Exclusive breast-feeding during the first 6 months of life increases mother–child bonding and is associated with healthier maternal and offspring outcomes( Reference Beyerlein and von Kries 1 – Reference Diniz and Da Costa 5 ). Maternal energy metabolism during human lactation is characterised by augmented glucose production and increased mobilisation of fat from maternal depots, ensuring adequate milk production for the increasing demands of the growing infant( Reference Tigas, Sunehag and Haymond 6 – Reference Lassek and Gaulin 8 ). To date, it is not known whether these changes in energy metabolism are related to differences in appetite-regulating hormones.
The neuroendocrine milieu of lactation is governed by increased circulating concentrations of prolactin and oxytocin, as well as inhibition of the gonadotropin-releasing hormone, leading to lactational amenorrhoea( Reference McNeilly 9 ). These and other hormonal changes during lactation might also affect glucose metabolism, fat metabolism and energy balance. Oxytocin reduces insulin resistance, increases lipolysis and exerts anti-orexigenic effects( Reference Leng, Onaka and Caquineau 10 – Reference Deblon, Veyrat-Durebex and Bourgoin 12 ). In contrast, prolactin increases the central resistance to leptin, stimulates appetite and promotes high-fat diet-induced obesity( Reference Augustine and Grattan 13 , Reference Auffret, Viengchareun and Carré 14 ).
Appetite-regulating hormones derived from the gut may also be affected by the complex neuroendocrine changes accompanying lactation, as they are subject to tight regulatory feedbacks within the gut–brain axis and are closely related to alterations of energy metabolism( Reference Maier, Riedl and Vila 15 , Reference Murphy, Dhillo and Bloom 16 ). Indeed, the gastrointestinal expression and circulating concentrations of the anorexigenic hormone peptide YY (PYY) were increased throughout the lactation period in rodents, whereas ghrelin levels remained unchanged( Reference Taylor, Patterson and Ghatei 17 ). In humans, at 4 weeks postpartum, there were no differences in plasma ghrelin levels and PYY between lactating and non-lactating women( Reference Larson-Meyer, Ravussin and Heilbronn 18 ). Nevertheless, 3 years postpartum, circulating concentrations of both ghrelin and PYY strongly correlated with the duration of breast-feeding( Reference Stuebe, Mantzoros and Kleinman 19 ).
The WHO recommends exclusive breast-feeding during the first 6 months. During this time, the infant gains weight rapidly and the amount of milk needed for exclusive breast-feeding increases accordingly( Reference Neville, Keller and Seacat 20 ). Therefore, it is expected that hormone differences at 3–6 months postpartum strongly reflect lactation-induced changes and are less influenced by peripartal events. Based on this hypothesis, we studied the effects of breast-feeding on appetite-regulating hormones in a cohort of lactating and non-lactating women 3–6 months postpartum, compared with a control group.
Methods
Design and participants
The protocol was approved by the institutional review board of the Medical University of Vienna and registered with clinicaltrials.gov (NCT00831818). The participants were twenty-nine healthy women, who were recruited after signed informed consent to three age- and BMI-matched groups: lactating group, non-lactating group and control group. All the participants underwent a thorough clinical examination and biochemical tests before being included in the study. The lactating group included ten mothers of 3 to 6-month-old infants (age range 14–23 weeks, mean age 16·2 weeks), who were exclusively breast-fed and had never taken formula milk. The non-lactating group included nine mothers of 3- to 6-month-old infants (age range 14–22 weeks, mean 15·9 weeks), who were not breast-fed during the previous 4 weeks or longer. The control group included ten healthy women with no history of pregnancy or breast-feeding during the previous 12 months. Women who were menstruating (two mothers in the lactating group, all mothers in the non-lactating group and all women in the control group) attended the sessions during the first 3 d of the follicular phase of the menstrual cycle. Inclusion criteria for lactating and non-lactating groups were as follows: singleton pregnancy, no gestational diabetes, no pregnancy complications, normal vaginal delivery and healthy normally growing infants (reported in the mother–child pass). Exclusion criteria for all three groups were BMI<18·5 kg/m2 or BMI>24·9 kg/m2, smoking, current endocrine and metabolic diseases, cardiovascular, kidney or liver diseases and malignancy. One lactating mother, five non-lactating mothers and six women from the control group were on hormonal contraception. Four mothers from the breast-feeding group, five mothers from the non-lactating group and four women from the control group were primiparous. Other participants of the lactating and non-lactating group had at least two children. Six participants of the control group were nulliparous.
The recruitment process was performed via flyers. We experienced difficulties in recruiting mothers with normal BMI who did not breast-feed, as >50 % of not breast-feeding women contacting the study centre were overweight or obese.
During the study day, all the participants came to the clinical research centre between 08.00 and 10.00 hours after fasting for at least 4 h. The timing of the visit was planned to be flexible, as mothers of infants were advised to arrive about 45 min before the next planned breast-feeding or bottle-feeding episode. Participants from the lactating and non-lactating groups received an intravenous cannula in the left antecubital vein and blood samples were collected at five time points during a time period of at least 90 min: start of the study (time point – 20 min), immediately before breast-feeding/bottle-feeding (time point 0 min) and 15, 30 and 60 min after starting breast-feeding/bottle-feeding. Mothers were instructed either to breast-feed their infants (lactating group) or to bottle-feed their infants (non-lactating group). Breast-feeding lasted from 8 to 20 min (mean 15 min).
Assays
All the study participants provided baseline blood samples for the routine measurement of glucose and lipid values, C-reactive protein, HbA1c and thyroid-stimulating hormone. All these measurements were performed in a routine laboratory (www.kimcl.at). Blood samples for the measurement of ghrelin, PYY, leptin, adiponectin, prolactin, growth hormone (GH), cortisol and insulin were centrifuged for 15 min at 1200 g , and serum or plasma was frozen at –20°C. Blood samples for the measurement of acylated ghrelin were obtained in ice-cold (4°C) vials containing EDTA and aprotinin (Trasylol, 500 000 Kallikrein inhibitor units, 40 µl/ml). After centrifugation, plasma aliquots of 1 ml were acidified with 110 µl 1 n-HCl and immediately frozen at –20°C.
All the frozen samples were analysed at the end of the study with kits of the same lot number with all samples obtained from one participant measured within the same kit and an equal proportion of samples from all three groups within each kit. Plasma concentrations of total ghrelin were determined using RIA kits from Peninsula Laboratories (Bachem) with intra-assay and inter-assay CV <9·9 %. Acylated ghrelin was measured using a sandwich ELISA kit from Linco Research with intra-assay and inter-assay CV both being <7·5 %. Concentrations of PYY, leptin, adiponectin and insulin were determined using RIA kits from Linco Research (Millipore) with intra-assay and inter-assay CV being 9 and 8·2 % for PYY and 5–8 % for leptin and adiponectin, respectively. Prolactin, cortisol and GH were measured in a routine certified laboratory using electro-chemiluminescence immunoassay, with sensitivity and CV being 0·05 ng/ml and 4 % for prolactin, 0·04 µg/dl and 6 % for cortisol and 0·01 ng/ml and 7·5 % for GH, respectively (www.kimcl.at).
Statistical analysis
Data are expressed as mean values with their standard errors and were analysed using the statistical software SPSS release 12.0.1 (SPSS Inc.). Baseline characteristics between the three groups were compared by one-way ANOVA followed by multiple t tests with the Bonferroni correction as post hoc statistics, where appropriate. Breast-feeding-induced changes over time were analysed using repeated measurements ANOVA with the interaction between time and treatment (time×treatment) being the term of interest. P<0·05 was considered statistically significant.
Results
Subject characteristics
The clinical characteristics of the three groups are given in Table 1. There were no differences in age, weight or BMI between the groups. Waist circumference was higher in breast-feeding women (Table 1). Parameters of glucose metabolism were similar in all the groups. Lactating mothers had lower circulating TAG levels and a lower LDL-cholesterol:HDL-cholesterol ratio when compared with non-lactating mothers (Table 2).
Table 1 Baseline characteristics of study participants(Mean values with their standard errors)

The lactating group includes women who exclusively breast-feed (n 10), the non-lactating group includes women who had not breast-fed during the previous 4 weeks or longer (n 9) and the control group includes healthy women with no history of pregnancy or breast-feeding during the previous 12 months (n 10).
* P=0·004 lactating v. non-lactating, † P<0·001 lactating v. control.
Table 2 Parameters of glucose and lipid metabolism(Mean values with their standard errors)
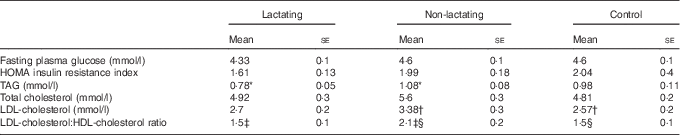
HOMA, homoeostasis model assessment.
The lactating group includes women who exclusively breast-feed (n 10), the non-lactating group includes women who had not breast-fed during the previous 4 weeks or longer (n 9) and the control group includes healthy women with no history of pregnancy or breast-feeding during the previous 12 months (n 10).
* P=0·006 lactating v. non-lactating, † P=0·025 non-lactating v. control, ‡ P=0·036 lactating v. non-lactating, § P=0·048 non-lactating v. control.
Baseline circulating hormone levels during lactation
Full lactation was associated with nearly 4-fold higher circulating prolactin concentrations (Table 3), but no differences in baseline GH, cortisol and insulin levels.
Table 3 Hormone concentrations of the study subjects(Mean values with their standard errors)
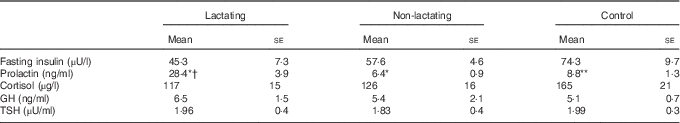
GH, growth hormone; TSH, thyroid-stimulating hormone.
The lactating group includes women who exclusively breast-feed (n 10), the non-lactating group includes women who had not breast-fed during the previous 4 weeks or longer (n 9) and the control group includes healthy women with no history of pregnancy or breast-feeding during the previous 12 months (n 10).
* P<0·001 lactating v. non-lactating, † P<0·001 lactating v. control.
At baseline, there were no differences in circulating concentrations of ghrelin, leptin and adiponectin between the groups (Fig. 1(a, c and d)). However, plasma PYY concentrations were significantly higher in lactating women (100·3 (se 6·7) pg/ml) when compared with non-lactating women (73·6 (se 4·9) pg/ml, P=0·008) or control women (70·2 (se 9) pg/ml, P=0·021) (Fig. 1(b)).

Fig. 1. Baseline concentrations of appetite-regulating hormones. Concentrations of (a) ghrelin, (b) peptide YY (PYY), (c) leptin and (d) adiponectin in the three groups. The lactating group included women who exclusively breast-feed (n 10), the non-lactating group included women who had not breast-fed during the previous 4 weeks or longer (n 9), control group included healthy women with no history of pregnancy or breast-feeding during the previous 12 months (n 10). Data are shown as mean values with their standard errors. * P=0·008 v. non-lactating, † P=0·021 v. control group.
Profile of hormone changes during breast-feeding sessions
During the process of breast-feeding, the already basally elevated prolactin concentrations increased further, but there were no changes in the profiles of plasma ghrelin, acylated ghrelin, cortisol and insulin (Fig. 2(a, c–f)). Plasma PYY remained significantly higher throughout the study period (Fig. 2(b)).

Fig. 2. The profile of hormone changes during breast-feeding. The profile of changes in (a) prolactin, (b) peptide YY (PYY), (c) ghrelin, (d) acylated ghrelin, (e) cortisol and (f) insulin concentrations during breast-feeding v. bottle-feeding. ●, breast-feeding; ○, bottle-feeding; the shaded area indicates the duration of breast-feeding/bottle-feeding. Data are shown as mean values with their standard errors.
Discussion
The main findings of this study are that full lactation is associated with increased baseline concentrations of maternal PYY, but the act of breast-feeding does not acutely influence the profile of circulating maternal ghrelin and PYY. Furthermore, full lactation is accompanied by increased baseline prolactin concentrations, lower plasma TAG, a better LDL-cholesterol:HDL-cholesterol ratio and increased waist circumference.
To our knowledge, these data on higher PYY concentrations represent the only reported difference in gut-derived hormones during breast-feeding in humans. In reality, the differences in PYY concentrations between lactating and non-lactating women might be even greater than that observed in our study, as we compared groups with similar BMI. In general, non-lactating women are more obese than lactating women( Reference Verret-Chalifour, Giguère and Forest 21 ), and obesity is associated with decreased PYY levels( Reference Maier, Riedl and Vila 15 , Reference Murphy, Dhillo and Bloom 16 ); therefore, the general cohort of non-lactating women is expected to have even lower PYY concentrations.
Lactation is associated with reduced expression of proopiomelanocortin and increased expression of the orexigenic neuropeptide Y in the hypothalamus, increased prolactin concentrations and decreased oestradiol concentrations( Reference Chen, Williams and Grove 22 , Reference Woodside 23 ). All these central and peripheral mechanisms impact energy metabolism and might also lead to adaptations in the secretion of gut hormones.
PYY is known for its appetite-inhibitory and lipolytic effects that lead to reduced weight gain in obesity-prone models( Reference Manning and Batterham 24 , Reference Sloth, Holst and Flint 25 ). The higher PYY concentrations between the 3rd and the 6th month of lactation are consistent with data from rodent studies, and complement a previous publication reporting no differences in appetite-regulating hormones between lactating and non-lactating women 4–6 weeks postpartum( Reference Taylor, Patterson and Ghatei 17 , Reference Larson-Meyer, Ravussin and Heilbronn 18 ). Indeed, the positive effect of lactation on postpartum weight retention is observed after the 3rd month postpartum, a period which is also less influenced by peripartal events( Reference Stuebe and Rich-Edwards 26 ). The cross-sectional nature of our study does not allow us to differentiate whether the increase in PYY is a consequence of full lactation or whether reduced PYY is a risk factor for failed lactation. Nevertheless, the fact that PYY concentrations did not differ between lactating and non-lactating mothers at 4 weeks postpartum( Reference Larson-Meyer, Ravussin and Heilbronn 18 ) supports the hypothesis that full prolonged lactation leads to an increase in PYY. This change during the progress of breast-feeding is most likely an adaptation to the concomitant metabolic changes such as the increased energy intake (required for milk production) and mobilisation of maternal depots found to accompany lactation. Indeed, PYY enhances lipolysis and selectively increases carbohydrate consumption, despite reducing appetite( Reference Manning and Batterham 24 , Reference Sloth, Holst and Flint 25 ). We hypothesise that elevated PYY levels promote the selective acquisition of nutrients, and enhance utilisation of fat from maternal depots, ensuring adequate milk production for the infant. One of the main reasons for maternal fat mobilisation during breast-feeding is the critical need for long-chain PUFA, arachidonic acid and, especially, DHA, which are limited in the environment, but extremely important for the rapidly growing infant brain( Reference Lassek and Gaulin 8 ). Our findings on increased maternal waist circumference and improved plasma lipid profile during breast-feeding are in line with previous studies, which have confirmed an association of breast-feeding with increased waist circumference and a relative decrease in lower-body fat( Reference Lassek and Gaulin 8 , Reference Sohlstrom and Forsum 27 , Reference Brewer, Bates and Vannoy 28 ).
We found no differences in ghrelin concentrations during lactation, despite the close relationship between ghrelin and energy metabolism. The main reason for this could be the fact that BMI is the most significant predictor of ghrelin in all the cohorts studied thus far, and we compared three groups with similar BMI in our study( Reference Murphy, Dhillo and Bloom 16 ).
The secretion of ghrelin and PYY follows circadian changes and is strongly influenced by the feeding status. It is important to note that, in this study, we studied breast-feeding-associated differences in appetite-regulating hormones during fasting. In the fasted state, plasma ghrelin is inversely related to cortisol( Reference Espelund, Hansen and Højlund 29 ). Despite the well-known reduced hypothalamic–pituitary–adrenal axis activity in response to stressors, as well as changes in the profile of prolactin and oxytocin concentrations during breast-feeding, we did not find any relevant difference in gut-derived appetite-regulating hormones during one single breast-feeding episode( Reference Heinrichs, Meinlschmidt and Neumann 30 ).
Limitations of this study are the lack of data on maternal education, employment, income, feeding patterns and weight changes over time, as all these parameters could affect the metabolic profile. The strength of this study is the comparison of age- and BMI-matched groups of lactating, non-lactating and control women.
Taken together, in this study, we show that full lactation is associated with elevated baseline PYY concentrations at 3–6 months postpartum, but a single breast-feeding episode does not induce any acute changes in circulating appetite-regulating hormones. Increased plasma PYY concentrations during lactation might not only contribute to increased mobilisation of fat from maternal depots, ensuring adequate milk production in the short term, but also contribute to better maternal health outcomes in the long term.
Acknowledgements
The authors thank Astrid Hofer and Liliana-Imi Ionasz for their excellent technical assistance.
The authors declare that they have no conflicting interests.
G. V., A. L. and M. C. designed the research; G. V., J. H., G. G., S. M. B.-P. and A. K.-W. conducted research; G. V., M. C. and A. L. analysed the data; G. V. wrote the manuscript; and G. V. and A. L. had primary responsibility for the final content. All authors read and approved the final manuscript.








