Isoflavones are a class of bioactive phytochemicals that have been widely studied for their potential role in the prevention of various chronic diseases, such as cardiovascular diseases, neurodegenerative diseases, osteoporosis, cancers… Soy and its processed products (tofu, tempeh, miso, natto, soymilk, soy-based yoghurts and desserts) are the only sources providing high quantities of isoflavones in the human diet. Isoflavone intake has been estimated to 25–50 mg/day in Asian countries, with a maximum around 100 mg/d for elderly Japanese men(Reference Messina, Nagata and Wu1). Americans and Europeans, who have low soy content in their habitual diet, only consume few milligrams of isoflavones per day. However, advertising on the beneficial effects of isoflavones, especially for relieving postmenopausal symptoms, has led to self-supplementation through isoflavone-rich foods or dietary supplements. Isoflavones in soybeans mainly include daidzein, genistein and glycitein, which are present in glycosylated or aglycone forms (Fig. 1). The structural similarity of aglycones with 17β-estradiol gives them the capacity to bind estrogen receptors (ERs) and to induce hormone-like effects. Isoflavones and their protective role against various pathologies involving hormonal dysregulation have been extensively studied because of this particular property.
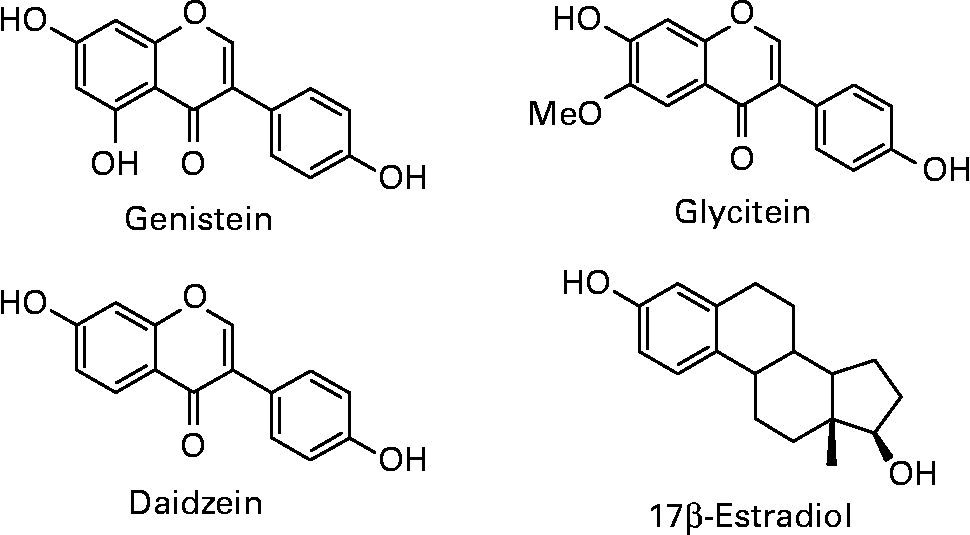
Fig. 1 Structures of the soy isoflavones genistein, daidzein and glycitein in comparison to 17β-estradiol.
The current evidence provided by preclinical studies on the role of isoflavones in reducing prostate and breast cancer risk has been recently reviewed(Reference Messina2–Reference Wietrzyk, Gryniciewicz and Opolski4). A summary of the main data available will be made here, to highlight the present limitations in our understanding of their mechanisms of action, and to evaluate the potential of nutrigenomic approaches to further clarify these mechanisms.
Human studies
The lower risk of prostate and breast cancer in areas of high soy and isoflavone intake, especially in Asia(Reference Hsing, Tsao and Devesa5), as well as the increased risk observed for Asian people who migrated to Western countries or adopted a westernised lifestyle(Reference Shimizu, Ross, Bernstein, Yatani, Henderson and Mack6–Reference Sim and Cheng8) are well known. A recent meta-analysis compiling 2 cohort studies and 6 case–control studies in Western and Asian populations estimated that high soy food intake can be related to a 30 % reduction in prostate cancer risk (odds ratio 0·70, 95 % CI 0·59–0·83)(Reference Yan and Spitznagel9). For breast cancer, a smaller risk reduction (odds ratio 0·86, 95 % CI 0·75–0·99), stronger for premenopausal women, was found in a meta-analysis compiling 6 cohort and 12 case–control studies(Reference Trock, Hilakivi-Clarke and Clarke10). Asian women whose soy intake was high during puberty experienced lower risk for breast cancer than women who did not consume soy products or did only as adults(Reference Shu, Jin, Dai, Wen, Potter, Kushi, Ruan, Gao and Zheng11, Reference Wu, Wan, Hankin, Tseng, Yu and Pike12).
Among hundreds of dietary components that have been proposed as potential cancer preventive agents, only a few have been used in clinical trials. The impact of isoflavone supplementation has been studied on some prostate cancer-related endpoints such as serum levels of Prostate-Specific Antigen (PSA), PSA velocity, plasma levels of testosterone, dihydrotestosterone (DHT), insulin-like growth factor 1 (IGF-1) and IGF binding protein 3 (IGFBP-3). Among the 11 trials recently reviewed by Messina et al. (Reference Messina, Kucuk and Lampe13), only 4 reported a significant effect on PSA levels. However, reduction in prostate cancer risk may occur without any reduction in PSA levels. No beneficial effects were observed on levels of steroids, or on IGF-1/IGFBP-3 ratio. One study which compared the incidence of apoptosis in prostate tumours of patients supplemented or not with red clover isoflavones (160 mg/d for 7–54 days), reported significantly higher apoptosis in supplemented patients than in control subjects (1·48 % v. 0·25 %, P = 0·0007), specifically in regions of low to moderate-grade cancer (Gleason grade 1–3)(Reference Jarred, Keikha, Dowling, McPherson, Clare, Husband, Pedersen, Frydenberg and Risbridger14). It is worth noting that no adverse effects were observed in any trial. The whole available data have been considered sufficiently encouraging to justify the funding of additional Phase II trials by the NIH(Reference Messina, Kucuk and Lampe13).
Messina et al. (Reference Messina, McCaskill-Stevens and Lampe15) recently discussed the published and ongoing clinical breast cancer studies. Three double-blind randomized controlled trials reported no effect of a 1 to 2-year isoflavone supplementation on mammographic density used as a marker of breast cancer risk(Reference Maskarinec, Williams and Carlin16–Reference Atkinson, Warren, Sala, Dowsett, Dunning, Healey, Runswick, Day and Bingham18). A 2-week administration of a soy supplement (45 mg/d isoflavones) increased epithelial cell proliferation and progesterone receptor (PR) expression in normal breast tissue, suggesting an estrogen agonist effect(Reference McMichael-Phillips, Harding, Morton, Roberts, Howell, Potten and Bundred19). However, the potential link with proliferation of breast cancer cells is difficult to assess. Conflicting results have been obtained regarding the impact of soy isoflavones on hormone-related breast cancer risk factors such as plasma steroid hormone levels, Sex Hormone Binding Globulin (SHBG) plasma levels, urinary 2:16α-hydroxyestrone ratio, and menstrual cycle length(Reference Rosenberg Zand, Jenkins and Diamandis20).
The inconsistencies in the results and the failure to observe clear clinical effects may be explained, at least in part, by disparities in experimental designs, in the form of isoflavone administration, the dose, or the duration of the study. Furthermore, diverse subpopulations may respond differently to isoflavone consumption due to age, sex, ethnic background, gene polymorphisms, history of cancer, known risk factors, nutritional status, hormonal status or colonic microbiota composition. In this regard, the most critical parameters affecting the biological responses to isoflavone intake remain to be identified. The populations or subpopulations which may benefit, or possibly may experience some adverse effects while consuming isoflavones, also need to be identified.
The factors likely to affect isoflavone bioavailability may first modulate physiological responses to isoflavone intake. A compilation of 15 bioavailability studies in humans showed that plasma metabolite concentrations usually reach about 2 μmol/l after consumption of 50 mg isoflavones (aglycone equivalent)(Reference Manach, Williamson, Morand, Scalbert and Remesy21). Inter-individual variability in isoflavone absorption and metabolism has never been assessed in large and ethnically non-homogeneous populations, however some bioavailability studies suggest that it may be high. Maximum plasma concentrations ranged between 4 and 27 μmol/l genistein among 20 American men with prostate cancer challenged with a high pharmacological dose of genistein(Reference Miltyk, Craciunescu, Fischer, Jeffcoat, Koch, Lopaczynski, Mahoney, Crowell, Paglieri and Zeisel22). Furthermore, a recent clinical study showed that genistein and daidzein bioavailability, evaluated by plasma pharmacokinetics, was significantly lower in Caucasian than in Asian men after single or 10-day isoflavone supplementation(Reference Vergne, Lamothe, Chantre, Potier, Asselineau, Perez, Durand, Moore, Bennetau-Pelissero and Sauvant23). It would therefore be extremely useful to accurately measure the real individual exposure to isoflavones rather than only the isoflavone intake in clinical studies.
The intestinal microflora also plays a crucial role in the metabolism of isoflavones. Various bacterial metabolites are known to be produced, among which some may exert biological activities(Reference Heinonen, Hoikkala, Wahala and Adlercreutz24–Reference Rufer, Glatt and Kulling26). Equol is the most studied metabolite since it has been shown to be more estrogenic than its precursor daidzein in many in vitro studies and in animal models(Reference Setchell, Brown and Lydeking-Olsen27). There is great inter-individual variability in the capacity to produce equol, and only 30–50 % of the Western population exhibits the ‘equol-producer’ phenotype(Reference Atkinson, Frankenfeld and Lampe28). Song et al. recently reported that the equol-producer phenotype was more frequent in Korean American than in Caucasian American women (51 % v. 36 %) living in the same area of the US(Reference Song, Atkinson, Frankenfeld, Jokela, Wahala, Thomas and Lampe29). Several studies suggested that equol-producers may gain more benefits from soy consumption than non-equol-producers. In a case–control study in Japanese and Korean men, the proportion of equol-producers was 1·5- to 2-fold higher in the control group than in the group of men with prostate cancer(Reference Akaza, Miyanaga, Takashima, Naito, Hirao, Tsukamoto, Fujioka, Mori, Kim, Song and Pantuck30). More favourable hormonal profiles (lower plasma concentrations of estrone, estrone-sulfate, testosterone, androstenedione, dehydroepiandrosterone (DHEA), DHEA-sulfate, and midluteal progesterone) and higher concentrations of SHBG were also found in equol-producing premenopausal women compared to non-equol-producers, which was consistent with a lower risk for breast cancer(Reference Duncan, MerzDemlow, Xu, Phipps and Kurzer31). Frankenfeld et al. did not find such hormonal differences in postmenopausal women, but equol-producing women had higher 2:16α-hydroxyestrone ratio, which has also been related to a lower breast cancer risk(Reference Frankenfeld, McTiernan, Tworoger, Atkinson, Thomas, Stanczyk, Marcovina, Weigle, Weiss, Holt, Schwartz and Lampe32). Similar results were obtained in breast cancer survivors(Reference Nettleton, Greany, Thomas, Wangen, Adlercreutz and Kurzer33). Mammographic density was 39 % lower in equol-producing postmenopausal women compared with non-producers, which is again consistent with a lower breast cancer risk in equol-producers(Reference Frankenfeld, McTiernan, Aiello, Thomas, LaCroix, Schramm, Schwartz, Holt and Lampe34). Niculescu et al. studied the effects of a high-dose isoflavone supplementation (900 mg/d for 84 days) on gene expression changes in lymphocytes of 30 postmenopausal women. Using microarray analysis they showed that isoflavone treatment in equol-producer subjects differentially affects gene expression as compared with non-producers, with a stronger effect on some estrogen-responsive genes in equol-producers(Reference Niculescu, Pop, Fischer and Zeisel35). It is not yet clear whether equol itself is the active metabolite responsible for these beneficial effects, or whether it is only a biomarker for a particular microbial community composition able to elicit favourable metabolisms in addition to equol production. Some fingerprinting methods, such as terminal restriction fragment length polymorphism (TRFLP) analysis, are now available to compare the overall patterns of gut microbial composition between individuals(Reference Li, Hullar and Lampe36). These high-throughput methods should enlighten the role of specific bacteria and help to adequately stratify individuals in future human intervention and observational population-based studies.
Metabolomics is another high-throughput method that may be useful to deal with inter-individual variability. Nuclear magnetic resonance (NMR) and mass spectrometry (MS) techniques allow the simultaneous detection of hundreds or thousands of compounds present in human plasma, urine or tissues(Reference Dettmer, Aronov and Hammock37). Multivariate statistical analyses of the datasets obtained for groups of individuals that differ for one controlled criterion, such as isoflavone intake, microflora composition or type of cancer, provide a list of variables discriminating the groups. The identification of these discriminating variables may lead to the discovery of novel biomarkers for the particular criterion studied. Metabolic profiling using these biomarkers could then be used in clinical or cohort studies to better assess individual exposure to isoflavones, as well as to stratify individuals on the basis of their metabolic capacities or other phenotypes.
Another new possibility, offered by nutrigenetics, is the access to valuable information on individual genotypes through sequencing of targeted single nucleotide polymorphisms (SNPs)(Reference Kaput and Rodriguez38). As some polymorphisms in genes coding for membrane transporters, protein carriers or metabolic enzymes such as glucuronosyl-transferases could affect the nature and concentrations of isoflavone metabolites present in target tissues, it may be useful to search for correlations between individual genotypes, isoflavone bioavailability and isoflavone effects. However, the relevant SNPs that would be related to the responsiveness to isoflavone consumption are not identified to date. Beyond the gene polymorphisms that may affect bioavailability, two recent studies demonstrated the major interest of considering the inter-individual variability in the genetic background of a population to evaluate the isoflavone biological effects. Hedelin et al. studied the interactions between dietary phytoestrogen intake, ERβ polymorphisms, and the risk of prostate cancer in a case–control study performed in 2096 Swedish men(Reference Hedelin, Balter, Chang, Bellocco, Klint, Johansson, Wiklund, Thellenberg-Karlsson, Adami and Gronberg39). For one particular SNP of the ERβ gene, a higher phytoestrogen and isoflavone consumption was associated to a significantly reduced prostate cancer risk (respectively 57 % and 27 %) in subjects who were homozygous or heterozygous for the variant allele, whereas no association could be found in subjects who were homozygous for the wild-type allele (58 % of the population). In another cohort of 1988 menopausal women from UK, isoflavone exposure was positively associated with SHBG levels only in the fraction of the population that carried the Asn variant of the SHBG D356N polymorphism, which is associated with a reduced SHBG clearance(Reference Low, Taylor, Grace, Dowsett, Folkerd, Doody, Dunning, Scollen, Mulligan, Welch, Luben, Khaw, Day, Wareham and Bingham40). As higher SHBG levels have been associated with a lower breast cancer risk, isoflavone consumption may be particularly beneficial to these women. It is worth noticing that the authors would have concluded that isoflavone exposure was not associated to SHBG levels if they had considered the population as a whole, without stratification according to the genotype. Gene-isoflavone interactions may partly explain some of the discrepancies observed in the first clinical studies conducted so far, especially if the allele frequencies differed between the populations considered. Nevertheless, high statistical powered studies will be required in order to take the relevant gene polymorphisms into account in the investigation of isoflavone effects.
Another element that may explain the inconsistencies observed in clinical studies is the relevance of the endpoint biomarkers used. Biomarkers for staging and monitoring the evolution of prostate and breast cancer development are critically needed to evaluate the impact of any therapeutic intervention. Considering that cancers are multi-factorial and variable diseases, it appears today unrealistic to rely on a single biomarker (such as PSA for prostate cancer) to assess diagnosis, prognosis, and prediction of response to therapy. Complex combinations of markers coming from transcriptomic, proteomic as well as metabolomic approaches are certainly more appropriate and promising perspectives can already be seen. Rhodes et al. have analysed gene expression profiles in 40 published cancer microarray data sets, comprising 38 million gene expression measurements from >3700 cancer samples(Reference Rhodes, Yu, Shanker, Deshpande, Varambally, Ghosh, Barrette, Pandey and Chinnaiyan41). This study generated a set of 69 genes that were commonly dysregulated in 12 types of cancers including prostate and breast cancers. Proteomic data have also been used on a blinded cohort to identify patients with prostate cancer with a high specificity(Reference Rhodes, Yu, Shanker, Deshpande, Varambally, Ghosh, Barrette, Pandey and Chinnaiyan41–Reference Adam, Qu, Davis, Ward, Clements, Cazares, Semmes, Schellhammer, Yasui, Feng and Wright43). There is now a great hope that these high-throughput methods will lead to the discovery of early biomarkers for preclinical metabolic dysregulations associated with the first stages of carcinogenesis. Many novel important genes involved in prostate carcinogenesis, such as Hepsin or Alpha-methylacyl-CoA racemase (AMACR), have been identified by using microarray analysis(Reference Calvo, Gonzalez-Moreno, Yoon, Huh, Desai, Nguyen and Green44). NMR-based metabolomic analyses to target biomarkers for prostate cancer are under development(Reference Jordan and Cheng45). Novel early biomarkers will improve our understanding of the initial stages of the diseases and will be essential to study the preventive effects of dietary intervention such as isoflavone supplementation.
Therefore, nutrigenomic approaches should allow to better assess individual metabolism and to better evaluate the effects of isoflavones at the individual level and at early stages of the diseases.
Animal studies
As demonstrated above, human studies are still hampered with major weaknesses, and more controllable animal and cellular models are very useful to investigate isoflavone effects. Different animal models of prostate cancer such as Lobund-Wistar rats (spontaneous tumours), rats treated with various type of carcinogens [N-methylnitrosourea (NMU), 7,12-dimethylbenz(a)anthracene (DMBA), 2-amino-1-methyl-6-phenylimidazopyridine (PhIP)] or implants of testosterone propionate, transgenic mice (TRAMP), xenograft models (e.g. immunodeficient mice implanted with human tumour cell lines) have been used to explore the effects of isoflavones on prostate cancers. In more than 40 such studies, isoflavones were shown to reduce both the incidence and the size of prostate tumours(Reference Messina2, Reference Magee and Rowland3, Reference Pollard and Suckow46). Isoflavones may act at different stages of carcinogenesis, by inhibiting the appearance of prostatic intra-epithelial neoplasia (PIN) or their progression into carcinomas, but the determination of their timing of action requires further experiments.
Much more conflicting results have been obtained in animal models of breast cancer, as isoflavones have been shown to inhibit as well as to promote the growth of mammary tumours, depending on the studies. Many parameters may explain such discrepancies including the various natures of the animal models used, the test compounds, the dose and the mode of administration, or the timing of isoflavone exposure. Overall, isoflavones were mainly shown to prevent or to delay chemically-induced mammary carcinogenesis in prepubertal or premenopausal adult female rats. The protective effects of isoflavones at the prepubertal period have been linked to a promotion of early mammary gland differentiation, leading to a decreased number of immature terminal end buds (TEBs), sites for malignant transformation, and an increased number of more differentiated lobules(Reference Murrill, Brown, Zhang, Manzolillo, Barnes and Lamartiniere47–Reference Cabanes, Wang, Olivo, DeAssis, Gustafsson, Khan and Hilakivi-Clarke51). Complex cross-talks between steroids, growth factors and other pathways are involved in the differentiation process. Hilakivi-Clarke et al. reported that the pro-differentiating effect of isoflavones was associated with a repression of ERα and PR expression but an increase of ERβ expression in mammary glands(Reference Hilakivi-Clarke, Cho, deAssis, Olivo, Ealley, Bouker, Welch, Khan, Clarke and Cabanes52). Lamartiniere et al. suggested that isoflavone exposure in the prepubertal period mainly promotes mammary epithelium differentiation through the transient activation of epidermal growth factor (EGF) signalling pathway(Reference Lamartiniere50).
The most frequent types of breast cancer are the estrogen-dependent breast tumours in postmenopausal women. Widely used animal models for these types of tumours are immunodeficient mice implanted with estrogen-dependent human breast cancer MCF-7 cells, combined with ovariectomy and in some cases with estradiol implants releasing high doses of estradiol that stimulate the growth of MCF-7 implanted cells. The group of Helferich published several consistent studies showing that genistein at nutritionally relevant doses (0·025–0·1 % in the diet) stimulates tumour growth or negates the inhibitory effect of tamoxifen in such xenograft models(Reference Hsieh, Santell, Haslam and Helferich53–Reference Ju, Doerge, Allred, Allred and Helferich56). In such models the circulating levels of endogenous estrogens are either much lower (ovariectomy) or much higher (estradiol implants) than the physiological levels observed in postmenopausal women. Hence the relevance of the xenograft models to evaluate the effects of weak estrogenic compounds, the activity of which may depend on the levels of endogenous estrogens, has been criticised. Recently, Ju et al. developed a mouse model with slow-growing estrogen-dependent tumours in ovariectomised athymic mice, using silastic implants to deliver estradiol at levels similar to those observed in postmenopausal women(Reference Ju, Allred, Allred and Helferich57). Again, genistein administered in the diet at 500 ppm was shown to act in an additive manner with the low levels of circulating estradiol and stimulated the growth of the tumorigenic MCF-7 cells in this model.
Several authors reported that in utero exposure to high-dose isoflavones (s.c. injections) increased the susceptibility of rats to chemically-induced mammary cancers later in life, in the same way as in utero exposure to high levels of endogenous estrogens(Reference Hilakivi-Clarke, Onojafe, Raygada, Cho, Skaar, Russo and Clarke49, Reference Yang, Nakagawa, Tsuta and Tsubura58). This effect has been related to an increased number of TEBs, and a reduction in epithelial differentiation in the mammary gland. However, other authors reported that a more nutritional exposure to soy protein isolate (but not to genistein alone) in utero through maternal diet protected young adult rats from NMU-induced mammary tumourigenesis(Reference Su, Eason, Geng, Till, Badger and Simmen59). Early exposure to isoflavones or at least to genistein before puberty may cause transient and persistent effects on mammary gland development, as well as on hormone receptor levels(Reference Padilla-Banks, Jefferson and Newbold60). These imprinting-like effects may thus differ depending on dose and timing of exposure and lead to adverse or beneficial effects in later life.
Dolinoy et al. provided the first evidence that early in utero exposure to genistein can produce permanent epigenetic changes(Reference Dolinoy, Weidman, Waterland and Jirtle61). They showed that maternal dietary genistein supplementation (250 ppm) of mice during gestation shifted the coat colour of heterozygous viable yellow agouti (A vy/a) offspring towards pseudoagouti and decreased the incidence of obesity during adulthood. These marked phenotypic changes were mediated by an increased methylation of six CpG sites located in a retrotransposon upstream of the Agouti gene which determines coat colour. An exciting hypothesis of research has been put forward with this study. DNA methylation is known to control cellular differentiation in the mammary epithelium. Thus, epigenetic events associated with in utero or prepubertal exposure to genistein may also contribute to the effects observed on animal mammary gland differentiation(Reference De Assis and Hilakivi-Clarke62). Such imprinting phenomena are extremely difficult to study in humans. Extrapolation of data from animal models must take into account some differences in isoflavone bioavailability in rodents and in humans. For example, all laboratory animals have been shown to be constitutive equol-producers, and equol is the major circulating metabolite in animals while it is only present in a low proportion in humans(Reference Setchell, Brown and Lydeking-Olsen27).
Animal studies also showed that isoflavones exert various effects depending on their structures and application forms. The large majority of studies have been conducted with genistein. When used alone, daidzein was shown to be equivalent to genistein at the same dose (250 ppm in the diet) in delaying mammary tumour development in MMTV-neu mice(Reference Jin and MacDonald63). In contrast, prepubertal exposure of Sprague–Dawley female rats to 250 ppm daidzein in the diet neither enhanced mammary gland differentiation nor suppressed DMBA-induced mammary tumour development as previously demonstrated for genistein(Reference Lamartiniere, Wang, Smith-Johnson and Eltoum64). Furthermore, dietary daidzein only had a slight stimulatory effect on MCF-7 tumour growth in athymic mice, whereas ( ± )-equol did not stimulate tumour growth(Reference Ju, Fultz, Allred, Doerge and Helferich65). Thus, the effects observed with genistein should not be extrapolated to other isoflavones, and the various isoflavones may affect different metabolic or signalling pathways. Moreover, consumption of genistein in pure or highly enriched forms (as in soy protein isolates) has a greater stimulatory effect on MCF-7 cells implanted in immunodeficient mice than the same content of dietary genistein in less purified soy flour(Reference Allred, Allred, Ju, Goeppinger, Doerge and Helferich66). Other authors reported a higher beneficial effect of soymilk or soy protein isolate toward chemically-induced cancer as compared to pure genistein(Reference Su, Eason, Geng, Till, Badger and Simmen59, Reference Ohta, Nakatsugi, Watanabe, Kawamori, Ishikawa, Morotomi, Sugie, Toda, Sugimura and Wakabayashi67). Soybeans contain a wide variety of other phytochemicals such as saponins, protease inhibitors like the Bowman-Birk inhibitor, phenolic acids, phytic acid, and lignans that may exert additional or synergistic effects with isoflavones. Taking supplements may thus have different effects than increasing soy food consumption.
Cell models
Isoflavones have been shown to inhibit proliferation and/or induce apoptosis in various androgen-dependent or androgen-independent prostate cancer cell lines. The concentrations used were generally high, allowing cytotoxic effects, but anti-proliferative effects have also been observed at lower concentrations (5 μm) of genistein(Reference Messina2, Reference Magee and Rowland3). Again, the impact of genistein is more complex in breast cancer cell lines than in prostate cell lines, since genistein stimulates cell growth of estrogen-dependent cells when used at concentrations lower than 10 μm but inhibits their growth at higher concentrations(Reference Magee and Rowland3, Reference Hsieh, Santell, Haslam and Helferich53, Reference Wang and Kurzer68, Reference Le Bail, Champavier, Chulia and Habrioux69). High concentrations of genistein also inhibit the growth of ER-negative cell lines such as MDA-MB-231, indicating that this isoflavone can exert cellular effects independently from ER.
One major limit of such in vitro studies is that they use isoflavone concentrations far exceeding the concentrations commonly achieved in human plasma or tissues after soy consumption. The available data indicate that concentrations as high as 1–2·5 nmol/g of each isoflavone may be achieved in human prostate or prostatic fluid after short-term supplementation with high nutritional doses of isoflavones and that in Asian men basal levels of total isoflavones are about 0·4 nmol/g in prostate(Reference Hedlund, Maroni, Ferucci, Dayton, Barnes, Jones, Moore, Ogden, Wahala, Sackett and Gray70–Reference Guy, Vedrine, Urpi-Sarda, Gil-Izquierdo, Al-Maharik, Boiteux, Scalbert, Rémésy, Botting and Manach74). Isoflavone concentrations in breast tissue are still unknown. Maubach et al. suggested in an isoflavone supplementation study in 9 women, that they may be markedly lower than serum concentrations(Reference Maubach, Depypere, Goeman, Van Der Eycken, Heyerick, Bracke, Blondeel and De Keukeleire75).
The question of the dose is critical, since increasing the dose of exposure does not necessarily produce more intense effects but may also lead to completely different effects. Dang et al. (Reference Dang, Audinot, Papapoulos, Boutin and Lowik76) clearly showed that genistein elicits different biological effects in osteoprogenitor KS483 cells depending on the concentration of exposure. At low concentrations ( < 1 μm), genistein acts as an estrogen, stimulating osteogenesis and inhibiting adipogenesis, whereas at high concentrations (>1 μm), it acts as a ligand for PPARγ, leading to the upregulation of adipogenesis and downregulation of osteogenesis.
Daidzein and genistein glycosides and aglycones, when ingested with soy foods, are extensively metabolised in the human intestine and liver and systematically recovered in plasma as 7-O- and 4′-O-glucuronides, along with low amounts of aglycones and sulfate esters(Reference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi77–Reference Zhang, Hendrich and Murphy79). Mono-glucuronides of genistein and daidzein were recently shown to also be the main metabolites present in the human prostate(Reference Guy, Vedrine, Urpi-Sarda, Gil-Izquierdo, Al-Maharik, Boiteux, Scalbert, Rémésy, Botting and Manach74). However, the effects of these conjugated forms of isoflavones on breast or prostate cell proliferation have not been investigated so far. The nature of the physiological metabolites should yet be taken into consideration, because the biological properties of conjugated metabolites have already been shown to differ from those of the corresponding aglycones. The affinity of isoflavone glucuronides for ERs has been reported to be 10–40 times lower than that of the aglycones(Reference Zhang, Song, Cunnick, Murphy and Hendrich80). Furthermore, sulfation of isoflavones was shown to decrease their antioxidant activity and their effect on platelet aggregation, inflammation, cell adhesion and chemotaxis(Reference Turner, Baron, Wolffram, Minihane, Cassidy, Rimbach and Weinberg81, Reference Rimbach, Weinberg, de Pascual-Teresa, Alonso, Ewins, Turner, Minihane, Botting, Fairley, Matsugo, Uchida and Cassidy82). Tumour cell lines may have lost some membrane carriers, which would limit the uptake of anionic metabolites such as glucuronides or sulfates by the cells. For example, MCF-7 and T47D cells do not express the OATP-B carrier which is normally expressed in the mammary gland and transports a wide range of sulfated and glucuronidated conjugates of endogenous and exogenous compounds(Reference Pizzagalli, Varga, Huber, Folkers, Meier and St-Pierre83). Thus, exposure to the same isoflavone concentration could result in quite different intracellular concentrations of isoflavone metabolites depending on the cell type used. To facilitate comparisons between in vitro studies, as well as extrapolation to the in vivo setting, information on intracellular concentrations of isoflavone metabolites should be provided in future studies on cultured cells. Another limit of in vitro studies is that they rarely used non-tumourigenic cells. Even if tumourigenic cell lines are of great interest to study specific signalling or metabolic pathways or to assess the potential therapeutic effects of isoflavones, these highly modified cells are not the best models to investigate the preventive effects of isoflavones.
Mechanisms of action
Although many studies have been conducted to understand the effects of soy isoflavones on breast and prostate cancer, available data are inconsistent and the mechanisms of action are still not entirely elucidated. Studies on animal and cellular models have firmly established that isoflavones are active compounds, showing quite variable effects depending on the dose, the form of administration, or the timing and duration of exposure. Serious concerns were raised about a possible estrogen-like detrimental effect of genistein through growth stimulation of pre-existing estrogen-sensitive mammary tumours. In the present state of our knowledge, the increasing isoflavone self-supplementation should be considered with caution until the mechanisms of action of isoflavones are better understood.
As questioned in the titles of S. Barnes, “Isoflavones = phytoestrogens and what else?”(Reference Barnes84), or M. McCarty “Isoflavones made simple – Genistein's agonist activity for the beta-type estrogen receptor mediates their health benefits”(Reference McCarty85), a largely debated issue is to establish whether isoflavones only act through their phytoestrogenic properties or whether ER-independent mechanisms of action may also play a role. Isoflavones have been reported to modulate steroid biosynthesis, transport and metabolism, as well as carcinogen activation and detoxification, to inhibit cell proliferation induced by growth factors, to induce cell cycle arrest or apoptosis, to favour cell differentiation, to reduce oxidative stress or to inhibit angiogenesis, cell invasiveness and metastasis(Reference Magee and Rowland3, Reference Sarkar and Li86). They may act through modulation of cell signalling (direct binding to nuclear receptors, modification of the phosphorylation state of some signal transduction proteins), regulation of gene expression and/or specific inhibition of some key enzyme activities.
The mechanisms of action of isoflavones are reviewed here in the context of breast and prostate cancer. Table 1 summarizes the most relevant studies that have been published for each putative mechanism of action and notifies whether in vivo data exist or whether the mechanism has only been observed in vitro. In vitro studies using isoflavone concentrations up to 10 μm have been distinguished from studies using higher pharmacological concentrations.
Table 1 Mechanisms of action of isoflavones in prostate and breast tissues from cell, animal and human studies

P, prostate; B, breast; X, other cells/tissues.
The major focus in isoflavone research so far lies on the modulation of steroid hormone receptor signalling. Estrogens regulate many physiological processes in hormone-dependent tissues, including cell growth and differentiation, apoptosis and tissue-specific gene regulation, but also influence the pathological processes of hormone-dependent diseases, such as breast and prostate cancers. The biological actions of estrogens are mediated by the binding to one of the two specific nuclear receptors, ERα and ERβ(Reference Pettersson and Gustafsson87), which can induce gene transcription of estrogen-responsive target genes. A general hypothesis is that estrogens acting via ERα exert strong proliferation stimulatory effects while those interacting with ERβ tend to reduce this stimulation. Furthermore, ERβ has been shown to repress ERα-controlled transcription(Reference Lindberg, Moverare, Skrtic, Gao, Dahlman-Wright, Gustafsson and Ohlsson88, Reference Liu, Albanese, Anderson, Hilty, Webb, Uht, Price, Pestell and Kushner89). Cell response would therefore depend on the balance between ERα and ERβ expression levels within a given cell type. This balance is altered in favour of ERα during tumour progression(Reference Leygue, Dotzlaw, Watson and Murphy90), emphasizing the protective role of ERβ signalling and the usefulness of ERβ-selective agonists in cancer prevention.
Eight in vitro and in vivo studies consistently reported a downregulation of ERα and an upregulation of ERβ mRNA and protein levels by genistein at 1–10 μm in breast cancer cells(Reference Maggiolini, Bonofiglio, Marsico, Panno, Cenni, Picard and Ando91–Reference Cappelletti, Miodini, Di Fronzo and Daidone93). A decrease in ERα protein expression was also observed in mammary tumours of Sprague–Dawley rats after consumption of a soy extract(Reference Gallo, Giacomelli, Cantelmo, Zannoni, Ferrandina, Fruscella, Riva, Morazzoni, Bombardelli, Mancuso and Scambia94). In the dorsolateral prostate of rats and mice, exposure to dietary genistein ( ≥ 25 ppm for rats; 500 μg/g bw for mice) resulted in a downregulation of ERα and ERβ mRNA and protein expression(Reference Dalu, Blaydes, Bryant, Latendresse, Weis and Barry Delclos95–Reference Wang, Eltoum and Lamartiniere97).
Activation of ERs is an established effect of isoflavones that has been documented in the low nanomolar range, concentrations commonly achieved at nutritional levels of soy isoflavone intake. It has been shown in vitro that genistein has an agonist activity for both ER subtypes, but its affinity for ERβ is considerably greater than for ERα, with binding affinities of 8·4 nm and 145 nm, respectively(Reference Kuiper, Carlsson, Grandien, Enmark, Haggblad, Nilsson and Gustafsson98, Reference Kuiper, Lemmen, Carlsson, Corton, Safe, van der Saag, van der Burg and Gustafsson99). For daidzein, the corresponding values are 100 nm and 420 nm, indicating a much lower affinity for these receptors(Reference Kuiper, Carlsson, Grandien, Enmark, Haggblad, Nilsson and Gustafsson98, Reference Kuiper, Lemmen, Carlsson, Corton, Safe, van der Saag, van der Burg and Gustafsson99). The transactivating functions of ERα and ERβ are mediated by two transcription activation functions (AF) located in the ligand-binding domain, AF-1 (N-terminal) and AF-2 (C-terminal). AF-1 is very active in ERα on a variety of estrogen-responsive promoters, but minimal in ERβ, whereas AF-2-mediated transcriptional activities of ERs are dependent on recruitment and interactions with cofactor proteins (coactivators and corepressors) to estrogen-responsive promoters(Reference Matthews and Gustafsson100). The conformational change of the AF-2 transactivation helix induced by the formation of the ERβ-genistein complex is closer to that induced by ER antagonists than by ER agonists and could therefore account for the partial agonistic behaviour of genistein(Reference Pike, Brzozowski and Hubbard101). The higher affinity of isoflavones for ERβ is paralleled by their ability to activate transcription with ERβ at lower concentrations than with ERα(Reference Morito, Hirose, Kinjo, Hirakawa, Okawa, Nohara, Ogawa, Inoue, Muramatsu and Masamune102). Genistein, daidzein and equol were reported to increase the binding rate of ERs to estrogen responsive elements (ERE), with a more prominent effect on ERβ than ERα. The concentrations of genistein, daidzein and equol which would increase this binding rate by 50 % were determined to be 30 nm, 350 nm and 400 nm for ERβ and 15 μm, >300 μm and 3·5 μm for ERα, respectively(Reference Kostelac, Rechkemmer and Briviba103).
The activation of ERs by isoflavones subsequently lead to a modulation of the expression of their target genes, and thus, to a modulation of cellular processes such as proliferation and apoptosis. Well-known target genes for the ERs are PR, pS2, bcl-2 and cyclin D1(Reference Limer and Speirs104, Reference Maggiolini, Vivacqua, Carpino, Bonofiglio, Fasanella, Salerno, Picard and Ando105). PR mRNA and protein levels were shown to be upregulated by isoflavones in the majority of the in vitro and in vivo studies. In MCF-7 cells, an upregulation was observed with 1–10 μm genistein(Reference Fioravanti, Cappelletti, Miodini, Ronchi, Brivio and Di Fronzo106), and in premenopausal cynomolgus macaques and ovariectomised athymic nude mice implanted with MCF-7 cells, the doses ranged between 240 and 750 ppm in the diet(Reference Allred, Allred, Ju, Goeppinger, Doerge and Helferich66, Reference Wood, Register, Franke, Anthony and Cline107). In contrast, a natural soy extract (0·7 % in the diet), containing 12 % isoflavones and 35 % saponins, decreased PR protein expression in mammary tumours of female Sprague–Dawley rats(Reference Gallo, Giacomelli, Cantelmo, Zannoni, Ferrandina, Fruscella, Riva, Morazzoni, Bombardelli, Mancuso and Scambia94). Furthermore, soy isoflavones stimulated the expression of the ER target gene pS2 in breast cells, as shown in twelve in vivo and in vitro studies. As for the modulation of PR, low isoflavone concentrations (between 0·001 and 10 μm) were effective in vitro in MCF-7 cancer cells(Reference Fioravanti, Cappelletti, Miodini, Ronchi, Brivio and Di Fronzo106, Reference Wang, Sathyamoorthy and Phang108, Reference Sathyamoorthy and Wang109), whereas in vivo doses above 500 ppm in the diet in mouse xenograft models or 45 mg daily in premenopausal women were used(Reference Ju, Allred, Allred, Karko, Doerge and Helferich55, Reference Allred, Allred, Ju, Goeppinger, Doerge and Helferich66, Reference Hargreaves, Potten, Harding, Shaw, Morton, Roberts, Howell and Bundred110). Only one in vivo study reported a converse decrease in mRNA expression of pS2 in premenopausal cynomolgus macaques after administration of 240 ppm isoflavones in combination with 0·09 mg/g estradiol(Reference Wood, Register, Franke, Anthony and Cline107). Other ER target genes such as bcl-2 and cyclin D1 were also significantly increased in breast cells after genistein treatment. At 1 μm, it increased bcl-2 mRNA expression in MCF-7 cells(Reference Leung and Wang111, Reference Po, Wang, Chen and Leung112). Moreover, 750 ppm genistein in the diet increased cyclin D1 mRNA expression in a mouse xenograft model(Reference Allred, Allred, Ju, Goeppinger, Doerge and Helferich66). Increased transcriptional and translational levels of the aforementioned estrogen-responsive target genes rather suggest estrogen-agonistic activities of isoflavones in breast cancer cells.
Since the androgen receptor (AR) pathway plays a pivotal role in prostate cell growth, differentiation and function, agents that minimise or eliminate AR transactivation are considered useful to prevent and treat prostate cancer. Although genistein does not seem to act as a ligand for AR(Reference Bektic, Berger, Pfeil, Dobler, Bartsch and Klocker113, Reference Gao, Liu and Wang114), it has been shown to exert anti-androgenic effects in prostate cells, and to downregulate the expression and secretion of the typical androgen-responsive gene PSA(Reference Bektic, Berger, Pfeil, Dobler, Bartsch and Klocker113, Reference Davis, Muqim, Bhuiyan, Kucuk, Pienta and Sarkar115). The mechanisms underlying isoflavone anti-androgenic effects are still not entirely elucidated. In more than seven in vitro and in vivo studies, a decrease in AR expression at mRNA and protein levels was detected in prostate cells exposed to low isoflavone concentrations (0·1–1 μmin vitro; 250 ppm in diet in vivo)(Reference Fritz, Wang, Eltoum and Lamartiniere96, Reference Bektic, Berger, Pfeil, Dobler, Bartsch and Klocker113, Reference Davis, Kucuk and Sarkar116). Long-term soy protein isolate consumption also lowered AR expression in prostate of men with high prostate cancer risk(Reference Hamilton-Reeves, Rebello, Thomas, Slaton and Kurzer117). Isoflavones may also decrease androgen levels(Reference Weber, Setchell, Stocco and Lephart118, Reference Zhou, Yu, Zhong, Nassr, Franke, Gaston and Blackburn119). Moreover, a direct binding of DHT by equol has been reported in vitro, as well as a stimulation of testosterone inactivation through intracellular glucuronidation by biochanin A(Reference Lund, Munson, Haldy, Setchell, Lephart and Handa120, Reference Sun, Plouzek, Henry, Wang and Phang121). Interestingly, Bektic et al. demonstrated with the use of a pure anti-estrogen that the downregulation of the AR was mediated by ERβ in LNCaP cells. A cross-talk between ER and AR was also described by Davis et al. (Reference Davis, Kucuk and Sarkar116), who reported that genistein (0·5–5 μM) enhanced 17β-estradiol-induced PSA expression in LNCaP cells. At a slightly higher concentration (10 μM), genistein stimulated AR-driven gene expression through activation of the Raf-MEK-ERK signalling pathway in PC3 cells, again demonstrating the critical importance of the dose. It is noteworthy that daidzein, at concentrations up to 50 μM, did not modulate PSA or AR protein levels in LNCaP cells(Reference Davis, Kucuk and Sarkar116).
Because steroid hormone levels influence the development of hormone-dependent cancers, the research also focused on the impact of isoflavones in the synthesis and metabolism of steroids. However, the potency of isoflavones to inhibit some key enzymes involved, such as 17β-hydroxysteroid dehydrogenase, 3β-hydroxysteroid dehydrogenase, aromatase and 5α-reductase, in humans is questionable. Although the inhibition of 17β-hydroxysteroid dehydrogenase, 3β-hydroxysteroid dehydrogenase and 5α reductase was observed at low doses of 1–10 μM, this was mostly shown in platelet microsomes or using isolated human enzymes(Reference Le Bail, Champavier, Chulia and Habrioux69, Reference Makela, Poutanen, Lehtimaki, Kostian, Santti and Vihko122–Reference Kao, Zhou, Sherman, Laughton and Chen124). Data for enzyme inhibition in breast and prostate cells are still limited. Makela et al. reported an inhibition of 17β-hydroxysteroid dehydrogenase with 1 μM genistein in breast cancer cells(Reference Makela, Poutanen, Kostian, Lehtimaki, Strauss, Santti and Vihko125). In rat prostate homogenates an inhibition of 5α-reductase activity was observed after treatment with 20 μM genistein(Reference Evans, Griffiths and Morton126). Genistein and equol at nanomolar concentrations also showed inhibition of steroid sulfotransferases in vitro, whereas daidzein sulfates at 1·5–6 μM inhibited steroid sulfatase significantly(Reference Kirk, Harris, Wood, Waring and Hughes127–Reference Harris, Wood, Bottomley, Blagg, Owen, Hughes, Waring and Kirk129). Finally, a favourable reduction in serum hormone levels in women and men as well as the stimulation of SHBG and the prolongation of the menstrual cycle in women supplemented with soy isoflavones have been observed in some studies, even if results are still quite inconsistent(Reference Duncan, MerzDemlow, Xu, Phipps and Kurzer31, Reference Hamilton-Reeves, Rebello, Thomas, Slaton and Kurzer117–Reference Lund, Munson, Haldy, Setchell, Lephart and Handa120, Reference Kumar, Cantor, Allen, Riccardi and Cox130–Reference Mousavi and Adlercreutz133). It should be noticed that these mechanisms in women were highly dependent on factors such as the menopausal status, the level of endogenous estrogens, the intake of oral contraceptives and the gut microbial composition. In a postmenopausal primate model as well as in postmenopausal women isoflavone supplementation (129 mg/1800 kcal and 2 mg/kg bw/d, respectively) decreased serum hormones such as estrone-sulfate, estrone, estradiol and increased urinary 2-hydroxyestrone levels as well as the urinary 2:16α-hydroxyestrone ratio(Reference Wood, Register and Cline134, Reference Duncan, Underhill, Xu, Lavalleur, Phipps and Kurzer135). Furthermore, it was shown that isoflavones (60, 120 and 240 mg/d) lowered serum estrone and estradiol concentrations significantly in a high estradiol environment but not in a low estradiol environment in postmenopausal primates(Reference Wood, Register, Franke, Anthony and Cline107). Studies in premenopausal women showed inconsistent results. While no changes in menstrual cycle length and serum hormones were observed after long-term intervention with high isoflavone doses (50 mg/d for 2 years or 100 mg/d for 1 year)(Reference Maskarinec, Takata, Franke, Williams and Murphy17, Reference Maskarinec, Williams, Inouye, Stanczyk and Franke136), a short-term intervention of 12 weeks with 40 mg genistein/d and of 93 days with 10, 64 and 128 mg isoflavones/kg bw/d increased menstrual cycle length and decreased serum free estradiol, estrone, DHEA-sulfate, follicle stimulating hormone (FSH) and luteinising hormone (LH) levels(Reference Kumar, Cantor, Allen, Riccardi and Cox130, Reference Duncan, Merz, Xu, Nagel, Phipps and Kurzer137). It was also demonstrated that gut microflora influenced serum hormone levels of premenopausal women, showing that equol-producers had lower serum hormone concentrations after isoflavone supplementation than non-producers(Reference Duncan, MerzDemlow, Xu, Phipps and Kurzer31).
Recent in vitro studies suggest that isoflavones may also act through activation of other nuclear receptors than ER and AR. Dang et al. showed for the first time in 2003 that genistein was able to bind a human full-length PPARγ receptor expressed in bacteria, with a Ki of 5·7 μM, comparable to that of some known PPARγ ligands(Reference Dang, Audinot, Papapoulos, Boutin and Lowik76). Furthermore, genistein dose-dependently (5–50 μM) stimulated PPARγ-directed gene expression in T47D and MDA-MD-231 breast cancer cells and other cell types transfected with PPRE-luciferase construct and expression plasmids encoding human PPARγ(Reference Dang, Audinot, Papapoulos, Boutin and Lowik76, Reference Mezei, Banz, Steger, Peluso, Winters and Shay138). A similar reporter assay and siRNA targeting approach confirmed the PPARγ agonist effect of genistein in HUVEC cells at a low physiological dose (1 μM)(Reference Chacko, Chandler, D'Alessandro, Mundhekar, Khoo, Botting, Barnes and Patel139). Genistein (2·5 μM) in combination with polyunsaturated fatty acids also induced PPARγ mRNA expression in MDA-MB-231 cells(Reference Horia and Watkins140). PPARγ agonists are known to act as modulators of cell cycle and estrogenic actions, to inhibit the proliferation of cultured human breast and prostate cancer cells, and to modify breast epithelial gene expression leading to a more differentiated and less-malignant state(Reference Elstner, Muller, Koshizuka, Williamson, Park, Asou, Shintaku, Said, Heber and Koeffler141–Reference Theocharis, Margeli, Vielh and Kouraklis144). Accumulating evidence also suggests that PPARγ may act as a tumour suppressor in prostate cancer. Phase II clinical trials using PPARγ ligands have been recently carried out as novel therapy for advanced breast and prostate cancer patients(Reference Mueller, Smith, Sarraf, Kroll, Aiyer, Kaufman, Oh, Demetri, Figg, Zhou, Eng, Spiegelman and Kantoff143, Reference Suzuki, Hayashi, Miki, Nakamura, Moriya, Sugawara, Ishida, Ohuchi and Sasano145, Reference Han and Roman146). A balance between ER and PPARγ activation, depending on the genistein concentration and the levels of ER and PPARγ in the tissue may thus explain some of the effects of isoflavones(Reference Suzuki, Hayashi, Miki, Nakamura, Moriya, Sugawara, Ishida, Ohuchi and Sasano145).
Isoflavones are capable of inhibiting proliferation by inducing G1 and/or G2/M cell cycle arrest in breast and prostate cancer cells(Reference Magee and Rowland3, Reference Zhou, Gugger, Tanaka, Guo, Blackburn and Clinton147, Reference Shen, Klein, Wei, Guan, Contois, Wang, Chang and Hursting148). While concentrations ranging between 5 and 10 μM genistein inhibited cell cycle in breast cancer cell lines, pharmacological doses of 20–60 μM were required to achieve the same effect in prostate cancer cells(Reference Zhou, Gugger, Tanaka, Guo, Blackburn and Clinton147, Reference Davis, Singh, Bhuiyan and Sarkar149–Reference Frey, Li and Singletary152). Progression in the cell cycle depends on the activity of a functional complex formed between cyclin B1 and the cyclin-dependent kinase (cdk) Cdc2(Reference Kuiper, Lemmen, Carlsson, Corton, Safe, van der Saag, van der Burg and Gustafsson99, Reference King, Jackson and Kirschner153). A significant downregulation of cyclin B1 and Cdc2 levels by genistein has been consistently observed in breast and prostate models in vitro, but only at high doses (20–100 μM)(Reference Shao, Wu, Shen and Barsky154–Reference Liao, Pan, Guh and Teng157). In order to achieve the inhibition of Cdc2 protein expression, supra-physiological doses of genistein ( ≥ 45 μM) were also required in non-tumour breast cells as well as in breast and prostate cancer cells(Reference Frey, Li and Singletary152, Reference Touny and Banerjee156). In addition, progression to mitosis can be affected by the phosphorylation status of Cdc2 mediated by Wee-1 protein kinases(Reference Touny and Banerjee156). Exposure of the non-tumorigenic breast cell line MCF-10F to 45 μM genistein resulted in an increase in phosphorylated Cdc2 protein levels(Reference Frey and Singletary158), and in TRAMP C2 prostate cells an increase in protein expression of protein kinase Myt-1 was observed upon exposure to 50 μM genistein(Reference Touny and Banerjee156). In contrast, the other protein kinase Wee-1 was suppressed(Reference Touny and Banerjee156). On the other hand, dephosphorylation of Cdc2 by the phosphatase Cdc25C increases the kinase activity of the cyclin B1–Cdc2 complex in the M phase(Reference Gautier, Solomon, Booher, Bazan and Kirschner159). The group of Frey et al. demonstrated a significant downregulation of Cdc25C protein expression using 50 μM genistein in the non-tumorigenic breast cell line MCF-10F(Reference Frey, Li and Singletary152). In summary, the effect on cdc2 phosphorylation was only observed at high genistein concentrations.
Cell cycle progression is also affected by the levels of cdk inhibitors p21WAF1 and p27KIP1, which were significantly upregulated upon genistein supplementation in breast and prostate cells. Eleven in vivo and in vitro studies on breast cancer showed a genistein-induced upregulation of p21WAF1 ( ≥ 0·5 mg/kg bw in mouse xenograft model, 250 ppm in the diet in Sprague–Dawley rats, ≥ 5 μM in breast cell lines MCF-7, MDA-MB-231) and p27KIP1 mRNA and protein levels ( ≥ 1 μM in breast cell lines MCF-7, MDA-MB-231)(Reference Shao, Wu, Shen and Barsky154, Reference Li, Upadhyay, Bhuiyan and Sarkar160–Reference Dave, Eason, Till, Geng, Velarde, Badger and Simmen163). In prostate cancer cells, an upregulation of p21WAF1 and p27KIP1 mRNA and protein levels was consistently observed upon exposure to high concentrations of genistein (20–60 μM), as summarised from eight in vitro studies(Reference Davis, Singh, Bhuiyan and Sarkar149, Reference Rice, Samedi, Medrano, Sweeney, Baker, Stenstrom, Furman and Shiverick155, Reference Kobayashi, Nakata and Kuzumaki164).
In addition to the inhibition of cell proliferation, isoflavones can induce apoptosis in human breast and prostate cells at concentrations over 25 μM and 20 μM, respectively(Reference Magee and Rowland3, Reference Limer and Speirs104). One in vivo study reported isoflavone-induced apoptosis in mammary glands of Sprague–Dawley rats (250 ppm genistein or 394 ppm mixed isoflavones)(Reference Dave, Eason, Till, Geng, Velarde, Badger and Simmen163). Pro-apoptotic proteins Bax, Bak and Bok were shown to be upregulated in breast cell culture models or rat mammary glands by genistein at respectively concentrations of 5 μM, 25 μM or 250 ppm in the diet(Reference Po, Wang, Chen and Leung112, Reference Li, Upadhyay, Bhuiyan and Sarkar160, Reference Dave, Eason, Till, Geng, Velarde, Badger and Simmen163), while anti-apoptotic proteins Bcl-2 and Bcl-xL were downregulated at genistein concentrations ≥ 5 μMin vitro (MDA-MB-231, 184-B5/HER)(Reference Po, Wang, Chen and Leung112, Reference Li, Upadhyay, Bhuiyan and Sarkar160). Data regarding apoptosis-associated proteins and their modulation by isoflavones in prostate cells are limited. However, it was shown by three different groups that genistein concentrations above 40 μM were necessary to upregulate Bax and downregulate Bcl-xL and Bcl-2 in vitro (Reference Shen, Klein, Wei, Guan, Contois, Wang, Chang and Hursting148, Reference Kazi, Daniel, Smith, Kumar and Dou165, Reference Li, Marani, Mannucci, Kinsey, Andriani, Nicoletti, Denner and Marcelli166). Furthermore, expression of cell cycle regulators such as p53, PTEN, p21WAF1 and p27KIP1, also involved in the apoptotic process, were modulated by isoflavones. The modulation of p21WAF1 and p27KIP1 has been described above. From two in vitro studies reporting p53 modulation in breast cancer and two studies (one in vivo, one in vitro) reporting p53 modulation in prostate cancer, it was shown that only high genistein concentrations ( ≥ 25 μM) were able to increase this protein expression(Reference Leung and Wang111, Reference Bemis, Capodice, Desai, Buttyan and Katz167). In contrast, the tumour suppressor gene PTEN was upregulated after incubating MCF-7 cells with a very low concentration of 0·1 nM genistein(Reference Waite, Sinden and Eng161). In vivo, doses of 250 ppm genistein or 394 ppm mixed isoflavones were also reported to induce PTEN protein expression in the mammary gland(Reference Dave, Eason, Till, Geng, Velarde, Badger and Simmen163). An induction of PTEN gene expression by genistein (20 μM) was similarly described for LNCaP and PC3 prostate cells(Reference Cao, Jin and Zhou168).
Inhibition of angiogenesis is another biological effect of isoflavones that has been reported in vivo in the context of breast and prostate cancer. Genistein (0·1–0·5 mg/kg bw) or a soy phytochemical concentrate significantly reduced vessel density in breast and prostate mouse xenografts(Reference Zhou, Gugger, Tanaka, Guo, Blackburn and Clinton147, Reference Shao, Wu, Shen and Barsky154). In highly invasive MDA-MB-231 breast cancer cells, 10 μM genistein suppressed cell migration as well as cell adhesion(Reference Valachovicova, Slivova, Bergman, Shuherk and Sliva169). Furthermore, the invasive potential of several breast and prostate cancer cell lines was reduced by high physiological doses of genistein (5–10 μM)(Reference Horia and Watkins140, Reference El Touny and Banerjee170, Reference Skogseth, Larsson and Halgunset171). The group of El Touny observed a genistein-induced upregulation of the metastasis suppressor Kangai-1 gene in TRAMP mice with 250 ppm in the diet or in TRAMP-C2 cells when exposed to 5 μM genistein(Reference El Touny and Banerjee170). The potential of isoflavones to inhibit invasion of breast and prostate tumour cells is furthermore characterised by the downregulation of matrix metalloproteinases (MMP)-2 and 9, as demonstrated in vivo and in vitro with doses ≥ 0·1 mg genistein/kg bw in breast cancer mouse xenografts and ≥ 18 μM genistein in PC3 and LNCaP cells, respectively(Reference Shao, Wu, Shen and Barsky154, Reference Kumi-Diaka, Hassanhi, Merchant and Horman172, Reference Xu and Bergan173). An upregulation of tissue inhibitor of metalloproteinase (TIMP)-1 was only reported for breast cancer with doses ≥ 0·1 mg genistein/kg bw in breast cancer mouse xenografts and 74 μM in MCF-7 and MDA-MB-231 cells(Reference Shao, Wu, Shen and Barsky154). In normal, early cancerous as well as established prostate cancer cells, 10 nM genistein inhibited the TGFβ-mediated induction of MMP-2(Reference Kumi-Diaka, Hassanhi, Merchant and Horman172–Reference Huang, Chen, Xu, Liu, Deb, Platanias and Bergan174). Osteopontin, an extracellular matrix protein secreted by macrophages infiltrating prostate tumours, was reduced at the transcriptional level by genistein (250, 500 mg/kg bw) in TRAMP mice prostate(Reference Mentor-Marcel, Lamartiniere, Eltoum, Greenberg and Elgavish175).
A physiological impact of isoflavones that has been well documented for breast and prostate cells in vitro and in vivo is the modulation of cell signal transduction through several signalling pathways such as EGF, IGF-1, Akt, NF-κB and MAPK signalling.
The capacity to inhibit mitogen-stimulated growth in breast and prostate cells has been presumed to be due to the inhibition of tyrosine kinase activities associated with critical growth factor receptors(Reference Fioravanti, Cappelletti, Miodini, Ronchi, Brivio and Di Fronzo106, Reference Peterson and Barnes176–Reference Akiyama, Ishida, Nakagawa, Ogawara, Watanabe, Itoh, Shibuya and Fukami178). EGFR protein expression was shown to be inhibited in rodent prostate with dietary doses of genistein between 250 and 1000 ppm(Reference Dalu, Haskell, Coward and Lamartiniere179, Reference Wang, Eltoum and Lamartiniere180). The studies by the group of Lamartiniere showed an age-dependent effect of isoflavones on EGFR protein expression in the mammary gland of Sprague–Dawley rats exposed to 500 μg genistein/g bw(Reference Brown, Wang, Cotroneo, Zhao and Lamartiniere181–Reference Cotroneo, Wang, Fritz, Eltoum and Lamartiniere183). In young 21-day old rats an initial upregulation of EGFR expression in TEBs was observed, followed by a downregulation in adult 50-day old rats. EGF-stimulated DNA synthesis was also inhibited by isoflavones in breast and prostate cell lines. In LNCaP and DU-145 prostate cells, genistein and daidzein at 15–60 μM showed significant inhibition of EGF-stimulated growth(Reference Peterson and Barnes176), whereas the effect in MCF-7 breast cells is dependent on the genistein concentration added, showing growth inhibition with 0·1 μM and promotion with 1–10 μM(Reference Wang and Kurzer68).
In the prostate of TRAMP mice, a decrease in IGF-1R protein was observed following administration of 250 ppm genistein in the diet(Reference Wang, Eltoum and Lamartiniere180). In contrast, an in vitro study in MCF-7 breast cancer cells reported an increase in IGF-1R protein expression after exposure to 1 μM genistein(Reference Chen and Wong184). While IGF-1-stimulated proliferation of rat prostate cancer cells was inhibited by 10 μM genistein(Reference Wang, DeGroff and Clinton177), physiological genistein concentrations (0·1–10 μM) further increased IGF-1-stimulated growth of MCF-7 cells. Only pharmacological doses of genistein (>25 μM) showed an inhibitory effect on breast cancer cell growth(Reference Wang and Kurzer68).
Decreased IGF-1 serum levels were detected in male rats and mice after administration of soy protein and soy isoflavone-enriched diets(Reference Zhou, Gugger, Tanaka, Guo, Blackburn and Clinton147, Reference Aukema and Housini185). However, contrasted effects of soy or isoflavones on IGF-1 were observed in human studies. Cross-sectional studies in Asian and European women showed no association with soy, isoflavones and soy protein intake and IGF-1 levels(Reference Nagata, Shimizu, Takami, Hayashi, Takeda and Yasuda186–Reference Vrieling, Voskuil, Bueno de Mesquita, Kaaks, van Noord, Keinan-Boker, van Gils and Peeters188). Experimental studies in women gave controversial results. Consumption of 40 g soy protein for 3 months led to increased IGF-I plasma levels in postmenopausal women(Reference Arjmandi, Khalil, Smith, Lucas, Juma, Payton and Wild189). Again, increased plasma IGF-1 and IGFBP-3 concentrations were observed in women consuming 80 mg phytoestrogens (including soy isoflavones)(Reference Woodside, Campbell, Denholm, Newton, Honour, Morton, Young and Leathem190) and in premenopausal women after consumption of a low-isoflavone enriched diet (1 mg/kg bw +d)(Reference Wangen, Duncan, Merz-Demlow, Xu, Marcus, Phipps and Kurzer191). In contrast, a high-isoflavone diet (2 mg/kg bw +d) for 3 months resulted in decreased serum IGF-1 concentrations in postmenopausal women(Reference Wangen, Duncan, Merz-Demlow, Xu, Marcus, Phipps and Kurzer191). In men the effect of isoflavone supplementation on serum IGF-1 levels was also inconsistent. A positive correlation was found between intake of soy protein and serum IGF-1 levels in Asian and Caucasian men(Reference Probst-Hensch, Wang, Goh, Seow, Lee and Yu192, Reference Khalil, Lucas, Juma, Smith, Payton and Arjmandi193) but two intervention studies showed no effect of soy isoflavones(Reference Adams, Newton, Chen, Emerson, Potter, White and Lampe194, Reference Hussain, Banerjee, Sarkar, Djuric, Pollak, Doerge, Fontana, Chinni, Davis, Forman, Wood and Kucuk195).
Inhibition of the Akt signalling pathway through inhibition of Akt phosphorylation was observed in breast and prostate cell lines exposed to 1–10 μM genistein(Reference Waite, Sinden and Eng161, Reference Jagadeesh, Kyo and Banerjee196), as well as in male TRAMP/FVB mice fed with 250–1000 ppm genistein(Reference El Touny and Banerjee197). An abrogation of EGF-stimulated Akt activation was also observed with a high dose (50 μM) of genistein(Reference Li and Sarkar198, Reference Gong, Li, Nedeljkovic-Kurepa and Sarkar199). As PTEN expression is known to be upregulated in prostate and breast cells by low genistein concentrations, as described above, it may mediate the repression of the Akt pathway.
Furthermore it was shown that genistein concentrations above 10 μM inhibited the constitutively activated transcription factor NF-κB in highly invasive MDA-MB-231 breast cells(Reference Valachovicova, Slivova, Bergman, Shuherk and Sliva169). Pharmacological doses of genistein (>30 μM) inhibited NF-κB DNA-binding activity and abrogated NF-κB activation induced by DNA damaging agents, EGF and Akt in LNCaP, PC3 prostate and MDA-MB-231 breast cells(Reference Li and Sarkar198–Reference Vanden Berghe, Dijsselbloem, Vermeulen, Ndlovu, Boone and Haegeman202). Moreover, protein expression of IkB, the inhibitor of NF-κB in the cytosol, was induced in MCF-7 breast cancer cells by 100 μM genistein(Reference Kazi, Daniel, Smith, Kumar and Dou165). Conversely, an inhibition of IkB was reported for prostate cells in vitro (Reference Davis, Kucuk and Sarkar201). These effects have been observed using very high isoflavone concentrations. However, Borras et al. recently reported that a dose as low as 0·5 μM genistein efficiently activated NF-κB and subsequently elevated Mn-superoxide dismutase (SOD) expression in MCF-7 cells(Reference Borras, Gambini, Gomez-Cabrera, Sastre, Pallardo, Mann and Vina203).
Expression of different members of the MAPK cascade is modified by genistein in breast cells. Protein expression of JNK and p38 MAPK was increased when MCF-7 and MCF-10F cells were incubated with 0·1–45 μM genistein(Reference Leung and Wang111, Reference Frey and Singletary158). In contrast, ERK1/2 protein expression was decreased in MCF-10F cells when using a high genistein concentration (45 μM)(Reference Frey and Singletary158) and in the prostate of TRAMP mice fed genistein at 250 ppm in the diet(Reference Wang, Eltoum and Lamartiniere180). Genistein (1–10 μM) also increased ERK1/2 activity in non-tumorigenic epithelial RWPE-1 prostate cells via an estrogenic-dependent mechanism, while supra-physiological concentrations (>25 μM) decreased ERK1/2 signalling(Reference Gao, Liu and Wang114, Reference Frey and Singletary158, Reference Wang, DeGroff and Clinton177, Reference Wang, Clubbs and Bomser204, Reference Clubbs and Bomser205). Downstream targets c-jun and c-fos, members of the AP-1 complex which is located in promoter regions of several genes discussed in this paragraph, were demonstrated to be upregulated by genistein in breast cancer models in vivo and in vitro. While the dose inducing this effect was 0·1 mg/kg bw in mice, it was much higher (74 μM) in ER positive and negative breast cell lines(Reference Shao, Wu, Shen and Barsky154).
Isoflavones such as genistein were also reported to have a possible effect on DNA repair. The tumour suppressor genes BRCA1 and BRCA2 are high penetrance genes associated with an increased risk of estrogen-responsive hereditary cancers such as breast and ovarian cancer and their mutations are also linked to an increased risk of developing androgen-dependent pathologies such as prostate cancer(Reference Thompson and Easton206, Reference Boulton207). The corresponding proteins are involved in crucial cellular processes like cell proliferation, DNA repair and control of gene expression, notably through the regulation of ER- and AR-dependent transcriptional activities(Reference Boulton207, Reference Rosen, Fan, Pestell and Goldberg208). Physiological doses of genistein (0·5–2·5 μM) upregulated BRCA1 and BRCA2 protein expression in MCF-7 and T47D breast and LNCaP and DU-145 prostate cells in vitro (Reference Fan, Meng, Auborn, Carter and Rosen209). An in vivo study reported that administration of 1·25–3·3 μg genistein/g bw during prepuberty resulted in a long-lasting increase in BRCA1 mRNA levels in mammary glands of 3 and 8-week old female Sprague–Dawley rats(Reference Cabanes, Wang, Olivo, DeAssis, Gustafsson, Khan and Hilakivi-Clarke51). Furthermore, BRCA2 mRNA expression was upregulated in mammary glands of ovariectomised Wistar rats fed an isoflavone-formulated diet (soylife) providing 8 μg isoflavones/g bw for 90 days(Reference Vissac-Sabatier, Coxam, Dechelotte, Picherit, Horcajada, Davicco, Lebecque, Bignon and Bernard-Gallon210). Isoflavones are thus candidate regulators of BRCA1 expression, possibly through their interaction with hormone receptors.
The modulation of antioxidant and xenobiotic enzyme systems towards reduction of oxidative stress and carcinogen detoxification is another key player in cancer prevention. Although the direct antioxidant capacity of soy isoflavones by scavenging free radicals or preventing LDL oxidation was mostly observed at high doses in vitro (Reference Rufer and Kulling211–Reference Patel, Boersma, Crawford, Hogg, Kirk, Kalyanaraman, Parks, Barnes and Darley-Usmar213), evidence exists that isoflavones may induce antioxidant enzyme systems as shown in vitro in breast and prostate cells at concentrations observed in plasma, or in vivo after soy consumption. Genistein at 10 μM induced gene and protein expression of several antioxidant enzymes such as glutathione peroxidase (GPx), glutathione reductase (GSR), microsomal glutathione S-transferase 1 (mGST-1) and metallothionein 1X (MT-1X) in LAPC-4 prostate cancer cells(Reference Raschke, Rowland, Magee and Pool-Zobel214). A lower concentration (0·5 μM) of genistein also induced the transcription of Mn-SOD gene in MCF-7 cells(Reference Borras, Gambini, Gomez-Cabrera, Sastre, Pallardo, Mann and Vina203). Furthermore, isoflavone supplementation of 138 mg/d for 24 days increased Cu/Zn-SOD activities in erythrocytes of breast cancer survivors(Reference DiSilvestro, Goodman, Dy and Lavalle215). Several xenobiotic enzymes involved in the activation of pro-carcinogens or in the detoxication of the carcinogens were influcenced by isoflavones as shown in non-tumorigenic and tumorigenic breast cells using low doses of isoflavones, as well as in rat and human mammary glands. Levels of toxifying Phase I enzymes, such as cytochrome P450 (CYP) 1A1, 1A2 and 1B1 were decreased by 430 ppm isoflavones in the diet of female Sprague–Dawley rats(Reference Rowlands, He, Hakkak, Ronis and Badger216), by 0·7–15 μM genistein in MCF-7 cells(Reference Chan and Leung217) and in premenopausal women after supplementation with 400 mg daidzein/d(Reference Peng, Li and Zhou218). Detoxifying Phase II enzymes such as GSTP1 and quinone reductase (QR) were upregulated at 1–10 μM genistein in non-tumourigenic MCF-10A and tumourigenic MCF-7 and MDA-MB-231 breast cells(Reference Steiner, Peters, Gallagher, Magee, Rowland and Pool-Zobel219, Reference Bianco, Chaplin and Montano220).
The influence of isoflavones in the metabolism of vitamin D has also been explored. However, the potentially protective effect of genistein through an increase in the synthesis of the antiproliferative metabolite 1,25-dihydroxy-vitamin D3 in breast and prostate cancer cells was only observed at high non-physiological concentrations(Reference Farhan, Wahala and Cross221, Reference Cross, Kallay, Lechner, Gerdenitsch, Adlercreutz and Armbrecht222). Similarly, inhibition of topoisomerase II and modulation of telomerases were only shown in cell culture models exposed to supra-physiological concentrations of genistein, thus, are unlikely to play a role in the prevention of breast and prostate cancer(Reference Jagadeesh, Kyo and Banerjee196, Reference Markovits, Linassier, Fosse, Couprie, Pierre, Jacquemin-Sablon, Saucier, Le Pecq and Larsen223, Reference Chinni, Alhasan, Multani, Pathak and Sarkar224).
To summarise the mechanisms of action of isoflavones discussed herein for breast cancer it is suggested that the inhibition of cell cycle and induction of apoptosis, promotion of differentiation, as well as the inhibition of angiogenesis, invasion and metastasis are probable modes of action of isoflavones as they were observed at physiologically relevant doses in vivo as well as in vitro. Modulation of the ER pathways and the metabolism of steroid hormones are certainly involved, whereas the regulation of cell signalling pathways gave inconsistent results. Some supportive evidence exists for the modulation of EGF, IGF-1, MAPK, Akt and PPARγ pathways, whereas the effects of isoflavones on NF-κB signalling need to be further explored. Furthermore, the stimulation of antioxidant enzyme systems, the modulation of the xenobiotic metabolism towards enhanced detoxification and boosting of DNA repair have also been demonstrated at physiological isoflavone concentrations in breast cells.
The most likely mechanisms of action of isoflavones involved in the prevention of prostate cancer include interaction with AR and ER pathways and improvement of DNA repair. While inhibition of cell cycle was reported to occur only at supra-physiological concentrations, inhibition of angiogenesis, invasion and metastasis may occur at lower doses compatible with dietary exposure. Further studies are needed to establish the effects of isoflavones on the metabolism of steroids, on the modulation of antioxidant enzyme systems and xenobiotic metabolism, as well as on EGF, IGF-1, PPARγ and Akt signalling pathways in prostate cells.
Modulation of vitamin D metabolism, effect on telomerases, direct scavenging of free radicals or inhibition of topoisomerases II were only described at isoflavone concentrations far exceeding the physiological serum levels, raising doubts on their role in the prevention of breast and prostate cancer.
Effects on gene expression and microarray analyses
Targeted approaches focusing on the activity of one compound on one particular gene or protein, as described in the previous section, have been widely used in pharmacology, generally with success; however, they do not seem to be sufficient to elucidate the mechanisms of action of isoflavones at dietary levels of exposure. Diet is a very complex system, consisting of a huge number of possible combinations of diverse nutrients and micronutrients. Each constituent may have an impact on several metabolic or signalling pathways, and dietary constituents can exert opposite, additional or synergistic effects(Reference Lila and Raskin225).
Furthermore, there is abundant evidence of cross-talks between ERs and AR, EGF and IGF-1 signalling in breast and prostate cancer cells(Reference Lee, Cui and Oesterreich226). AR and ERs can be activated by growth factors and compounds that modulate the MAPK cascade(Reference Ueda, Mawji, Bruchovsky and Sadar227, Reference Kato, Masuhiro, Watanabe, Kobayashi, Takeyama, Endoh and Yanagisawa228) or the phosphatidylinositol-3 kinase (PI3K)/Akt-mediated cell survival pathway. In prostate cancers that have evolved to androgen independence, growth factor receptors may activate androgen receptors in the absence of androgens(Reference McCarty229). Bidirectional interactions exist. For example, the IGF-1R is directly activated by liganded ER, IGF signalling transcriptionally activates the ER, and IGF-1 and estrogens have synergistic effects on cell cycle signalling cascades and proliferation(Reference Hamelers and Steenbergh230). Signal cross-talk also exists bidirectionally between PPARγ and ER in breast cancer cells(Reference Wang and Kilgore231). ERα and PPARγ pathways have an opposite effect on the regulation of the PI3K/Akt transduction cascade(Reference Bonofiglio, Gabriele, Aquila, Catalano, Gentile, Middea, Giordano and Ando232). A variety of studies have shown that activated ER and PPAR can inhibit the activity of the transcription factor NF-κB, which plays a key role in the control of genes involved in inflammation, cell proliferation and apoptosis(Reference De Bosscher, Vanden Berghe and Haegeman233). A cross-talk between NF-κB and Akt signalling pathways has been suggested in genistein-treated MDA-MB-231 breast cancer cells(Reference Gong, Li, Nedeljkovic-Kurepa and Sarkar199). Moreover, NF-κB activation was reported to inhibit the tumour suppressor PTEN expression, which functions as a negative regulator of the PI3K/Akt pathway(Reference Vasudevan, Gurumurthy and Rangnekar234), whereas activated PPARγ has been shown to upregulate PTEN transcription(Reference Waite, Sinden and Eng161). These non-exhaustive examples of established cross-talks potentially involved in breast and prostate carcinogenesis show how complex the interactions between the various signalling pathways are.
Inter-organ relationships must also be considered. For example, the liver and the immune system certainly play a role in the aetiology of breast and prostate cancer. Therefore it appears evident that targeted approaches are not sufficient to study the effects of dietary compounds on the prevention of these diseases. High-throughput methods such as transcriptomics, proteomics and metabolomics have been recently introduced in the nutrition field.
Large scale gene expression profiling through the use of DNA microarrays consists in measuring simultaneously the expression of hundreds or thousands of genes in a given sample using a single hybridization step. The comparison of these transcriptomic signatures obtained in different experimental conditions offers the possibility to reveal gene networks and signal transduction pathways modulated by a dietary intervention. It allows venturing new hypotheses about mechanisms of action. This is a valuable progress since targeted approaches may favour the systematic focus on a few genes or proteins that are already known to be implicated but may not be the only or the most important actors.
Similarities and differences between phytoestrogen and estrogen activities have been investigated in this way, by comparison of transcriptomic signatures induced by isoflavones or estradiol exposure in MCF-7 cells or mouse estrogen-responsive tissues. Results have been conflicting with some studies describing very similar transcriptional responses(Reference Terasaka, Aita, Inoue, Hayashi, Nishigaki, Aoyagi, Sasaki, Wada-Kiyama, Sakuma, Akaba, Tanaka, Sone, Yonemoto, Tanji and Kiyama235–Reference Moggs, Ashby, Tinwell, Lim, Moore, Kimber and Orphanides237) and others reporting only limited overlapping between expression patterns elicited by estradiol and genistein(Reference Naciff, Jump, Torontali, Carr, Tiesman, Overmann and Daston238, Reference Konstantakopoulos, Montgomery, Chamberlain, Quinn, Baker, Rice, Georgiou and Campbell239). It was suggested that differences in transcriptional signatures may arise from concentration-dependent variations in the magnitude and kinetics of gene expression rather than from modulation of distinct pathways. The question of the dose therefore appears crucial. The number of genes whose expression is affected by estradiol increased with its concentration in MCF-7 cells and in the uterus of immature female rats(Reference Naciff and Daston240). In human endometrial cancer cells, a strong dose-dependency in the number of genes regulated by genistein was also observed: 2, 95 and 508 genes were affected by genistein exposure at 0·5, 5 and 50 μM respectively(Reference Konstantakopoulos, Montgomery, Chamberlain, Quinn, Baker, Rice, Georgiou and Campbell239). Shioda et al. obtained a gene expression profile after exposure of MCF-7 cells to 30 pM estradiol undistinguishable from that obtained with exposure to 3 μM genistein, but clearly different to the profile obtained after exposure to 10 μM genistein(Reference Shioda, Chesnes, Coser, Zou, Hur, Dean, Sonnenschein, Soto and Isselbacher241). Gene expression profiling thus allows comparing different estrogenic compounds but the results must be interpreted with caution due to this strong dependence on the dose. The few studies that used physiological doses of genistein and estradiol rather showed similar gene expression profiles with genistein and estradiol(Reference Buterin, Koch and Naegeli242, Reference Thomsen, Almstrup, Nielsen, Sorensen, Petersen, Leffers and Breinholt243) but more studies are clearly needed.
Microarrays may also be useful to study molecular mechanisms. They have been used to identify novel androgen-responsive genes or genes induced in common by peptide growth factors and androgen in human prostate epithelial cells(Reference York, Plymate, Nelson, Eaves, Webb and Ware244). Microarrays have been used to study isoflavone mechanisms of action and build up new hypotheses of research. Only few studies, described below, have applied this technique on cell models exposed to physiologically relevant conditions, e.g. with exposures consistent with isoflavone concentrations achievable in humans.
Global gene expression profiles analysed after a 24-h exposure of MCF-7 and T47D cells to 1 μM genistein showed large similarities with those obtained after exposure to 30 pM estradiol(Reference Buterin, Koch and Naegeli242). Using a threshold fold-change of 3, the authors found 97 upregulated genes, and 36 downregulated genes in genistein-treated MCF-7 cells, mainly involved in cell cycle, apoptosis, DNA metabolism, growth stimulation, cell adhesion, as well as xenobiotic metabolism and transport systems. Fewer genes were regulated by genistein in T47D cells but the same functions were affected. Shioda et al. showed using Affymetrix pangenomic microarrays that exposure of MCF-7 cells to 3 μM genistein for 48 h resulted in the upregulation of genes mainly involved in cell cycle progression, purine and pyrimidine synthesis, as well as steroid biosynthesis(Reference Shioda, Chesnes, Coser, Zou, Hur, Dean, Sonnenschein, Soto and Isselbacher241).
Few studies have been carried out in vivo. Thomsen et al. investigated the effects of neonatal or prepubertal exposure to 270 ppm genistein in the diet at the transcriptional and histological levels by using cDNA microarrays and immunohistochemical analysis of selected regulated genes(Reference Thomsen, Almstrup, Nielsen, Sorensen, Petersen, Leffers and Breinholt243). Changes in gene expression were clearly related to an increase in the number of epithelial mammary cells expressing the genes rather than to a specific regulation within the cells. This finding supported the hypothesis of a role of genistein in promoting earlier differentiation of the mammary gland.
In the mammary gland of male rats life-long exposed to genistein through maternal supplementation then directly in the diet after weaning until postnatal day 90, 90 genes were up or downregulated by at least 2-fold. The following genes were upregulated: growth factors (FGF 5), receptors (IGF-2R, TGFβ- type 2 receptor, acetylcholine receptor β, inositol 1,4,5-triphosphate receptor type 1), kinases (adenylate kinase 1 and 3, pyruvate dehydrogenase kinase). Genistein downregulated expression of various enzymes involved in lipid metabolism (phospholipase C β3, phospholipase C δ4, phospholipase Cγ1, pancreatic lipase, hepatic lipase), small molecule receptors (glutamate receptor, prostaglandin F receptor) and solute transporters (solute carrier family 2, 10, 13, 18, voltage-gated sodium channel) as well as organic cationic transporter-like 1 and hydroxysteroid dehydrogenase 11β type 1(Reference You and Bartolucci245). The effects on energy metabolism and transport systems constitute new putative mechanisms of action of isoflavones that will have to be investigated further.
In the context of prostate cancer, a series of elegant studies using pangenomic microarrays to investigate the chemopreventive mechanisms of action of isoflavones have been published(Reference Takahashi, Lavigne, Hursting, Chandramouli, Perkins, Barrett and Wang246–Reference Takahashi, Hursting, Perkins, Wang and Wang248). The authors exposed the human androgen-responsive prostate cancer cells LNCaP to 1, 5 and 25 μM genistein, daidzein or equol for 48 h, and showed that the number of regulated genes increased markedly with the concentration to which cells were exposed: 521, 484 and 101 genes were regulated by 25 μM equol, genistein and daidzein respectively whereas only 14, 3 and 29 genes were regulated by the same compounds at 1 μM. The nature of the regulated pathways also depended on the concentration: 1 μM genistein affected androgen-regulated genes and IGF-1 pathway, concentration of 5 μM affected cell cycle control genes and PPARα signalling, whereas genes related to DNA damage and stress response pathways were upregulated at a 25 μM concentration. Genistein, daidzein and equol induced similar effects on the androgen-responsive genes and IGF-1 pathways, but resulted in a different modulation of steroid/xenobiotic metabolism genes and cell cycle-related genes.
Targeted microarrays may also be useful and are often easier to interpret. Raschke et al. (Reference Raschke, Rowland, Magee and Pool-Zobel214) firstly showed with the comet assay that genistein (1–30 μM) protected prostate LAPC-4 cells from hydrogen peroxide-induced DNA damage. Using a targeted cDNA macroarray containing 192 genes involved in biotransformation or stress response, the authors showed that genistein modulated the expression of 3 of these genes at 1 μM and 19 genes at 10 μM. Real-time PCR confirmed the induction of three genes encoding products with antioxidant activities, namely GSR, mGST-1 and MT-1X possibly contributing to prevent DNA damage.
Seven other studies reported the use of the microarray technique on prostate cancer cells exposed to very high concentrations of isoflavones (45–100 μM)(Reference Rice, Samedi, Medrano, Sweeney, Baker, Stenstrom, Furman and Shiverick155, Reference Li and Sarkar200, Reference Li, Che, Bhagat, Ellis, Kucuk, Doerge, Abrams, Cher and Sarkar249–Reference Handayani, Rice, Cui, Medrano, Samedi, Baker, Szabo and Shiverick252). Although these studies may be useful to investigate the putative therapeutic effects of pharmacological doses of isoflavones, they are not useful to examine the mechanisms of action involved in the preventive effects of nutritional doses of isoflavones and thus are beyond the scope of this review. No studies are available to date on gene expression profiles induced by isoflavones in animal prostate.
Although the microarray technology has greatly progressed in the last few years, there are still some limitations. One important issue is the study design and the choice of the appropriate model. Due to high cost of the microarray approach, frequently only one dose or time point is tested, and this does not allow understanding properly the dynamics of changes in gene expression and cell signalling. Another debate is related to the use of cell lines or freshly isolated tissues. Results obtained with cell lines cannot be easily extrapolated to humans due to differences in their genome and to their different environment. On the other hand, isolated tissues are complex and made of a variety of cell types, and reproducibility is hampered by inter-individual differences. However, the heterogeneity of tissue samples and the interactions of the different cell types reflect more precisely the biological gene expression profile. Hence, in order to obtain statistically significant data, a rather large set of samples is required resulting in costly experiments.
Data analysis and interpretation of the large data sets using public databases such as gene network, gene pathway and genome ontology databases are critical issues. The defined cut-off level for differentially expressed genes, based on the fold changes, is also questioned. To date, relatively high fold change thresholds (above 2-fold) have been chosen. However, in microarray analyses using nutritional doses of micronutrients only low fold changes are frequently observed. Low fold changes may actually have a physiological impact, since a small regulation of several genes in one pathway might give an additive effect on the outcome. One must bear in mind that the microarray technology is a precious tool to build up new hypotheses of research while considering the complexity of metabolic and signalling pathways however classical approaches such as quantitative measurement of RNA levels (northern blot, RT-PCR, or in situ hybridization) or protein levels (immunohistochemistry or western blot), as well as biochemical analyses are still necessary for the final validation of mechanistic hypotheses and the ultimate establishment of a causal relationship between altered gene expression and a given disease or dietary intervention.
Proteomics
Rowell et al. used a proteomic approach to investigate the effects of prepubertal exposure to genistein before chemically induced mammary carcinogenesis(Reference Rowell, Carpenter and Lamartiniere253) and found the GTP cyclohydrolase1 protein (GTP-CH1) significantly upregulated in mammary glands. The authors used extensive literature and web searching to find information about related pathways that might explain how this particular protein might play a role in mammary cancer chemoprevention. They speculated that downstream signalling from GTP-CH1 would upregulate tyrosine hydroxylase, resulting in dynamic upregulation of catecholamines. This in turn, would decrease vascular endothelial growth factor receptor 2 (VEGFR2) levels, resulting in decreased ability to promote angiogenesis. Immunoblot and immunohistochemistry analyses confirmed that the proteins of interest (GTP-CH1, tyrosine hydroxylase, and VEGFR2) were modulated in their expression in the lining of mammary epithelial cells of 50-day-old rats. This first study demonstrated the usefulness of proteomics for the discovery of novel pathways that may be involved in cancer prevention by isoflavones.
Metabolomics
Few metabolomics studies have still been published in the field of nutrition. However, two NMR-based studies have been carried out to investigate the effects of soy isoflavone consumption(Reference Solanky, Bailey, Beckwith-Hall, Bingham, Davis, Holmes, Nicholson and Cassidy254, Reference Solanky, Bailey, Beckwith-Hall, Davis, Bingham, Holmes, Nicholson and Cassidy255). The 1H NMR spectral profiles of plasma and urine from premenopausal women before and following soy or miso consumption revealed significant changes in metabolites associated with energy metabolism. The results suggested an inhibitory effect of isoflavones on glycolysis, resulting in a general shift in energy metabolism from carbohydrate metabolism to lipid metabolism.
Metabolomics is theoretically a better tool to characterize metabolic phenotypes and the effects of dietary interventions on these phenotypes than transcriptomics or proteomics, since changes in gene or protein expression do not necessarily lead to changes in metabolite concentrations. However many technical hurdles still hamper progresses in this approach, as discussed in other reviews(Reference Dettmer, Aronov and Hammock37, Reference Gibney, Walsh, Brennan, Roche, German and van Ommen256). The present limit of the use of NMR-based metabolomics is the low sensitivity of the technique. The alternative is the use of mass spectrometry-based metabolomics but problems of standardization and robustness still have to be addressed. Furthermore, more efficient methods must still be developed to improve the automated identification of markers.
Epigenetic regulation
DNA methylation, usually occurring at promoter CpG islands, as well as acetylation and methylation of critical lysine residues on histones are key epigenetic mechanisms for the silencing of many genes involved in carcinogenesis, including tumour suppressor genes (such as BRCA1, p53, and caveolin-1), genes encoding hormone receptors, DNA repair enzymes, mediators of the apoptosis pathway, and detoxification enzymes(Reference Fay, Crowell and Kopelovich257). Histone modifying enzymes have been shown to directly regulate the expression and activity of ERα, AR and PPARγ(Reference Leader, Wang, Fu and Pestell258). Various techniques, such as methylation-specific oligonucleotide microarrays (MSO), methylation target array (MTA), cytosine extension assay and pyrosequencing now allow assessing global and gene specific DNA methylation. For example, using methylation-specific oligonucleotide microarrays, Yu et al., demonstrated that 25 genes among the 105 studied were methylated in prostate cancer cell lines but not in normal primary prostate tissue samples(Reference Yu, Paranjpe, Nelson, Finkelstein, Ren, Kokkinakis, Michalopoulos and Luo259). A few available reports indicate that genistein could regulate cancer-related gene transcription by modulating epigenetic events such as DNA methylation and/or histone acetylation, either directly or through an estrogen receptor-dependent process. Day et al. reported that the consumption of a genistein diet (300 ppm) by adult male mice was associated to a partial change in the global DNA methylation pattern in the mouse prostate(Reference Day, Bauer, DesBordes, Zhuang, Kim, Newton, Nehra, Forsee, MacDonald, Besch-Williford, Huang and Lubahn260). In LNCaP and PC3 cells, genistein reversed DNA hypermethylation and reactivated the methylation-silenced genes retinoic acid receptor β (RARβ), p16, and O-methylguanine methyltransferase (MGMT), but only when used at a high concentration (20 μM)(Reference Fang, Chen, Sun, Jin, Christman and Yang261). However, genistein at 5 μM enhanced the activity of the demethylating agent 5-aza-2-deoxycytidine and the histone acetylating drug trichostatin in the reactivation of these genes. Moreover, genistein, equol and to a lesser extent daidzein were shown to stimulate in vitro histone acetylation mediated by ERα or ERβ and their coactivators, an effect likely to be associated to stimulated transcription(Reference Hong, Nakagawa, Pan, Kim, Kraus, Ikehara, Yasui, Aihara, Takebe, Muramatsu and Ito262). Epigenetic effects of isoflavones thus undoubtedly constitute a novel promising axis of research.
In conclusion, although many isoflavone effects have already been established using classical targeted approaches, the exact mechanisms of action have not yet been established. Classical molecular approaches have been used, often in in vitro models, to explore specific hypotheses. The results often still need to be validated in vivo in nutritional conditions. The more recent studies using transcriptomic, proteomic and metabolomic high-throughput techniques obviously demonstrated their potential to describe the complexity of the biological effects of isoflavones and provide us with more comprehensive insights into how isoflavones may contribute to prevent breast and prostate cancers. New hypotheses on the molecular and cellular mechanisms of action of isoflavones have already been opened and there is no doubt that others will rapidly emerge from the increasing use of nutrigenomics approaches in the near future. Major discoveries regarding their mechanisms of action and their variability according to individuals, dose or timing of exposure still seem conceivable.
Acknowledgements
The publication of this paper was made possible by the financial support of the European Co-operation in the field of Scientific and Technical (COST) Research Action 926 “Impact of new technologies on the health benefits and safety of bioactive plant compounds” (2004–2008). The authors had no conflicts of interest to disclose.




