Large prospective studies have now demonstrated that a healthy diet is beneficial in reducing the risk of developing cardio-metabolic diseases such as diabetes and CVD(Reference Ford, Bergmann and Kroger1–Reference Stampfer, Hu and Manson3). Emerging data suggest that the relationship between dietary habits and CVD may, in part, be mediated via the microcirculation(Reference Kaushik, Wang and Flood4–Reference Serre and Sasongko6).
The microvasculature of the retina can be easily viewed and non-invasively imaged, and thus, can be a surrogate for the systemic microvasculature(Reference Wong and Mitchell7, Reference Wang, Mitchell and Leung8). There is now a large body of accumulating evidence to suggest that some retinal microvascular changes (narrower retinal arteriolar calibre and wider venular calibre) are markers of poorer microvascular health and could predict systemic vascular diseases(Reference Wong and Mitchell9), including hypertension, diabetes, stroke and CVD(Reference Wong and Mitchell7, Reference McGeechan, Liew and Macaskill10, Reference Wang, Baker and Hand11).
There is some recent research that has examined the relationship of individual nutrients and foods on the retinal microvasculature. For instance, in older adults, aged 50 years and older, a high glycaemic index was associated with poorer retinal microvascular health (wider retinal venular calibre)(Reference Kaushik, Wang and Wong5), while high fish consumption was associated with better retinal microvascular health (wider retinal arterioles and narrower venules)(Reference Kaushik, Wang and Flood4).
Because nutrients are not consumed independently but together within a variety of foods in the diet(Reference Russell, Flood and Rochtchina12), there remains a need to examine overall diet quality in association with changes to the retinal microvascular structure. The measurement of overall diet quality has been previously used to determine the associations between whole foods and health status(Reference Kim, Yang and Yang13). A healthy diet has been characterised in many ways, although there is no consensus about what best defines a healthy diet(Reference Zamora, Gordon-Larsen and Jacobs14). One approach groups foods ‘a priori’ that are representative of present nutrition knowledge in the form of dietary guidelines or other dietary recommendations(Reference Waijers, Feskens and Ocke15). This may be a more useful tool in public health practice to assess a population's adherence to present dietary guidelines based on empirical evidence(Reference Russell, Flood and Rochtchina12).
To the best of our knowledge, no population-based study has investigated the association between overall diet quality and microvascular health. We previously developed a tool(Reference Russell, Flood and Rochtchina12) modelled using both Australian and US diet quality indices(Reference Fogli-Cawley, Dwyer and Saltzman16, 17). Using a large, representative cohort of adults aged 50 years and over, we aimed to assess the association between diet quality, using a tool based on dietary guidelines for the Australian population and retinal vascular calibre, a marker of systemic microvascular health.
Methods
Study population
The Blue Mountains Eye Study (BMES) is a population-based study of participants aged ≥ 49 years, living in two postcodes of the Blue Mountains region, west of Sydney, Australia, which has studied age-related eye diseases and other health outcomes in an older urban Australian population. Details of the study methods have previously been described(Reference Attebo, Mitchell and Smith18). Fig. 1 shows the distribution of participation over a period of 5 years (from 1992–4 to 1997–9) in the BMES. The study was approved by the Human Research Ethics Committee of the University of Sydney, and was conducted adhering to the tenets of the Helsinki Declaration. Signed informed consent was obtained from all the participants at each examination.
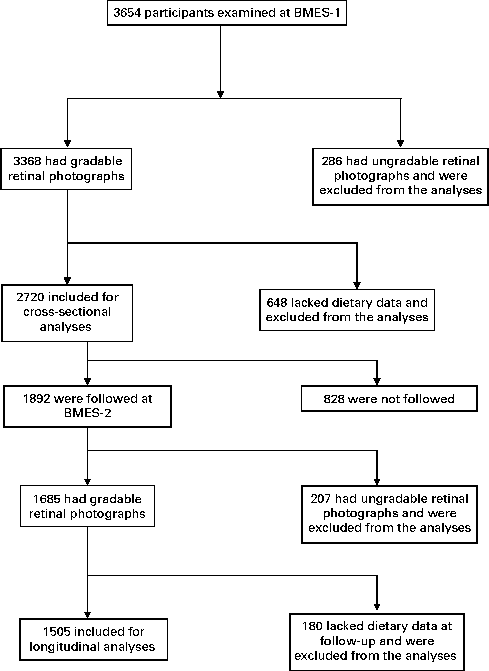
Fig. 1 Participation flowchart in the Blue Mountains Eye Study (BMES) from 1992–4 (BMES-1) to 1997–9 (BMES-2).
Dietary assessment
At baseline, dietary data were collected using a 145-item self-administered FFQ, modified for the Australian diet and vernacular from an early Willett FFQ(Reference Willett, Sampson and Browne19) and including reference portion sizes. Participants used a nine-category frequency scale to indicate the usual frequency of consuming individual food items during the past year. The FFQ was validated by comparing nutrients from the FFQ to 4-d weighed food records collected over 1 year (n 79). Most nutrient correlations were between 0·50 and 0·60 for energy-adjusted intakes, similar to other validated FFQ studies(Reference Barclay, Flood and Brand-Miller20, Reference Smith, Mitchell and Webb21). A dietitian coded data from the FFQ into a customised database that incorporated the Australian Tables of Food Composition 1990 (NUTTAB 90)(Reference Foster-Powell, Holt and Brand-Miller22).
A modified version of the Australian diet quality index(17), based on the Dietary Guidelines for Australian Adults(23) and the Australian Guide to Healthy Eating (AGHE)(Reference Smith, Kellet and Schmerlaib24), was used to establish the total diet score (TDS) assessing adherence to the Australian dietary guidelines. The methodology used to develop TDS has been previously reported(Reference Russell, Flood and Rochtchina12). Briefly, TDS were allocated for intakes of selected food groups and nutrients for each participant as described in the Dietary Guidelines for Australian Adults (details for FFQ food groupings available in Table S1, available online). The TDS is divided into ten components, and each component has a possible score ranging from 0 to 2. A maximum score of 2 was given to subjects who met the recommendations, with pro-rated scores for lower intakes (Table S1, available online). These were then summated, providing a final score ranging between 0 and 20, with higher scores indicating closer adherence to the dietary guidelines(Reference Russell, Flood and Rochtchina12).
The TDS account for both food intake and optimal choice, with scores allocated to reflect intake characteristics from both sources. Food intake scores were based on total intakes of vegetables, fruit, cereals and breads, meat, fish, poultry and/or alternatives and dairy products, as well as Na, alcohol, sugar and extra foods. Optimal choices' scores determined intakes of foods with greater dietary benefits, including servings of whole-grain cereals, lean red meat, low- or reduced-fat milk v. whole milk, low saturated fat intake and fish consumption. Cut-points for scores were determined from recommended number of servings given in the AGHE with some exceptions(Reference Smith, Kellet and Schmerlaib24). We replaced the AGHE's recommended two servings per d of fruit with three servings per d and the number of vegetables consumed per d from five to seven servings to allow for self-reported FFQ over-estimation, as determined by the validity study(Reference Smith, Mitchell and Webb21). The alcohol cut-points reflect guidelines about alcohol consumption in Australia, in which it is recommended that men consume a maximum of two standard drinks per d (20 g of alcohol) and women one standard drink per d (10 g of alcohol)(23).
To follow dietary guideline recommendations as closely as possible, the non-dietary component of the Australian Guide to Healthy Eating, preventing weight gain, was included in the TDS. A maximum score of two points was allocated to this non-dietary component, which contributed to the maximum TDS of 20. Half the score was assigned to energy balance, calculated as the ratio of energy intake to energy expenditure, with a maximum score given for ratios falling between 0·76 and 1·24, defined as the 95 % confidence levels of agreement between energy intake and expenditure(Reference Black25). The other half of the score was assigned to leisure-time physical activity. Details of walking exercise and the performance of moderate or vigorous activities were used to calculate metabolic equivalents(Reference Craig, Marshall and Sjostrom26). Subjects in the highest metabolic equivalents tertile scored one point, reducing to a 0 point score for subjects in the lowest metabolic equivalents tertile(Reference Russell, Flood and Rochtchina12).
Retinal photography
Detailed methods for grading the calibre of retinal arterioles and venules are described elsewhere(Reference Wang, Mitchell and Leung8). In brief, at the baseline examination, 30° photographs of the macula, optic disc and other retinal fields of both eyes were taken, after pupil dilation, using a Zeiss FF3 fundus camera (Zeiss). We used methods developed by the University of Wisconsin–Madison(Reference Hubbard, Brothers and King27) to measure the internal calibre of retinal arterioles and venules from digitised photographs. These were then summarised using established formulas(Reference Sherry, Wang and Rochtchina28) that account for branching patterns and combine individual vessel calibres into summary indices, and are presented as the central retinal artery equivalent or central retinal vein equivalent, representing the mean calibre of these vessels. Arteriole-to-venule ratio was calculated from central retinal artery equivalent and central retinal vein equivalent. Intra- and inter-grader reliability of this method was high(Reference Sherry, Wang and Rochtchina28), with quadratic weighted κ values of 0·85 (central retinal artery equivalent) and 0·90 (central retinal vein equivalent) found for inter-grader reliability and between 0·80 to 0·93 and 0·80 to 0·92 for intra-grader reliability of the two graders, respectively. Vessel diameters for only right eyes were used in the analyses.
Collection of potential confounder information
At face-to-face interviews with trained interviewers, a comprehensive medical history, which included information about demographic factors, socio-economic characteristics and lifestyle factors like smoking, was obtained from all the participants. History of smoking was defined as never, past or current smoking. Current smokers included those who had stopped smoking within the past year. Information on physician-diagnosed history of stroke and CHD were also obtained. BMI was calculated as weight divided by height squared (kg/m2). Blood pressure (BP) was measured using standard auscultatory methods. Mean arterial BP was defined as 0·33 × systolic BP+0·67 × diastolic BP. Hypertension was categorised into stage I (140/90–160/100) and stage II (>160/100 or treated). Fasting blood samples were processed the same day for leucocyte count and glucose. Diabetes was defined either by history or from fasting blood glucose ≥ 7·0 mmol/l.
Statistical analysis
SAS statistical software (SAS Institute, Inc.) version 9.1 was used for analyses, including t tests, χ2 tests and linear regression analyses. Retinal vessel calibre associations with TDS were assessed as continuous variables (per unit increase in TDS) using linear regression models. ANCOVA (general linear model) was used to assess associations between TDS, with adjusted means of retinal arteriolar and venular calibre. We conducted two analyses. First, the cross-sectional association of diet score and retinal vessel calibre was examined in linear regression models and general linear model using baseline data and was initially adjusted for age and sex, and then further adjusted for BMI, mean arterial BP, smoking, serum glucose, leucocyte count and history of diagnosed stroke and CHD for cross-sectional analyses. We then conducted longitudinal analyses that examined baseline dietary intake in relation to retinal vascular calibre measured at the 5-year follow-up as well as 5-year change in retinal vascular calibre, defined as the difference in the calibre measures between baseline and the 5-year follow-up visits and adjusted for age, sex, smoking, mean arterial BP, BMI and baseline retinal vascular calibre. Additionally, to assess the vessel calibre values while avoiding collinearity between arteriolar and venular diameters(Reference Wright29), we adjusted arteriolar diameter for venular diameter, and venular diameter for arteriolar diameter, using the residual method suggested by Willett(Reference Willett and Willett30). We also tested for statistically significant interactions between age and TDS in relation to retinal vascular calibre by adding a product term for age and TDS in the final, multivariable model. Linear regression analyses indicated a marginally significant interaction between age and TDS in association with retinal arteriolar calibre (P interaction= 0·05). Therefore, supplementary analyses were performed in subgroups stratified by age, i.e. age ≤ 65 and >65 years.
Results
Of the 3654 participants examined at BMES-1, 2720 had complete data on retinal vascular calibre and TDS data (Fig. 1). Study participants were, on average, overweight (BMI of 27·6 kg/m2) and 71 % had either stage I or II hypertension (Table 1). Mean TDS at baseline was 9·47 (sd 2·2).
Table 1 Demographic and clinical characteristics of Blue Mountains Eye Study participants included for analyses (Mean values and standard deviations; number of participants and percentages)
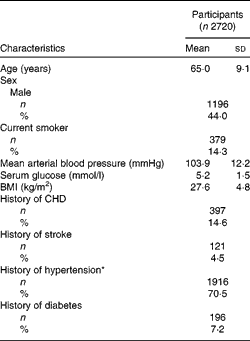
* Hypertension stage I, 140/90–160/100; stage II,>160/100 or treated.
Cross-sectional association between total diet score and retinal vascular calibre
The distribution of nutrient and food group intake by quartiles of TDS is shown in Table 2. Comparing the highest with the lowest quartile of TDS, a significant widening (approximately 3 μm) of mean retinal arteriolar calibre was observed (multivariable-adjusted P trend= 0·0001; Table 3). Conversely, increasing TDS was associated with narrower mean retinal venular calibre (multivariable-adjusted P trend= 0·02; Table 3). After multivariable adjustment, each unit increase in TDS was associated with a 0·54 μm increase in mean retinal arteriolar calibre (P= 0·0001) and a 0·44 μm decrease in mean retinal venular calibre (P= 0·01).
Table 2 Dietary intakes of nutrients and food groups stratified by quartiles of total diet score at baseline among Blue Mountains Eye Study participants in 1992–4 (n 2720) (Mean values and standard deviations)

GI, glycaemic index; GL, glycaemic load.
Table 3 Cross-sectional association between total diet score and retinal vascular calibre among Blue Mountains Eye Study participants in 1992–4 (n 2720) (Adjusted mean values and 95 % confidence intervals)

* Further adjusted for retinal vascular calibre, BMI, smoking, mean arterial blood pressure, serum glucose, leucocyte count and history of diagnosed stroke and CHD.
Among those aged ≤ 65 years, a significant, approximately 4·4 μm widening of mean retinal arteriolar (P trend< 0·0001) and approximately 2·3 μm narrowing of mean retinal venular vessel diameter (P trend= 0·03) was associated with increasing TDS from the lowest to the highest quartiles (Table 4). Non-significant associations were observed among persons aged >65 years (Table 4).
Table 4 Cross-sectional association between total diet score and retinal vascular calibre stratified by age among Blue Mountains Eye Study participants in 1992–4 (n 2720) (Adjusted mean* values and 95 % confidence intervals)

* Adjusted for age, sex, fellow retinal vascular calibre, BMI, smoking, mean arterial blood pressure, serum glucose, leucocyte count and history of diagnosed stroke and CHD.
Longitudinal association between total diet scores and retinal vascular calibre
We analysed the temporal relationship between baseline TDS and retinal vascular calibre measured at the 5-year follow-up (n 1505). Mean TDS at the 5-year follow-up was 9·51 (sd 2·2), and a change of − 0·04 (P= 0·45) in mean TDS was observed over the period of 5 years. Change was normally distributed with sd of 2, and approximately 67 % of the participants had a change in TDS of < 2 units. After adjusting for age, sex, smoking, mean arterial BP, BMI and baseline retinal vascular calibre, baseline TDS were not associated with retinal arteriolar calibre (P trend= 0·89) or venular calibre (P trend= 0·25) measured 5 years later. Additionally, after multivariable adjustment, baseline TDS were not associated with change from baseline to 5-year follow-up examination in retinal arteriolar calibre (P= 0·29) or venular calibre (P= 0·07).
Discussion
The potential for microvascular benefits from a higher overall diet quality among adults has not previously been explored. We found that consumption of a high-quality diet, consistent with the national dietary guidelines for Australian adults, was associated with better retinal microvascular health (wider mean retinal arteriolar calibre and narrower retinal venular diameter). These associations were independent of CVD risk factors (e.g. smoking and BMI) and morbidity (e.g. CHD). Stratified analyses suggest that these associations were stronger in the younger ( ≤ 65 years) than in the older (>65 years) persons. However, baseline TDS was associated neither with retinal vascular calibre measures 5 years later, nor with the 5-year change in retinal vascular calibre.
Retinal vascular calibre changes reflect ageing and cumulative response to CVD and related risk factors, inflammation, NO-dependent endothelial dysfunction and other processes(Reference Sun, Wang and Mackey31). Narrower retinal arteriolar calibre has been found to be associated with older age; higher levels of past, current, and future BP; and obesity, and predicts the incidence of diabetes and CHD(Reference Wong and Mitchell7, Reference McGeechan, Liew and Macaskill10, Reference Liew and Wang32–Reference Gopinath, Baur and Teber37). Wider retinal venular calibre, in contrast, has been found to be associated with impaired fasting glucose and diabetes; dyslipidaemia; obesity; systemic marker of inflammation, endothelial dysfunction and cigarette smoking; and to predict the risk of stroke and CHD(Reference McGeechan, Liew and Macaskill10, Reference Sun, Wang and Mackey31–Reference McGeechan, Liew and Macaskill33, Reference Wang, Liew and Klein35, Reference Gopinath, Baur and Teber37–Reference Wong, Islam and Klein39). The TDS, which encompasses several dimensions of a health-promoting diet, was associated with healthier retinal vessels (wider retinal arteriolar calibre and narrower venular calibre). These data extend our previous findings from the BMES cohort, showing that high fish consumption was associated with better retinal vascular health (wider retinal arterioles and narrower retinal venules)(Reference Kaushik, Wang and Flood4), while high-glycaemic index and low-cereal fibre diets were associated with worse retinal vascular health (wider retinal venular calibre)(Reference Kaushik, Wang and Wong5).
The physiological influence of diet on the retinal microcirculation is likely to be cumulative, long-term and probably complex(Reference Serre and Sasongko6). A recent Korean study(Reference Kim, Yang and Yang13) demonstrated that different food-based diet quality scores were inversely related to biomarkers of oxidative stress in adults. Additionally, Fung et al. (Reference Fung, McCullough and Newby40) showed that higher diet quality scores were associated with reduced endothelial dysfunction. Reduced oxidative stress and improved endothelial function have previously been shown to elicit the dilation of retinal arterioles(Reference Nagaoka, Kuo and Ren41) and could be potential mechanisms that mediate the positive relation between higher diet quality and retinal arteriolar diameter.
Higher diet quality scores, characterised by high intake of fruits, vegetables, whole grains, nuts and fish, were previously shown to be associated with lower concentrations of inflammatory markers such as IL-6 and C-reactive protein(Reference Fung, McCullough and Newby40, Reference Hoebeeck, Rietzschel and Langlois42). The retinal microvasculature is known to be influenced by inflammatory factors(Reference Klein, Klein and Knudtson43), and could be a likely pathway by which diet quality mediates retinal microvascular signs, particularly retinal venular narrowing, in adults with higher diet quality(Reference Klein, Klein and Knudtson43). However, in the present study, adjusting for leucocyte count (an inflammatory marker) in the final, multivariable model only slightly reduced the magnitude of retinal venular narrowing, i.e. approximately 2·5 compared with approximately 2·3 μm. This suggests that other inflammatory markers or another pathway independent of inflammation might mediate the association between diet quality and retinal venular calibre. Further studies are warranted to clarify the underlying causal mechanisms.
Associations between diet quality and retinal vessel diameter were primarily observed among adults aged ≤ 65 years. This observed association was not due to a significant difference in TDS between participants aged ≤ 65 and >65 years, 9·19 v. 9·35, respectively (P= 0·06). We speculate, therefore, that the protective influence of a healthy diet may decline with age due to age-related changes in small vessel walls, leading to rigidity (sclerosis) of small vessels.
We highlight that the differences in arteriolar calibre between the lowest and highest quartiles of TDS were modest (approximately 2–3 μm). Nevertheless, we and others have shown that even such small reductions in retinal arteriolar calibre can be associated with moderate changes in BP, e.g. each 10 mmHg increase in systolic BP was associated with a 1·1 μm reduction in arteriolar calibre(Reference Kaushik, Wang and Wong5, Reference Ikram, de Jong and Vingerling44). The relationship of arteriolar calibre is also graded and small differences in adulthood can translate into meaningful differences in CHD risk(Reference McGeechan, Liew and Macaskill10). Therefore, the present findings could have relevant public health implications, as encouragement of healthy lifestyle choices and nutritional patterns could potentially prevent subclinical changes in the microvasculature and have a role in the reduction of CVD morbidity and mortality risk.
The lack of a longitudinal association between TDS and retinal vascular calibre over a 5-year interval is not completely unexpected. In general, retinal microvascular structure over a lifetime does not change appreciably with the cumulative effects of CVD risk factors; for instance, BP measured 9 years ago was associated with narrower retinal arterioles, independent of current BP levels in adults(Reference Wong, Hubbard and Klein45). This suggests that the observed cross-sectional association between diet quality and retinal vascular calibre is likely to represent the cumulative beneficial effects of a healthy diet rather than a single ‘snapshot’ of the current diet. Moreover, the FFQ is limited as it only measures diet at one time and not over a lifetime; however, older adults often have established eating patterns that remain constant over time(Reference Cade, Burley and Warm46), which further supports our hypothesis on the cumulative effects of a healthy diet on the retinal microvasculature. Second, the follow-up period of 5 years may not be sufficiently long enough to observe any sustained effects of diet quality on the retinal microvasculature. This notion agrees with other population-based studies, which did not find significant associations between diet quality and 4-year all-circulatory disease mortality(Reference Seymour, Calle and Flagg47) and risk of ischaemic and haemorrhagic strokes(Reference Fung, Rexrode and Mantzoros48). Third, the lack of a prospective link could be due to a low level of compliance to dietary guidelines in our cohort. Our earlier study(Reference Russell, Flood and Rochtchina12) showed poor compliance with dietary guidelines in the BMES cohort, with the maximum score reaching approximately 75 % compliance to dietary guidelines, which is consistent with other findings in the Australian population(Reference McNaughton, Ball and Crawford49). Therefore, if overall diets were improved, we might have observed an appreciable influence of diet quality on the retinal microcirculation. Finally, the dietary guidelines for Australian adults (used to construct the TDS) were developed to provide guidelines for diet targeting overall health and well-being, but they are not disease specific and may provide protective benefits for some diseases and not others(Reference Ibiebele, Parekh and Mallitt50). Hence, the non-significant temporal findings observed in the present study do not necessarily suggest that a healthy dietary pattern is ineffective in preventing retinal microvascular disease, but rather a different definition of a healthy diet may be needed to achieve optimal effects(Reference Zamora, Gordon-Larsen and Jacobs14, Reference McCullough, Feskanich and Stampfer51).
Strengths of the present study include its population-based sample, use of a validated food questionnaire to collect dietary data, availability of rich confounder information and the quantitative computer-assisted measurement of retinal vessel diameters from digitised fundus photographs. Limitations using FFQ for self-reported dietary intake can underestimate energy intake(Reference Cade, Burley and Warm46) or overestimate fruit, vegetable and dairy product intakes(Reference Ibiebele, Parekh and Mallitt50). However, a comprehensive assessment of the whole diet is less subject to measurement error than is the assessment of energy intake alone(Reference Zamora, Gordon-Larsen and Jacobs14, Reference Schatzkin, Kipnis and Carroll52). That is because even when people under- or over-estimate the total amount they consume, the ratios of the foods that they self-report are still likely to be reflective of actual consumption(Reference Zamora, Gordon-Larsen and Jacobs14). An additional limitation is the assumption that the dietary guidelines used to define diet quality indices are based on the best-available scientific knowledge, which may not necessarily be correct, as it is difficult to keep the dietary guidelines up to date(Reference Michels and Schulze53). Finally, we cannot exclude the possibility of residual confounding from unmeasured lifestyle (e.g. sedentary behaviours) or societal parameters that could have influenced observed associations with the retinal vascular structure.
In summary, we found that diet quality, measured with a TDS reflecting present intake guidelines, could influence the retinal microvascular health of older adults, with better diet quality associated with better retinal microvascular health (wider retinal arterioles and narrower venules). Additional research is warranted to assess whether adherence to dietary guidelines is associated with the retinal microvascular structure in the longer term, and the possible public health and clinical implications of such relationships.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512005491
Acknowledgements
The authors' contributions were as follows: B. G. and P. M. aided in study concept and design; P. M. carried out acquisition of data; analysis and interpretation of data was done by B. G., E. R., J. J. W., T. Y. W. and P. M.; drafting of the manuscript was done by B. G. and P. M.; B. G., V. M. F., E. R., J. J. W., T. Y. W. and P. M. carried out critical revision of the manuscript. We would like to acknowledge Joanna Russell who also contributed to the development of the TDS. The authors declare no conflict of interest. Sources of funding are as follows: The BMES was supported by the Australian National Health and Medical Research Council (grant no. 974159, 991407, 211069 and 262120) and Westmead Millennium Institute. This work was also supported by the Meat and Livestock Australia. B. G. is supported by a National Health and Medical Research Council Centre for Clinical Research Excellence grant (grant no. 529923).







