Altering the frequency and timing of meal consumption is often suggested as a weight loss strategy(Reference Schoenfeld, Aragon and Krieger1). It is hypothesised that consumption habits influence cardiometabolic health partly through a circadian mechanism, as food intake is known to entrain peripheral circadian oscillators(Reference Oosterman, Kalsbeek and la Fleur2). The oscillators represent a feedback loop of circadian-related gene expression and proteins, which have downstream metabolic effects that influence weight status(Reference Kumar Jha, Challet and Kalsbeek3–Reference Scott, Carter and Grant5). Breakfast consumption may be particularly important in entraining circadian oscillators compared with other meal times. Animal studies have shown that the first meal of the day determines the circadian phases of peripheral clocks, possibly because it is the first meal following a prolonged overnight fast(Reference Hirao, Nagahama and Tsuboi6,Reference Wu, Sun and ZhuGe7) . Further, evidence from human research has indicated that infrequent breakfast consumption is associated with poor weight status and indicators of cardiometabolic risk(Reference Ma, Bertone and Stanek8–Reference St-Onge, Ard and Baskin14).
In addition to behaviours related to the consumption or omission of meals, the timing of food intake may have important metabolic effects. There is evidence that eating late at night or consuming a higher proportion of energy later in the day has adverse metabolic consequences(Reference Wang, Patterson and Ang15–Reference Gallant, Lundgren and Drapeau17), though results from human observational studies are limited. Regularity in the day-to-day timing of consumption behaviours may be important. Circadian misalignment from irregular sleep patterns due to shift work or social jetlag (i.e. when people have different sleep patterns on weekends compared with weekdays) results in poor weight status(Reference Depner, Stothard and Wright18). Therefore, it is plausible that de-synchronisation of these rhythms from irregular meal consumption patterns may have similar unfavourable consequences.
Research on meal timing is difficult to investigate in free-living populations because 24-h diet recalls or dietary records are needed over multiple time points to accurately classify these complex exposures. The Cancer Prevention Study-3 (CPS-3) Diet Assessment Sub-study (DAS) presents a unique opportunity with up to six 24-h recalls collected per participant over a 1-year period. Based on the established relationships between food intake, circadian rhythms and metabolic health, we used information collected in the CPS-3 DAS to investigate breakfast consumption and food intake timing behaviours in relation to body weight status and waist circumference (WC). We also examined food intake behaviours and their association with an inflammatory biomarker, as chronic low-grade inflammation can be a consequence of circadian misalignment(Reference Leproult, Holmback and Van Cauter19). It was hypothesised that irregular consumption patterns (i.e. inconsistent breakfast consumption and timing of intake) and consumption habits occurring later in the day would be associated with higher weight status and inflammation.
Methods
Study population
Participants were drawn from CPS-3, a prospective cohort of 303 682 men and women enrolled between 2006 and 2013 from thirty-five states and Puerto Rico(Reference Patel, Jacobs and Dudas20). The CPS-3 DAS was designed to test the validity and reproducibility of the CPS-3 modified FFQ. Before completing the 2015 follow-up survey, invitations to preregister for the CPS-3 DAS were mailed to 10 000 CPS-3 participants living in five different geographical regions of the USA, among whom 1801 participants expressed willingness to participate. Of these, 866 (48 %) were preliminarily enrolled in the DAS after they returned the CPS-3 2015 survey. The survey contained separate sections for specific questions on demographics, medical history, sleeping habits, smoking and tobacco use and physical activity, which were evaluated using a validated instrument. Participants provided self-reported race/ethnicity during enrolment into the CPS-3(Reference Patel, Jacobs and Dudas20). DAS participants were asked to complete six 24-h recalls approximately 2 months apart (four on week days, two on weekends), to provide two fasting blood and urine samples 6 months apart at a Quest Diagnostics Inc. Patient Services Center, and to complete a follow-up FFQ at the end of the 1-year study period. Among the 866 participants initially enrolled in the DAS, exclusions were made for the present analysis if participants did not respond to inquiries regarding the availability for 24-h recall telephone interviews (n 47), moved outside of the five study regions (n 4), did not complete at least one recall (n 146), had missing outcome data (n 3), reported shift work (n 20) or had an implausible BMI (<15 or >50 kg/m2; n 2), bringing the final analytic cohort to 644 men and women. Participants in the present analytic cohort were similar to CPS-3 cohort participants with respect to age, BMI and prevalence of healthy behaviours, though there were more men and they were slightly more racially/ethnically diverse (due to oversampling) and had a higher proportion of graduate degrees. All aspects of CPS-3 were approved by the institutional review board at Emory University.
Breakfast consumption and meal timing definitions
The 24-h recall interviews were conducted via telephone and included detailed questions about everything the participant had to eat and drink over the previous 24-h period. The participants were queried for food preparation, portion size and time and place of consumption. They were also asked to name the eating occasion with one of the following: breakfast, brunch, lunch, dinner, snack, beverage only and other. If a participant reported consumption of breakfast or brunch, regardless of the time, they were considered to have consumed breakfast that day.
The meal timing exposures included the time of first intake, duration of intake window (i.e. time between the first intake and the last intake of the day) and midpoint of intake window to the nearest minute. We chose these measures of meal timing because the first intake of the day has been implicated in setting the phase of circadian clocks(Reference Hirao, Nagahama and Tsuboi6,Reference Wu, Sun and ZhuGe7) , and the midpoint of the daily intake window indicates whether consumption habits are typically centred around an earlier or a later time in the day. The duration of the intake window provides insight to overnight fasting, which may be associated with weight status(Reference Halberg, Henriksen and Soderhamn21). Eating occasions in which at least 210 kJ were consumed were used to identify the first and last intake of the day. Using the 210 kJ criterion has been suggested to define an eating occasion because it best predicts variation in total energy intake(Reference Leech, Worsley and Timperio22). Caffeine has been shown to have circadian effects(Reference Burke, Markwald and McHill23); therefore, coffee or tea consumption was also included as the first or last intake time, regardless of the energy content. The average time of all six 24-h recalls was taken for first intake, intake window and midpoint of the window to see whether increasing times (i.e. later in the day or longer window) were associated with weight status and inflammation. To determine whether an irregular pattern in meal timing behaviours is associated with the outcomes, we calculated the intra-individual standard deviation (isd) in each exposure across the six recalls for each participant. Accordingly, individuals with a high isd in the behaviour exhibit an irregular consumption timing pattern.
Weight status
Participants were asked to report their body weight and were mailed a tape measure with instructions to measure their WC at two time points during the study, coinciding with the times of urine and fasting blood sample collection. Participants were instructed to measure WC over only a single layer of clothes at the navel for men and just above the navel for women. Self-reported height collected from the baseline CPS-3 survey was used with the DAS self-reported weights to calculate BMI (kg/m2). To reduce variation from measurement error, the average of the two reports for BMI and WC was used in cross-sectional analyses. BMI was categorised as overweight (25·0–29·9 kg/m2) and obese (≥30·0 kg/m2) outcomes(24). WC was dichotomised as having visceral obesity in women if ≥88 cm and in men if ≥102 cm(25).
Pro-inflammatory glycoprotein acetyls
Two fasting blood samples were collected by a trained, certified phlebotomist from Quest Diagnostics, Inc., approximately 6 months apart. A total of 40 ml of blood was collected and transported to a Quest Diagnostics regional processing laboratory for specimen aliquoting and initially frozen at −80°C. Specimen aliquots were then shipped on dry ice to a central repository at Fisher BioServices, Inc. where they are stored long-term in liquid N2 vapour phase. A high-throughput proton NMR metabolomics platform was used to quantify serum concentrations of glycoprotein acetyls (GlycA) at Nightingale Health Ltd laboratories(Reference Soininen, Kangas and Wurtz26). It is recognised that protein glycosylation affects the innate immune system, particularly in the form of inflammatory responses(Reference Gornik and Lauc27). It has been established that GlycA strongly correlates with high-sensitivity C-reactive protein, thus making it a good marker of low-grade systemic inflammation(Reference Otvos, Shalaurova and Wolak-Dinsmore28,Reference Akinkuolie, Buring and Ridker29) . The average of the two GlycA measurements was used for this analysis.
Statistical approach
Logistic and linear regression models were used in cross-sectional analyses of breakfast consumption and food intake timing behaviours in relation to weight status and GlycA. Individuals who reported consumption of breakfast on all six 24-h recalls were the referent group in models with breakfast consumption as the exposure. A small proportion of participants reported consuming breakfast on less than three recalls (n 40), so they were combined into a single group. The individual average and isd in intake timing behaviours were treated as continuous exposures, with OR and betas (β) reported for a 1-h later intake time (average) or 1-h increase (for isd) in each exposure. Polytomous logistic regression was used for categorical BMI outcomes with the OR and 95 % CI reported for overweight and obese, respectively, compared with normal weight. Standard logistic regression was used for the categorical WC outcome. Multiple linear regression models were used for continuous outcomes of BMI, WC and GlycA, with β and corresponding 95 % CI reported. Results from models with GlycA were reported for a 1-sd increase (sd = 0·20 mmol/l) in the marker. Potential covariates were chosen from a priori knowledge and through the use of directed acyclic graphs (DAG). Those potential covariates that changed the point estimates by greater than 10 % were included in the final models. All models included adjustment for age (years), sex (male; female), daily energy intake (kJ/d), diet quality (Healthy Eating Index-2015)(Reference Krebs-Smith, Pannucci and Subar30), sleep duration (average sleep hours), moderate-vigorous physical activity (metabolic equivalent of task (MET)-hours/week), race/ethnicity (White, Black, Hispanic), employment status (full-time, part-time, unemployed, retired, other), smoking status (current, former, never, missing) and alcohol consumption (abstainer, ≤1 drink/d for women and ≤2 for men, >1 drink/d for women and >2 for men); with additional adjustment for BMI (kg/m2) in models with GlycA as the outcome. Diet quality was assessed using the Healthy Eating Index-2015, which quantifies the concordance of an individual’s diet with the 2015–2020 Dietary Guidelines for Americans and is comprised of adequacy components for healthy foods and moderation components for nutrients(Reference Krebs-Smith, Pannucci and Subar30).
Multiple sensitivity analyses were performed. Food intake timing exposures were evaluated without inclusion of coffee or tea consumption to see whether results changed substantially. In a separate analysis, we calculated a weighted average of the six recalls, so that responses more accurately characterise the 5 week days and 2 weekend days that make up a week. Furthermore, individuals who reported participation in a weight loss diet over the past year on the post-FFQ (n 44) were excluded in an additional sensitivity analysis as their responses may reflect reverse causation. We additionally included models of WC outcomes with and without adjustment for BMI to examine whether any of the meal timing behaviours were associated with central adiposity independent of general adiposity. The mean and isd of first intake were included as covariates in models of mean and isd intake window and midpoint of intake window in separate sensitivity analyses as they may be important confounders in models of weight status and inflammation. Lastly, there may be some degree of misreporting of dietary intake in the study population and that misreporting of energy intake may correlate with misreporting of eating occasions. To evaluate the potential of bias from misreporting, we calculated the ratio of expected to observed energy intake using the revised Goldberg equation(Reference Black31), with suggested physical activity level multipliers from the WHO(Reference Schofield32) to account for different energy needs among individuals with inactive (multiplier of 1·21 for both sexes), low (multiplier of 1·55 for men, 1·56 for women), medium (1·78 for men, 1·64 for women) and high (2·10 for men, 1·83 for women) moderate-vigorous physical activity levels. Physical activity levels were estimated from the responses to the CPS-3 2015 survey questions on physical activity(Reference Rees-Punia, Matthews and Evans33). The Pearson correlations for the ratio with BMI and our meal timing exposures were subsequently examined.
Results
Descriptive characteristics stratified by breakfast consumption frequency are shown in Table 1. Over half of the participants (n 399) reported breakfast consumption on all six 24-h recalls. Participants who consumed breakfast on all 6 d were typically older, consumed the most energy but had the highest diet quality (as measured by the Healthy Eating Index-2015), were most active, had higher BMI, more likely to be White, work full-time and never smoke compared with those who reported skipping breakfast at least 1 d. Those who reported breakfast consumption on 3 d or less had the lowest energy intake, lowest dietary quality, lowest BMI and the highest proportion of alcohol abstainers. Centre and dispersion statistics for the meal timing exposures can be seen in Table 2. The mean time at first intake was 07.58 hours. The average length of the intake window was 11 h and 54 min, with an average midpoint of the intake window occurring at 13.55 hours. The mean isd of time at first intake, length of intake window and midpoint of intake window was 1 h and 8 min, 1 h and 43 min and 54 min, respectively.
Table 1. Descriptive characteristics of Cancer Prevention Study-3 Diet Assessment Sub-study stratified by breakfast consumption frequency
(Mean values and standard deviations; frequencies and percentages)
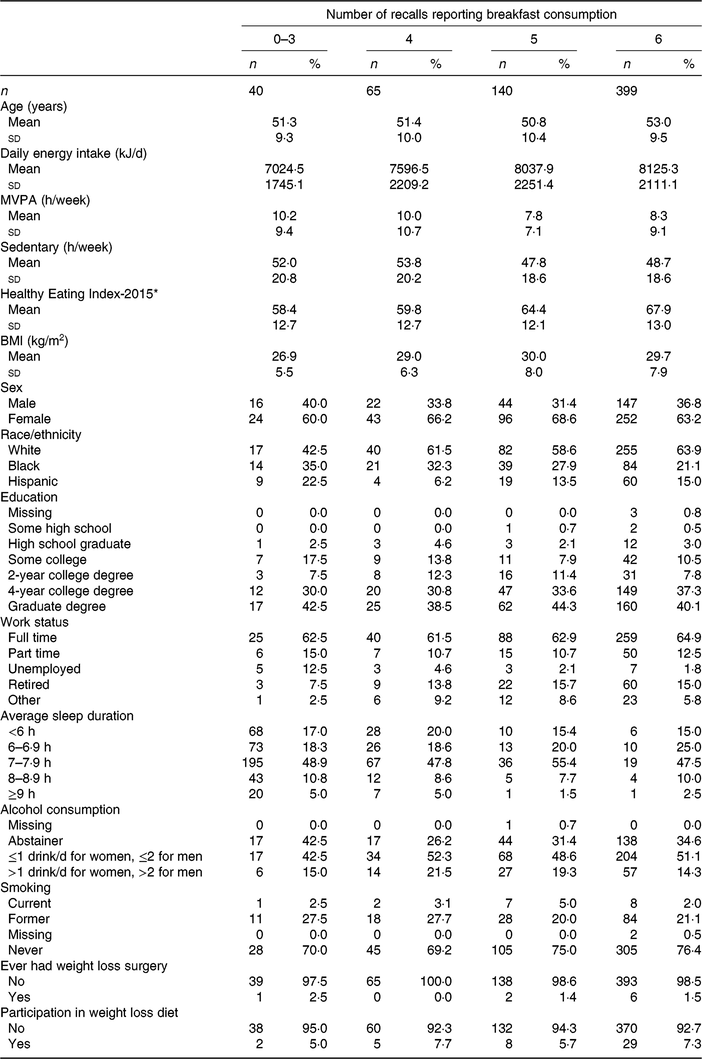
MVPA, moderate-vigorous physical activity.
* Krebs–Smith et al.(Reference Krebs-Smith, Pannucci and Subar30).
Table 2. Descriptive statistics for meal timing exposures (24-h:min) (Mean values and standard deviations; minimum and maximum values; percentiles)
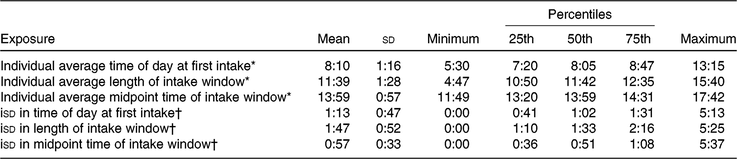
* Average of each individual’s exposure across all six 24-h recalls.
† Intra-individual standard deviation (isd) in exposure across all six 24-h recalls.
There was a similar prevalence of overweight (30·7 %) and obesity (29·5 %) as defined by BMI. Results from models with BMI are shown in Table 3. Polytomous logistic regression models showed that, compared with individuals who consumed breakfast on every day, those who consumed it on 5 d had 2·60 (95 % CI 1·57, 4·31) and 1·86 (95 % CI 1·07, 3·24) greater odds of being overweight and obese, respectively. In linear regression models, individuals who consumed breakfast on 5 or 4 of the days had a 1·29 (95 % CI 0·19, 2·38) and 1·64 (95 % CI 0·12, 3·16) kg/m2 higher BMI than those who consumed breakfast on all 6 d. A non-significant inverse association was seen among participants who consumed breakfast less than or equal to 3 d. None of the average meal timing exposures was associated with the categorical BMI outcomes, but a 1-h later average time of first intake was associated with a 0·44 (95 % CI 0·04, 0·84) kg/m2 higher BMI. Further, a 1-h increase in the isd of first intake, length of intake window and midpoint of intake window was significantly associated with a 1·12 (95 % CI 0·49, 1·75), 0·70 (95 % CI 0·15, 1·25) and 0·97 (95 % CI 0·11, 1·83) kg/m2 higher BMI. However, polytomous logistic regression models showed no statistically significant associations.
Table 3. Breakfast consumption and intake timing behaviours in relation to weight status measured by BMI* (Odds ratios and 95 % confidence intervals; β-coefficients and 95 % confidence intervals)
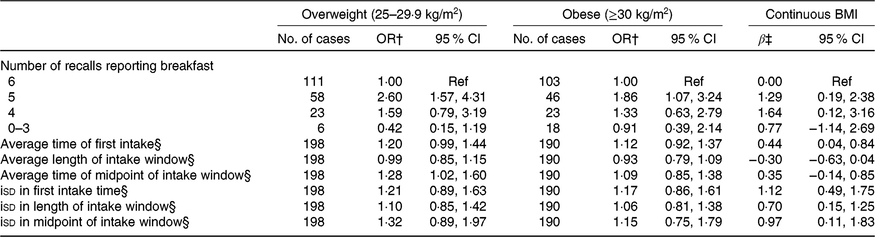
Ref, reference; iSD, intra-individual standard deviation.
* All models include adjustment for age, sex, total energy intake, Healthy Eating Index-2015, sleep duration, physical activity, race, employment status, smoking status and alcohol consumption.
† OR and corresponding 95 % CI from polytomous logistic regression are reported.
‡ Regression coefficients and corresponding 95 % CI from multiple linear regression are reported.
§ OR and regression coefficients are for a 1-h increase in exposure (average time or isd).
The prevalence of obesity was substantially larger when using WC criterion (43·6 %) compared with BMI. Using WC to define central adiposity (Table 4) resulted in no statistically significant associations from logistic or linear regression models with breakfast consumption frequency as the exposure. A 1-h longer average length of intake window was associated with a 0·98 (95 % CI −1·79, −0·16) cm lower WC. Similar to BMI, we saw a 1·57 (95 % CI 0·01, 3·13) cm higher WC for a 1-h increase in isd of first intake time, but not for any other meal timing regularity exposures. However, these statistically significant results were attenuated towards the null and no longer met the significance threshold after additional covariate adjustment for BMI (online Supplementary Table S1).
Table 4. Breakfast consumption and intake timing behaviours in relation to weight status measured by waist circumference* (Odds ratios and 95 % confidence intervals; β-coefficients and 95 % confidence intervals)

Ref, reference; iSD, intra-individual standard deviation.
* All models include adjustment for age, sex, total energy intake, Healthy Eating Index-2015, sleep duration, physical activity, race, employment status, smoking status, and alcohol consumption.
† OR and corresponding 95 % CI from logistic regression are reported.
‡ Regression coefficients and corresponding 95 % CI from multiple linear regression are reported.
§ OR and regression coefficients are for a 1-h increase in exposure (average time or isd).
Individuals who ate breakfast on 5 d had higher GlycA levels (β: 0·21; 95 % CI 0·03, 0·40) compared with those who consumed breakfast on all 6 d, but no other exposure groups showed statistically significant associations (Table 5). Results with GlycA corroborated WC results of an association for increasing length of intake window, as a 1-h longer window was associated with lower levels of GlycA (β: −0·07; 95 % CI −0·13, −0·02). A 1-h increase in isd for first intake time (β: 0·15; 95 % CI 0·04, 0·26) and midpoint of intake window (β: 0·16; 95 % CI 0·02, 0·31) were associated with higher GlycA, but no association was observed of isd in length of intake window.
Table 5. Breakfast consumption and intake timing behaviours in relation to pro-inflammatory glycoprotein acetyl (GlycA)* (β-Coefficients and 95 % confidence intervals)
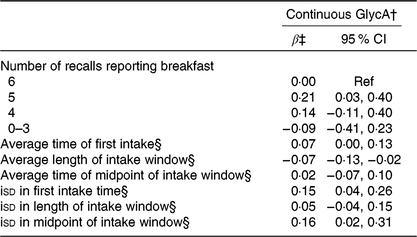
Ref, reference; iSD, intra-individual standard deviation.
* All models include adjustment for age, sex, total energy intake, Healthy Eating Index-2015, BMI, sleep duration, physical activity, race, employment status, smoking status and alcohol consumption.
† GlycA standardised to have a sd of 1.
‡ Regression coefficients and corresponding 95 % CI from multiple linear regression are reported.
§ Regression coefficients are for a 1-h increase in exposure (average time or isd).
All null associations remained null when excluding coffee and tea for defining intake timing exposures in a sensitivity analysis, and regularity in intake timing exposures remained statistically significant but were slightly attenuated toward the null (online Supplementary Table S2). Results were slightly attenuated but remained significant when using a weighted average of the days to represent a full week (online Supplementary Table S3). No substantive changes in our estimates for the associations between consumption timing and weight status or inflammation were observed when excluding individuals who self-reported participation in a weight loss diet (online Supplementary Table S4). Interestingly, no results remained statistically significant after adjustment for first intake timing, with point estimates sometimes crossing the null value, except for the estimate for average length of intake window with GlycA, which remained statistically significant (online Supplementary Table S5). The ratio of expected to observed energy intake did not correlate with BMI or any of our meal timing exposures (online Supplementary Table S6).
Discussion
In this cross-sectional analysis of free-living adults, we observed some evidence that breakfast consumption and intake timing behaviours are related to BMI and the inflammatory marker, GlycA. More specifically, participants who consumed breakfast on 4 or 5 d had higher BMI than those who consumed breakfast on all 6 d. No association was seen in individuals who consumed breakfast on 3 or fewer days, perhaps due to a small number of participants in that group (n 40). A 1-h later time in the day’s first intake was associated with a higher BMI, but not WC, and no associations were observed for a 1-h later average midpoint of the intake window. Interestingly, a 1-h longer time in the average length of intake window was associated with lower WC and inflammation, though the association with WC was not independent of the first intake time. With respect to regularity in intake timing behaviours, as measured by a higher isd, results showed that irregular patterns were individually associated with higher BMI and heightened levels of inflammation in continuous models. However, none was significant when accounting for regularity in first intake time. No statistically significant associations were observed for these relationships with categorical outcomes, but point estimates were above the null value and it may have been a result of limited power. Results from many, but not all, of our models suggest regularity in meal behaviours may be related to weight status and systemic inflammation, particularly for the first intake of the day as they often impact consumption habits(Reference Yoshimura, Hatamoto and Yonekura34) and metabolic responses to food later in the day(Reference Astbury, Taylor and Macdonald35).
Our results for breakfast consumption frequency and weight status are consistent with the previously published studies showing that breakfast skipping may be associated with heavier weight status(Reference Ma, Bertone and Stanek8–Reference Horikawa, Kodama and Yachi10,Reference van der Heijden, Hu and Rimm12,Reference Song, Chun and Obayashi36–Reference Watanabe, Saito and Henmi38) . We did not observe significant associations among the frequent breakfast skippers but power was severely limited in those groups. A recent study of women showed that regular omission of breakfast may be inversely associated with higher weight status if it is skipped every day, as would be consistent with our hypothesis of regularity in promoting better circadian health, but we were unable to investigate regular omission as only three participants reported skipping breakfast on all six 24-h recalls.
Meal timing behaviours that occurred later in the day were generally not associated with any of the outcomes. To our knowledge, no study has investigated the effects of a later first intake or midpoint of intake window in relation to weight status. One small human trial showed that shifting meals to later in the day caused a delay in the phase of peripherally controlled circadian glucose rhythms, but no changes were observed in measures of the central circadian clock phase and weight was not assessed(Reference Wehrens, Christou and Isherwood39). The authors hypothesised that a change in the peripheral, but not central, clocks indicates circadian misalignment caused by the later meal times.
It is unclear why a longer duration of intake window was inversely associated with WC and GlycA, the latter of the two remaining significant independent of the first intake time. We expected a longer intake window, indicating a shorter overnight fast, to be positively associated with weight and inflammation. Evidence from animal studies has shown that an increasing fasting period (such an overnight fast) followed by a time-restricted feeding regimen is associated with weight loss and improved metabolic health, but human studies have been inconsistent(Reference Rothschild, Hoddy and Jambazian40). Much of the published research in humans was conducted during Ramadan in which the intake occurs during the night, thus more research is needed in the field of time-restricted feeding during normal hours.
The most consistent relationships in the present analysis were observed for exposures related to irregular food intake patterns, as measured by isd. More specifically, irregularities in the time of first intake and in the midpoint of the intake window were associated with higher weight status and inflammation in continuous models; there was low power in categorical models to identify statistically significant associations. Results of irregularity in midpoint of the intake window became null after accounting for irregularity in first intake time, which is backed by mechanistic studies showing the importance of the day’s first intake as it relates to metabolism(Reference Hirao, Nagahama and Tsuboi6,Reference Wu, Sun and ZhuGe7,Reference Yoshimura, Hatamoto and Yonekura34) . To our knowledge, there has only been one other published study to investigate regularity in food intake timing behaviours(Reference Zimmerman, Johnson and Brunstrom41). In the cross-sectional study, Zimmerman et al.(Reference Zimmerman, Johnson and Brunstrom41) defined irregular meal timing through a ‘chaotic eating index’ which characterises the number of 30-min time intervals in which a participant eats divided by the number of eating episodes. The index was not associated with BMI in two separate study populations. In one of the populations in which multiple diet records were available, the variation in the time intervals between meals was not related to BMI. It is possible the authors did not observe any associations because they did not characterise their exposures to relate to potential circadian mechanisms, but rather attempted to capture the overall variability in possible consumption times through an index that has yet to be validated. In doing so, the concepts related to food intake timing that have been shown to influence circadian phase (i.e. first intake or overnight fast) may not have been adequately classified, as was the objective herein.
The established impact of food intake on circadian rhythms served as the basis for our hypothesis and is also a plausible mechanism to explain the observed associations. There is sufficient evidence that food intake entrains peripheral circadian oscillators thus potentially inducing misalignment between central and peripheral clocks(Reference Oosterman, Kalsbeek and la Fleur2). Misalignment occurs when inputs from peripheral circadian oscillators operate in rhythms that are out of sync from the central oscillators. Further, it is known that circadian misalignment can lead to adipocyte dysfunction, increased ectopic fat and systemic inflammation, all of which are related to obesity(Reference Golombek, Casiraghi and Agostino42). It is also possible that irregular food intake timing patterns result in subsequent behaviours that influence weight or poor cardiometabolic health. For instance, research has shown that high variability in timing may be associated with overeating or snacking on nutrient-deficient foods(Reference Bellisle43). However, the associations observed in the present study between irregularity in meal timing and weight status were independent of diet quality and energy intake.
There are limitations in the present study that must be noted. Foremost, our exposure and outcome data were self-reported and may contain bias. We would expect individuals who are overweight to underreport their weight. There is also evidence that individuals who are overweight tend to underreport meals(Reference Bellisle, McDevitt and Prentice44). However, we found no evidence that misreporting of dietary intake (defined as expected/observed energy intake) for 24-h recalls was associated with our exposures or BMI; thus, we suspect that underreporting of BMI may have attenuated our results towards the null. Detailed information on sleep and circadian phase was not available concurrently with the dietary recalls; therefore, making it difficult to rule out confounding. Average sleep duration was included as a variable in all models, but residual confounding may be present as we were unable to account for regularity in sleep patterns which may be related to both intake time and weight status(Reference Depner, Stothard and Wright18). Future studies of food intake regularity should take into account sleep and wake time. Due to the cross-sectional design, it is possible that reverse causation may be biasing our results. However, results remained similar when excluding individuals who reported participation in a weight loss diet during the study period, as those participants may have reported their exposure level because of their poor weight status. Sample size limited our power to examine differences across strata, such as sex. Although there are inherent limitations to observational research, results of the present study are potentially more generalisable than some trials that take place in rigid and tightly controlled settings making it difficult to translate to free-living populations. Other strengths of the analysis include multiple criteria to define weight status and inclusion of a novel biomarker for chronic low-grade inflammation. We also defined irregularity in meal behaviours in a novel way that has been effective in identifying associations with cardiometabolic disease in the literature pertaining to irregular sleep(Reference Bei, Wiley and Trinder45).
In conclusion, the results of the present analysis provide evidence that irregular breakfast consumption and intake timing patterns may be related to weight status and inflammation. Specifically, behaviours pertaining to the first intake of the day seem to be most relevant to weight status and systemic inflammation. More research is required in larger prospective studies with information on sleep and wake time. Further, mechanistic work is needed to support, or refute, biological plausibility.
Acknowledgements
M. A. G. was supported by the American Cancer Society’s Cancer Prevention Studies Post-Doctoral Fellowship Program. All other authors were supported by American Cancer Society funds for the creation, maintenance and updating of the Cancer Prevention Study-3 cohort. We sincerely appreciate all Cancer Prevention Study-3 participants and each member of the study and bio-specimen management group.
All authors contributed to the development of the research questions, study design and writing of the article; M. A. G. conducted the analysis.
The authors report no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519002125








