Postmenopausal women have a greater risk of developing heart failure with preserved ejection fraction due to higher aortic stiffness contributing to isolated systolic hypertension and augmented left ventricular pulsatile load (aortic pulse pressure (PP))(Reference Coutinho, Borlaug and Pellikka1,Reference Sun2) . In adults older than 50 years, increased aortic systolic blood pressure (SBP) and PP are attributed to elevated pressures of the forward (Pf) and backward (Pb) waves(Reference Hodson, Norton and Ballim3,Reference Weber and Chirinos4) . The Pf is mainly influenced by stroke volume and proximal aorta stiffness(Reference Li, Gu and Fok5), while the Pb is affected by waves reflected back to the aorta from peripheral sites(Reference Chirinos and Segers6). Elevated resting Pf and Pb in postmenopausal women cause left ventricular pulsatile load that predisposes them to heart failure development(Reference Coutinho, Borlaug and Pellikka1).
Elevated SBP during moderate- to high-intensity exercise is a predictor of cardiovascular mortality due to left ventricular remodeling in healthy middle-aged women(Reference Sarma, Howden and Carrick-Ranson7). Increased systolic and pulsatile load are also apparent during low-intensity exercise(Reference Choi, Stebbins and Nho8,Reference Kang, Maharaj and Dillon9) , an exertion comparable to normal activities of daily living. Healthy postmenopausal women have exaggerated aortic SBP and Pf responses to low-intensity isometric handgrip exercise (IHG) compared with young counterparts(Reference Kang, Maharaj and Dillon9). The blood pressure (BP) response to exercise is induced by an increase in sympathetic-mediated vasoconstriction via skeletal muscle mechano- and metaboreceptor activation. Post-exercise muscle ischaemia (PEMI) following IHG is a manoeuver commonly used to isolate the metaboreflex by trapping muscle metabolites(Reference Figueroa, Maharaj and Johnson10) and maintain sympathetic-mediated BP elevation(Reference Delaney, Greaney and Edwards11). Evidence suggests that metaboreflex overactivation in postmenopausal women(Reference Choi, Stebbins and Nho8,Reference Wenner, Greaney and Matthews12) causes amplified aortic SBP, PP, Pf and Pb responses(Reference Kang, Maharaj and Dillon9), indicating greater left ventricular pulsatile load(Reference Coutinho, Borlaug and Pellikka1). This pulsatile overload during exercise may cause ventricular hypertrophy and stiffness(Reference Sarma, Howden and Carrick-Ranson7,Reference Chirinos, Segers and Raina13) , leading to heart failure in middle-aged women(Reference Sarma, Howden and Carrick-Ranson7). Proposed mechanisms for augmented SBP responses to exercise and PEMI are reduced nitric oxide (NO) bioavailability(Reference Michishita, Ohta and Ikeda14) and increased sympathetic activity in healthy women(Reference Wenner, Greaney and Matthews12), suggesting that endothelial dysfunction contributes to the increased sympathetic-mediated vasoconstriction. Endothelial dysfunction is characterised by reduced NO production due to arginine (ARG) deficiency commonly seen in postmenopausal women(Reference Klawitter, Hildreth and Christians15,Reference Moreau, Hildreth and Meditz16) .
Increasing NO bioavailability via ARG precursors may be an effective approach to improve vasodilatory capacity and reduce exercise hypertension in postmenopausal women(Reference Maharaj, Fischer and Dillon17). Evidence indicates oral L-citrulline supplementation (CIT) is more efficient at increasing circulating ARG levels than a similar dose of ARG(Reference Schwedhelm, Maas and Freese18), resulting in greater NO bioavailability(Reference Shatanawi, Momani and Al-Aqtash19). Previous data demonstrated that CIT increases plasma levels of ARG(Reference Maharaj, Fischer and Dillon20) and NO and reduces aortic SBP and PP at rest in postmenopausal women(Reference Wong, Alvarez-Alvarado and Jaime21). Moreover, evidence indicates that CIT can reduce aortic SBP, PP, and pressure wave amplitude responses to sympathetic overactivation induced via cold exposure in older adults(Reference Jaime, Nagel and Maharaj22). Similarly, acute L-citrulline (6 g) and chronic CIT (6 g/d) attenuated aortic SBP responses to exercise and to PEMI combined with cold exposure in hypertensive postmenopausal women(Reference Maharaj, Fischer and Dillon17) and healthy young men(Reference Figueroa, Alvarez-Alvarado and Jaime23), demonstrating the ability of CIT to effectively control BP responses to conditions with excessive sympathetic activity. However, a single dose of CIT had no BP lowering effect during dynamic exercise without cold exposure in postmenopausal women(Reference Maharaj, Fischer and Dillon17), suggesting that a higher dose may be necessary to reduce exercise BP. The highest CIT dose proposed for clinical use in older adults is 10 g(Reference Moinard, Nicolis and Neveux24). It was recently shown that CIT (10 g) was effective for reducing resting aortic BP via improved endothelial function in postmenopausal women with hypertension(Reference Maharaj, Fischer and Dillon20). Since it has been shown that antihypertensive medications are ineffective at controlling BP during low-intensity IHG and PEMI(Reference Ubolsakka-Jones, Sangthong and Aueyingsak25,Reference Chant, Bakali and Hinton26) , 10 g of CIT may be an effective alternate strategy to attenuate hypertensive responses to low-intensity exercise.
The purpose of this study was to investigate the effects of CIT on aortic BP and pressure waves during exercise and metaboreflex activation in postmenopausal women compared with placebo (PL). We hypothesise that CIT for 4 weeks would attenuate aortic BP and pressure wave responses to IHG and PEMI.
Methods
Participants
Participants were postmenopausal women between 52 and 71 years of age, BMI < 40 kg/m2 and brachial SBP ≤ 160 mmHg. All participants were postmenopausal for at least 1 year and sedentary (< 120 min/week of exercise). Exclusion criteria included BMI ≥ 40 kg/m2, brachial SBP > 160 mmHg, diagnosis of cardiovascular diseases (CVD), type 2 diabetes or other chronic diseases. Participants were excluded if they were current or previous tobacco users, consumed more than twelve alcoholic drinks per week, started hormone replacement therapy within the last 6 months and/or medications that may affect the outcome variables (β-blockers, more than one vasoactive antihypertensive medication). The study protocol was approved by the Institutional Review Board of Texas Tech University (IRB2018-463) and conformed to the standards set by the Declaration of Helsinki. Participants provided written informed consent before data collection. This study is registered in ClinicalTrials.gov under NCT05227781.
Study protocol
This was a randomised, double-blind, PL-controlled, parallel study design. Participants were asked to maintain their usual physical activity throughout the study. Participants were instructed to come to the laboratory testing visits following an overnight fast and 24-h abstinence from caffeine, medications, alcohol and physical activity. During their first visit, participants signed an informed consent, completed a health history questionnaire and were familiarised with all testing protocols. Participants were randomised to either CIT (10 g) or PL (maltodextrin) (NOW Foods) and were asked to take six capsules in the morning and seven in the evening for 4 weeks. Randomisation was performed by a member of the research group that was not involved in laboratory measurements using a block scheme stratified by age and brachial SBP. The last dose of CIT or PL was ingested 10–12 h before their last laboratory visit and participants returned the capsule bottles for compliance assessment (≥80 %) at their last visit.
Participant characteristics
Height and weight were measured using a stadiometer (Free-Standing Portable Height Rod, Detecto) and beam scale (Weigh Beam, Detecto) to calculate BMI (body weight (kg) divided by height squared (m2)). Waist circumference was measured at the midpoint between the superior border of the iliac crest and lower border of the ribs using a nonelastic tape measure. Participants then performed three maximal voluntary contractions with the dominant hand using a handgrip dynamometer (Lafayette Instrument CO., Lafayette). The highest maximal voluntary contractions of the three trials were used to calculate the IHG exercise intensity (30 %). Participants rested in the supine position for 20 min in a quiet, dimly lit and temperature-controlled room (22–24°C). After rest, brachial BP was measured twice using an automated oscillometric device (HEM-705CP; Omron Healthcare, Vernon Hill). A third reading was performed if SBP and/or diastolic BP (DBP) were ≥ 5 mmHg to ensure a stable resting measurement.
Skeletal muscle metaboreflex
Following the 20-min rest period, pulse wave analysis was obtained in the supine position. Then, a pressure cuff (E20 Rapid Cuff Inflator, Hokanson) was positioned around the upper arm. Participants performed 2 min of IHG at 30 % of their maximal voluntary contractions while receiving visual and auditory feedback to ensure the target force was sustained throughout the 2 min. To isolate the metaboreflex, 10 s prior to cessation of exercise, the pressure cuff was rapidly inflated to 250 mmHg for 3 min of PEMI. Brachial BP and aortic pressure waves were obtained during IHG and PEMI.
Blood pressure and aortic pressure waves
Radial applanation tonometry (SphygmoCor CPV, AtCor Medical) was used to estimate aortic BP and pressure wave characteristics at rest, during IHG and PEMI. Radial artery pressure waves were calibrated using brachial DBP and mean arterial pressure that were obtained at rest, during IHG and PEMI and transformed to aortic pressure waveforms via a validated transfer function to obtain aortic pressures. Wave separation analysis was used to determine Pf, Pb and transit time of the reflected wave (RPPT). A minimum of two high-quality wave recordings (operator index ≥ 80 %) during rest and the second and third minute of IHG and PEMI, respectively, were averaged for analysis. Brachial and aortic PP was calculated as the difference between SBP and DBP.
Statistical analysis
Based on a previous study(Reference Jaime, Nagel and Maharaj22), it was calculated that eleven participants per group would be needed to provide 80 % power (α = 0·05) and an effect size of 1·26 to observe a significant decrease in aortic PP response to IHG and PEMI following CIT supplementation. Normality of data was determined by the Shapiro–Wilk test. An independent t-test was used to assess between-group differences at baseline and the changes in haemodynamics during IHG and PEMI between CIT and PL. A two-way repeated-measures analysis of variance with Bonferroni adjustments was used to detect a group (CIT and PL) by time (0 and 4 week) interaction in haemodynamics at rest and during IHG and PEMI as well as the changes from rest to IHG and PEMI. If group-by-time interactions were identified, t-tests were used for post hoc comparisons. Pearson’s correlations were used to examine relationships between the changes (Δ) in aortic Pf and Pb responses to PEMI with the aortic ΔPP response to PEMI from 0 to 4 weeks. Statistical analyses were performed using SPSS 26.0 (IBM SPSS). Statistical significance was set a priori at P < 0·05. All results are reported as mean ± se.
Results
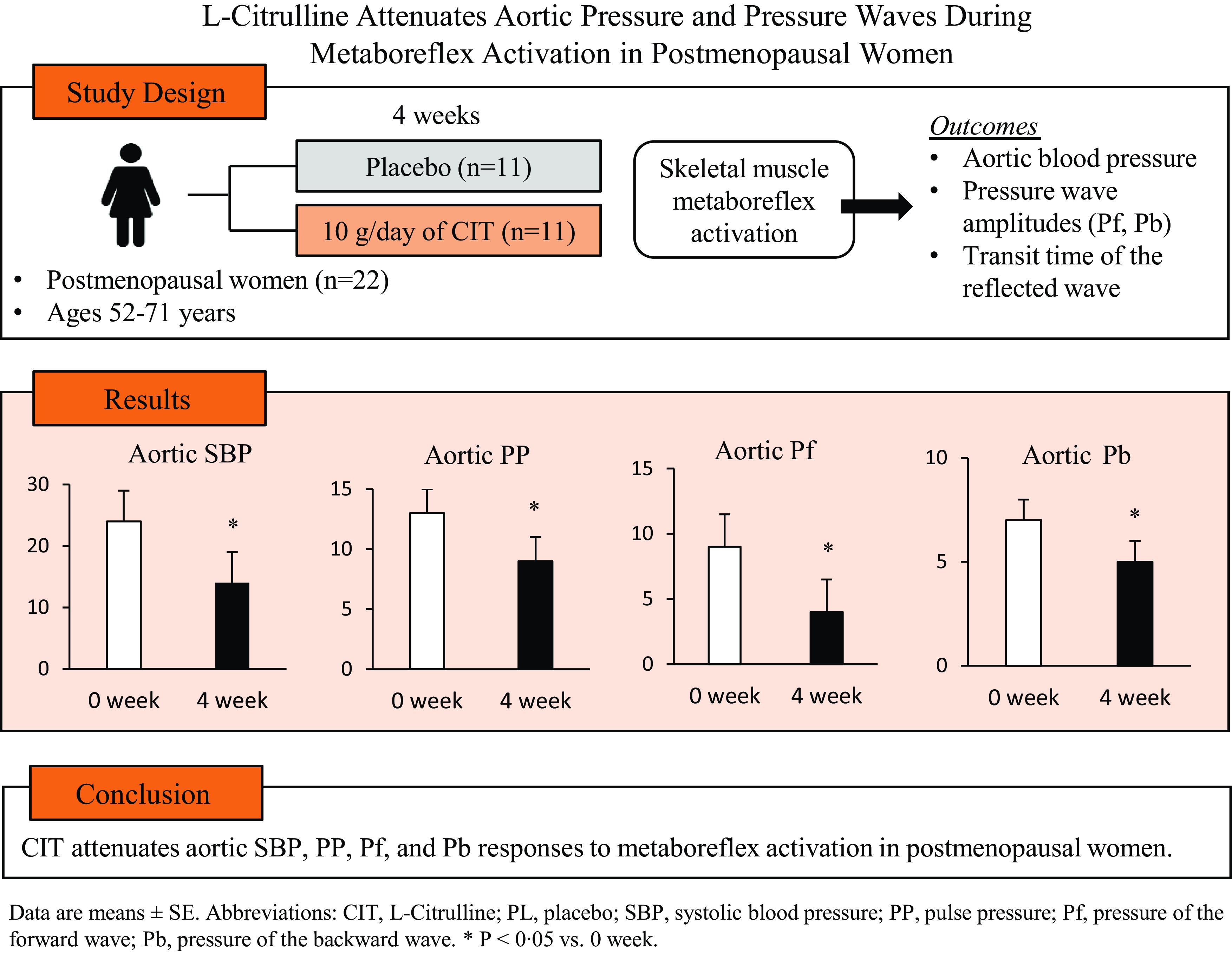
Twenty-eight participants were randomised to CIT or PL groups. Fourteen and eleven participants in the CIT and PL group, respectively, completed their supplementations. However, PEMI data of three women in the CIT group were defective, and thus data of eleven were used in the analysis (Fig. 1). Compliance to the supplements was 95 ± 5 % and 93 ± 7 % for CIT and PL, respectively. There were no adverse effects to the supplements reported by participants during the study. Participant characteristics and medications are presented in Table 1. Participants had elevated BP (CIT n 6 and PL n 3) and hypertension (CIT n 5 and PL n 8). Two and four women with hypertension in the CIT and PL groups, respectively, were on medication. There were no significant differences between groups in age, height, weight, waist circumference, BMI or maximal voluntary contractions at baseline (all P > 0·05).

Fig. 1. Consort study flow chart. CIT, L-citrulline; PL, placebo.
Table 1. Participant characteristics and medications
(Mean values with their standard errors; numbers and percentage)

CIT, L-citrulline; PL, placebo; WC, waist circumference; MVC, maximal voluntary contraction; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme; Ca2+, calcium.
Brachial and aortic BP, pressure waves and RPPT at rest and during IHG and PEMI at 0 and 4 weeks are presented in Table 2. There were no significant differences between groups in haemodynamics at rest or in response to IHG and PEMI at 0 weeks (all P > 0·05). CIT had no effect (P > 0·05) on resting BP and on aortic BP responses to IHG. However, the aortic SBP response to IHG was trending towards significance (P = 0·12). There were significant group-by-time interactions for aortic SBP, PP, Pf and Pb (P < 0·05), but no interactions were found for brachial pressures, aortic mean arterial pressure or RPPT. CIT significantly decreased aortic SBP (Δ-9 ± 2 v. Δ-1 ± 1 mmHg, P = 0·006, Fig. 2(a)), PP (Δ-5 ± 2 v. Δ0 ± 1 mmHg, P = 0·03, Fig. 2(b)), Pf (Δ-6 ± 2 v. Δ-1 ± 1 mmHg, P = 0·01, Fig. 2(c)) and Pb (Δ-3 ± 1 v. Δ0 ± 1 mmHg, P = 0·02, Fig. 2(d)) responses to PEMI compared with PL. The aortic ΔPP was positively correlated with ΔPf (r = 0·743, P < 0·001, Fig. 3(a)) and ΔPb (r = 0·724, P < 0·001, Fig. 3(b)).
Table 2. Blood pressure and pressure waves at rest and during IHG and muscle metaboreflex activation before and after supplementation
(Mean values with their standard errors)

CIT, citrulline; PL, placebo; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; Pf, pressure of the forward wave; Pb, pressure of the backward wave; RPPT, transit time of the reflected wave; IHG, isometric handgrip; PEMI, postexercise muscle ischaemia.
* P < 0·05.
† P < 0·01 change in response to PEMI from rest following supplementation.
‡ P < 0·05.
§ P < 0·01 v. 0 weeks.

Fig. 2. Changes (Δ) in aortic systolic blood pressure (SBP, a), pulse pressure (PP, b), pressure of the forward (Pf, c) and reflected wave (Pb, d) from rest to PEMI following L-citrulline and placebo supplementation. Values are means with standard errors. Solid circles and triangles represent hypertensive participants. *P < 0·05; † P < 0·01 v. placebo.

Fig. 3. Relationship between changes (Δ) in aortic pulse pressure (PP) and pressure of the forward (Pf, a) and reflected wave (Pb, b) from rest to PEMI from 0 to 4 weeks. Abbreviations: CIT, L-citrulline; PL, placebo.
Discussion
The novel findings of this study are that 4 weeks of CIT (10 g/d) attenuated aortic SBP, PP and pressure wave amplitude (Pf and Pb) responses to metaboreflex activation in postmenopausal women compared with PL. Attenuated aortic PP was related to decreases in both Pf and Pb responses to PEMI. Conversely, there were no decreases in BP or pressure wave amplitude responses to IHG. These findings suggest that CIT may be a viable dietary strategy to reduce left ventricular systolic and pulsatile load during skeletal muscle metaboreflex activation in postmenopausal women.
In this study, 4 weeks of CIT had no effect on BP or aortic pressure waves at rest. These findings are similar to a previous study using CIT for 2 weeks in older adults (69 % with hypertension)(Reference Jaime, Nagel and Maharaj22). In contrast, 6 weeks of watermelon supplementation, a natural rich source of CIT, reduced resting brachial and aortic BP and wave reflection amplitude in obese hypertensive adults(Reference Figueroa, Wong and Kalfon27,Reference Figueroa, Wong and Hooshmand28) . In the current study, women in the CIT group had either hypertension or elevated BP at rest. A recent study from our laboratory has shown that 4 weeks of CIT reduced resting aortic DBP and mean arterial pressure in hypertensive postmenopausal women. Although not statistically significant, resting aortic SBP tended to decrease by ∼3 mmHg(Reference Maharaj, Fischer and Dillon20). This non-significant reduction may have been due to a relatively low mean aortic SBP at rest and because 50 % of the women in the CIT group were treated for hypertension. Thus, CIT may only be beneficial at rest in individuals with hypertension.
During exercise, increases in stroke volume and sympathetic-mediated vasoconstriction contribute to augment SBP responses in young adults(Reference Choi, Stebbins and Nho8,Reference Milia, Roberto and Mulliri29) . Evidence suggests that postmenopausal women have exaggerated sympathetic activation associated with increases in peripheral vasoconstriction during IHG(Reference Choi, Stebbins and Nho8,Reference Wenner, Greaney and Matthews12) , which contributes to greater SBP responses compared with young women(Reference Kang, Maharaj and Dillon9). Exaggerated SBP and PP responses to maximal and IHG exercise are associated with increased left ventricular mass in middle-aged healthy women and individuals with hypertension(Reference Sarma, Howden and Carrick-Ranson7,Reference Chirinos, Segers and Raina13) . This uncontrolled exercise SBP predisposes non-hypertensive women to develop arterial stiffening, leading to chronic systolic hypertension and progression to heart failure(Reference Sarma, Howden and Carrick-Ranson7). In the present study, CIT had no effect on aortic BP or pressure wave responses to low-intensity IHG exercise. Although not statistically significant, the aortic SBP (∼4 mmHg) and DBP (∼3 mmHg) response to IHG decreased slightly after CIT. The aortic SBP, but not the aortic DBP, response to IHG was trending toward significance (P = 0·12). However, although not significant, the simultaneous decrease of both aortic SBP and DBP explains the non-significant change in aortic PP. Similarly, CIT (6 g) for 2 weeks did not attenuate aortic DBP response to IHG in young overweight males(Reference Figueroa, Alvarez-Alvarado and Jaime23), providing context of the findings in the current study.
An overactive muscle metaboreflex is the main contributor to the amplified SBP response to exercise in postmenopausal women(Reference Choi, Stebbins and Nho8,Reference Wenner, Greaney and Matthews12) . The exaggerated BP response to PEMI is primarily regulated by sympathetic vasoconstriction since the increase in cardiac output is not greater in postmenopausal than young women(Reference Choi, Stebbins and Nho8,Reference Wenner, Greaney and Matthews12) . We previously demonstrated an increased aortic PP response to PEMI was attributed to both augmented Pf and Pb in normotensive postmenopausal women(Reference Kang, Maharaj and Dillon9). Although IHG and PEMI did not increase Pf in young women and men(Reference Kang, Maharaj and Dillon9,Reference Stock, Chouramanis and Chirinos30) , Pf increased in postmenopausal women(Reference Kang, Maharaj and Dillon9). Similarly, the increase in aortic SBP during supine cycling results from forward compression waves (Pf) produced by early stroke volume ejection in middle-aged adults(Reference Schultz, Davies and Roberts-Thomson31). Therefore, the increase in Pf could be produced by stroke volume ejection into a stiffer aorta(Reference Mitchell, Parise and Benjamin32). Considering that stroke volume does not increase during IHG(Reference Chirinos, Segers and Raina13) and PEMI(Reference Milia, Roberto and Mulliri29) in middle-aged and older adults, the Pf response to PEMI was most likely driven by an increased aortic stiffness in postmenopausal women(Reference Kang, Maharaj and Dillon9). Increased arterial stiffness and vasoconstriction result in increased wave reflection from peripheral sites back to the aorta during systole, increasing the magnitude of Pb(Reference Stock, Chouramanis and Chirinos30). Despite an augmented Pb response to PEMI, the increase in Pf has a predominant effect on the exaggerated SBP and PP responses to metaboreflex activation in postmenopausal women(Reference Kang, Maharaj and Dillon9). The elevated aortic SBP during PEMI may contribute to greater left ventricular pulsatile load and development of heart failure(Reference Tsao, Lyass and Larson33). Thus, attenuation of aortic pulsatile load during low-intensity exercise similar to activities of daily living is of clinical importance to prevent left ventricle damage(Reference Chirinos, Segers and Raina13).
In the current study, 4 weeks of CIT significantly attenuated aortic SBP, PP, Pf (∼6 mmHg) and Pb (∼3 mmHg) responses to PEMI. We found both Pf and Pb were positively correlated to aortic PP responses to PEMI. Therefore, the decrease in aortic PP was due to attenuation of both Pf and Pb responses. We have previously shown that 2 weeks of CIT (6 g/d) attenuated aortic SBP and wave reflection (augmented pressure) responses to metaboreflex activation combined with cold exposure (a manoeuver to increase sympathetic activity) in young overweight men(Reference Figueroa, Alvarez-Alvarado and Jaime23). Notably, CIT did not reduce wave reflection during PEMI without cold exposure, indicating that CIT is less efficient to attenuate haemodynamic responses to mild sympathetic-mediated vasoconstriction in healthy young men(Reference Figueroa, Alvarez-Alvarado and Jaime23). Thus, CIT may be more efficacious in populations with exaggerated BP and sympathetic responses to exercise and cold exposure, such as hypertensive postmenopausal women and older adults(Reference Maharaj, Fischer and Dillon17,Reference Jaime, Nagel and Maharaj22) . The efficiency of CIT to attenuate Pf and Pb responses to cold-induced sympathetic vasoconstriction was previously demonstrated after 2 weeks of supplementation in older adults(Reference Jaime, Nagel and Maharaj22). Notably, CIT attenuated Pf to a greater extent than Pb, suggesting an efficiency to reduce aortic stiffness and forward compression pressure waves generated by stroke volume ejection(Reference Stock, Chouramanis and Chirinos30,Reference Schultz, Davies and Roberts-Thomson31) . The decrease in Pb suggests that CIT effectively reduced the impact of sympathetic-mediated peripheral vasoconstriction(Reference Stock, Chouramanis and Chirinos30,Reference Zamani, Rawat and Shiva-Kumar34) . Although Pb attenuation was of lesser magnitude, it is of clinical significance since Pb is an early predictor of cardiovascular mortality, independent of arterial stiffness(Reference Wang, Cheng and Sung35). We provide the first evidence that CIT is an effective dietary strategy to reduce systolic and pulsatile load on the left ventricle via attenuation of pressure waves during metaboreflex activation, which may reduce left ventricular damage(Reference Chirinos, Segers and Raina13,Reference Zuo, Chang and Tan36) in postmenopausal women, a population with increased risk for developing heart failure with preserved ejection fraction(Reference Coutinho, Borlaug and Pellikka1).
Mechanisms that contribute to exaggerated SBP and PP responses to exercise are endothelial dysfunction(Reference Michishita, Ohta and Ikeda14,Reference Thanassoulis, Lyass and Benjamin37,Reference Stewart, Sung and Silber38) and arterial stiffening(Reference Thanassoulis, Lyass and Benjamin37). Endothelial dysfunction is characterised by reduced NO bioavailability and vasodilatory capacity. During IHG, SBP increases due to augmented sympathetic-mediated peripheral vasoconstriction(Reference Kim and Ha39). To modulate BP, endothelial cells in conduit arteries produce NO in response to shear stress to promote endothelium-dependent vasodilation(Reference Thanassoulis, Lyass and Benjamin37). Conversely, endothelial dysfunction increases aortic stiffness, vasoconstriction and wave reflection, contributing to augmented systolic and pulsatile load on the left ventricle(Reference Thanassoulis, Lyass and Benjamin37,Reference Stewart, Sung and Silber38,Reference McEniery, Wallace and Mackenzie40) . In fact, Mitchishita and colleagues(Reference Michishita, Ohta and Ikeda14) found that the exaggerated SBP response to exercise is associated with NO bioavailability in middle-aged women with elevated BP and stage 1 hypertension. CIT is an ARG precursor and can increase NO bioavailability and endothelial function. Recent findings from our laboratory showed that 4 and 8 weeks of CIT can improve endothelial function assessed via brachial and superficial femoral flow-mediated vasodilation through increases in ARG and NO bioavailability in hypertensive and obese postmenopausal women(Reference Maharaj, Fischer and Dillon20,Reference Wong, Alvarez-Alvarado and Jaime21,Reference Kang, Dillon and Martinez41) . Moreover, our previous data demonstrated that CIT attenuates the acute increase in systemic arterial stiffness induced by sympathetic mediated vasoconstriction during PEMI and cold exposure(Reference Figueroa, Alvarez-Alvarado and Jaime23). Thus, the ability of CIT to attenuate aortic SBP, PP, Pf and Pb responses to metaboreflex activation may have occurred through reduced proximal aortic stiffness and increased peripheral vasodilatory capacity(Reference Maharaj, Fischer and Dillon20,Reference Kang, Dillon and Martinez41) . The impact of CIT on Pf may explain the observed aortic pulsatile load attenuation without affecting peripheral BP. Moreover, improved limb artery vasodilation may explain the reduction in Pb during PEMI. These findings are important considering postmenopausal women have increased pulsatile hemodynamic load on the left ventricle at rest(Reference Coutinho, Borlaug and Pellikka1) and during metaboreflex activation(Reference Kang, Maharaj and Dillon9).
This study is not without limitations. The sample size was relatively low, and a larger sample size could strengthen the findings of future studies. We did not account for CIT content in the diet. Although CIT is found in chickpeas, nuts and other vegetables, watermelon is the main natural source of CIT (0·7 to 3·6 g/kg)(Reference Rimando and Perkins-Veazie42). Thus, it would be necessary to ingest 1·6–8·6 kg and 2·7–14·3 kg of watermelon flesh or rind per day to get 6 g and 10 g of CIT, respectively. We have reported increases in ARG levels after 6 g(Reference Figueroa, Maharaj and Kang43) and 10 g(Reference Maharaj, Fischer and Dillon20) of CIT for 4 weeks in postmenopausal women. However, serum ARG levels did not increase significantly with 2 g of CIT(Reference Figueroa, Maharaj and Kang43). Thus, CIT in the diet may have not affected the results of the present study. Obtaining blood before and after supplementation to verify increases in circulating CIT, ARG and NO would have further strengthened our findings. Additionally, measurement of muscle sympathetic nerve activity, aortic characteristic impedance, stroke volume and total peripheral resistance could better confirm our hypothesis that CIT improves aortic SBP and PP through improvements in peripheral vasodilation, aortic stiffness and sympathoinhibition. Moreover, our participants were otherwise healthy postmenopausal women with elevated SBP and hypertension, and as such, there is a crucial need to examine the effects of longer duration of CIT supplementation on aortic SBP and PP at rest and during exercise in individuals with increased CVD risk, such as women with hypertension or metabolic syndrome.
In conclusion, 4 weeks of CIT attenuates aortic SBP, PP and pressure wave amplitude responses to metaboreflex activation in postmenopausal women. Our findings suggest that the reductions in Pf and Pb contributed to attenuation of aortic PP. Therefore, CIT may be an effective dietary strategy to reduce CVD risk in postmenopausal women.
Acknowledgements
We would like to thank participants who volunteered their time to make this project a success. We thank NowFoods for supplying the L-citrulline and placebo without charge.
This research did not receive specific grant from any funding agency.
A. F. conceived and designed the study; K. N. D., Y. K., A. M., S. M. F. and M. A. M. performed data collection; K. N. D. and A. F. analysed data, interpreted results and wrote the manuscript. A. F., Y. K., A. M., S. M. F. and M. A. M. edited and revised the manuscript. All authors read and approved to publish the final version of the manuscript.
The authors do not have financial or personal conflict of interest to declare.