have reported that treatment with nebivolol, which exerts a vasodilator effect via endothelial NO production, reduced MAP responses to IHG in hypertensives( Reference Price, Raheja and Wang 21 ). These findings suggest that improved peripheral vasodilation attenuates the exercise pressor response. During PEMI following IHG, sympathetic-mediated peripheral vasoconstriction maintains SBP and MAP higher than at rest but similar to that during IHG( Reference Delaney, Greaney and Edwards 8 , Reference McNulty, Moody and Wagenmakers 9 ). In healthy, young men, the BP response to PEMI is attributed to increases in peripheral resistance( Reference McNulty, Moody and Wagenmakers 9 ) and stroke volume( Reference Crisafulli, Scott and Wensel 22 , Reference Figueroa, Hooshmand and Figueroa 23 ). In the present study, IHG and PEMI elevated brachial SBP to 145 and 135 mmHg, respectively, at baseline. We found that l-CIT did not reduce SBP during PEMI, suggesting that l-CIT is less effective in reducing a small SBP response to PEMI in young, healthy men. Importantly, l-CIT reduced aortic DBP and wave reflection (AP and AIx) during PEMI, which may indicate a decreased vasomotor tone. Previous studies have demonstrated that acute NO-donor or antihypertensive therapy acutely decreases resting DBP and wave reflection magnitude independently of reflection timing (Tr) via reduction in vascular tone in the small arteries but not in the aorta( Reference Kelly, Millasseau and Ritter 24 , Reference Hashimoto, Westerhof and Westerhof 25 ). Therefore, despite persistent elevation of sympathetic activation during PEMI( Reference Delaney, Greaney and Edwards 8 ), l-CIT may counteract the increase in vasomotor tone during metaboreflex activation in young, healthy men.
Young, normotensive, overweight men have an exaggerated sympathetic reactivity to CPT( Reference Park, Middlekauff and Campese 2 ), which may be a mechanism for cold-induced hypertension( Reference Manou-Stathopoulou, Goodwin and Patterson 14 ). The CPT evokes a sympathoexcitation-mediated hypertensive response through skin cooling and pain sensation( Reference Manou-Stathopoulou, Goodwin and Patterson 14 ). Previous studies have shown that haemodynamic responses are greater during combined stimulation by IHG or PEMI with cold exposure compared with IHG or PEMI alone in young, healthy adults( Reference Geleris, Stavrati and Boudoulas 5 , Reference Koutnik, Figueroa and Wong 12 ). Similarly, we found greater haemodynamic response to PEMI+CPT compared with isolated PEMI in young men( Reference Kalfon, Campbell and Alvarez-Alvarado 13 ).
We noted attenuation of haemodynamic responses to PEMI+CPT after l-CIT. Consistent with these findings, we previously reported reductions in aortic SBP and AIx responses to CPT or whole-body cold exposure following 2–4 weeks of l-CIT in young, healthy men( Reference Figueroa, Trivino and Sanchez-Gonzalez 4 ). Similarly, l-citrulline from watermelon attenuated CPT-induced increases in aortic BP and AP, but not in AIx, in older, obese adults with hypertension. An explanation for this discrepancy could be that AP is a better index of wave reflection at rest( Reference Fantin, Mattocks and Bulpitt 26 ) and during PEMI( Reference Figueroa, Jaime and Johnson 27 ) in older adults( Reference Fantin, Mattocks and Bulpitt 26 ). On the other hand, AIx is a reliable measure of wave reflection in young adults( Reference Fantin, Mattocks and Bulpitt 26 ). Collectively, these findings indicate that l-CIT reduces cardiac afterload during cold exposure( Reference Figueroa, Wong and Kalfon 19 , Reference Sanchez-Gonzalez, Koutnik and Ramirez 28 ), and our present findings add to the existing knowledge that l-CIT has a cardioprotective effect during concurrent PEMI and cold exposure. Our findings may be important for the prevention of adverse cardiac events in individuals with increased cardiovascular risk, as cold stimulus combined with exercise, but not cold alone, may increase the risk of myocardial ischaemia due to excessive cardiac afterload( Reference Manou-Stathopoulou, Goodwin and Patterson 14 ).
Previous studies have shown that IHG or PEMI with concurrent CPT increases PWV by 1·2–1·8 m/s in young men( Reference Geleris, Stavrati and Boudoulas 5 , Reference Kalfon, Campbell and Alvarez-Alvarado 13 ). In the present study, l-CIT attenuated the baPWV response to PEMI+CPT by 1·06 m/s, whereas l-CIT was ineffective in reducing baPWV during isolated PEMI. At baseline, baPWV levels during PEMI with and without CPT were 13·6 and 12·8 m/s, respectively. Subsequently, l-CIT reduced baPWV during PEMI+CPT to relatively the same absolute level as that evoked during isolated PEMI. To the best of our knowledge, the efficacy of l-CIT on the PWV response to cold exposure has not been previously examined. A previous investigation has reported that the anti-stiffening effect of l-CIT on resting baPWV without cold exposure was attributed to an increased NO availability( Reference Ochiai, Hayashi and Morita 17 ); 1-week l-CIT and 6-week watermelon supplementation reduced baPWV by 1·2–1·3 m/s in middle-aged adults with a baseline baPWV >14 m/s( Reference Ochiai, Hayashi and Morita 17 , Reference Figueroa, Wong and Hooshmand 18 ). In the present study, only PEMI+CPT elevated baPWV close to this level, suggesting that l-CIT is able to exert a beneficial effect in conditions with increased PWV. Cold exposure( Reference King, Ahuja and Wass 29 ) and vasoconstrictor drugs (angiotensin II)( Reference Kelly, Millasseau and Ritter 24 ) predominantly increase peripheral (brachial) PWV. It is well known that femoral-ankle PWV (faPWV) is the largest peripheral arterial segment in baPWV( Reference Sugawara, Hayashi and Yokoi 30 ) and is increased by PEMI( Reference Davies, Frenneaux and Campbell 10 ). As Tr, an estimate index of aortic stiffness, was not affected by l-CIT, our findings suggest that l-CIT may have reduced faPWV compared with carotid-femoral PWV (cfPWV).
The present findings could be important for individuals with hypertension and heart failure, as they have an exaggerated sympathetically mediated haemodynamic response to exercise due to metaboreflex overactivation( Reference Delaney, Greaney and Edwards 8 , Reference Keller-Ross, Johnson and Joyner 31 ). In heart failure patients, PEMI-induced increase in BP may lead to reduced exercise tolerance due to increased myocardial work( Reference Keller-Ross, Johnson and Joyner 31 ). Evidence suggests that l-CIT improves resting BP and left ventricular function in heart failure patients( Reference Orozco-Gutiérrez, Castillo-Martínez and Orea-Tejeda 32 ). Future studies are needed to examine the effects of l-CIT on haemodynamic responses to exercise and PEMI in individuals with metaboreflex overactivation.
The exact mechanism by which l-CIT counteracts vascular reactivity to physical stress is unclear. Recent evidence supports that l-citrulline is a NO precursor, as its conversion to l-arginine leads to endothelial NO synthesis( Reference Bailey, Blackwell and Lord 15 , Reference Morita, Hayashi and Ochiai 16 ). In young men, the increase in MAP during PEMI following IHG may have been secondary to vasoconstriction and not to increase in stroke volume( Reference McNulty, Moody and Wagenmakers 9 ). BP and AIx reductions by vasodilator drugs including NO donors is through a decrease in vascular smooth muscle tone of the peripheral arteries( Reference Kelly, Millasseau and Ritter 24 , Reference Hashimoto, Westerhof and Westerhof 25 ). Although PWV is influenced by distending pressure (MAP), changes in PWV lead to changes in BP( Reference Weisbrod, Shiang and Al Sayah 7 ). Therefore, attenuated peripheral vasoconstriction during physical stress may account for our findings.
Our study is limited by the lack of measurement of stroke volume and plasma l-arginine and NO levels. We did not measure the main components of baPWV (cfPWV and faPWV) because of technical limitations (hip was passively flexed to submerge the foot in cold water). However, baPWV is highly correlated with cfPWV( Reference Sugawara, Hayashi and Yokoi 30 ), and increased values of both indices are similarly associated with cardiovascular risk factors( Reference Yamashina, Tomiyama and Arai 33 ). Our participants were young, healthy men and the present findings may not be translated to other populations. Finally, the multiple comparisons performed in our study are a statistical concern because they may lead to false-positive significant findings.
In conclusion, our findings demonstrate that 2-week l-CIT attenuated aortic BP, wave reflection and systemic arterial stiffness responses to stress induced by exercise-related metabolites and local pain/cold exposure. In addition, the vascular protective effect of l-CIT during metaboreflex activation was more pronounced during cold exposure.
Acknowledgements
The authors thank NOW foods for providing the l-citrulline and placebo capsules without charge. NOW foods had no role in the design, analysis or writing of this article.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The contributions of the authors are as follows: A. F. and R. K. designed the study and analysed the data. R. K., S. A.-A. and S. J. J. collected the data. A. F. wrote the manuscript. All the authors reviewed and approved the final version of the manuscript. A. F. had the primary responsibility of the study.
None of the authors has any conflicts of interest to declare.







