Micronutrient deficiencies are widespread in many low-income countries( Reference Ramakrishnan 1 ), especially among infants and children under 5 years of age. The most common micronutrient deficiencies are vitamin A, Fe, and Zn( Reference Ramakrishnan 1 , Reference Bailey, West and Black 2 ), although Se deficiency is an increasing concern( Reference Mutakin and Setiawan 3 ). Deficiency of these four micronutrients compromise the immune system( Reference Gibson 4 , Reference Raiten, Sakr Ashour and Ross 5 ), resulting in increases in morbidity and mortality during early childhood. Fe and Zn deficiency are also associated with an increased risk of impaired growth and cognition( Reference King, Brown and Gibson 6 – Reference Sandstead 8 ).
Micronutrient biomarkers in serum can be used to assess status of these four micronutrients. However the presence of inflammation or infection confounds their assessment( Reference Raiten, Sakr Ashour and Ross 5 , Reference Thurnham, McCabe and Northrop-Clewes 9 – Reference Brown, Lanata and Yuen 11 ). Consequently, the resulting deficiency prevalence estimates may not reflect the true burden unless inflammation or infection has been taken into account.
During the acute phase response initiated by inflammatory cytokines, the hepatic synthesis of several proteins, termed acute phase proteins (APP), increases( Reference Raiten, Sakr Ashour and Ross 5 ). Some of these APP respond to the acute phase response by increases in circulating plasma levels (i.e., positive APP) such as ferritin, and others (e.g. retinol-binding protein (RBP), Zn and selenoprotein P) by decreases (i.e. negative APP). Some APP, notably C-reactive protein (CRP) and α-1-glycoprotein (AGP), are recommended as inflammatory biomarkers by the World Health Organization( 12 ), providing a measure of the severity and duration of inflammation, respectively( Reference Raiten, Sakr Ashour and Ross 5 ).
In the past, estimates of micronutrient deficiencies have often been based on excluding participants with an elevated CRP and/or AGP concentration( Reference Thurnham, McCabe and Haldar 13 ). However, such an approach may reduce the sample size and/or introduce a sampling bias in low-income settings where the burden of inflammation is often high( Reference Raiten, Sakr Ashour and Ross 5 ). As a result, several approaches to adjust the concentrations of micronutrient biomarkers affected by the inflammatory response have been developed( Reference Thurnham, McCabe and Northrop-Clewes 9 , Reference Thurnham, McCabe and Haldar 13 – Reference Cichon, Ritz and Fabiansen 17 ). Recently a new approach involving regression modelling has been recommended by the Biomarkers Reflecting Inflammation and Nutritional Determinants Anemia (BRINDA) Project in which the inflammatory biomarkers (CRP and AGP) are treated as continuous variables so that greater corrections can be applied when the inflammatory biomarkers indicate severe inflammation( Reference Suchdev, Namaste and Aaron 18 – Reference Namaste, Aaron and Varadhan 22 ). This regression approach is in contrast to the earlier adjustment method of Thurnham et al.( Reference Thurnham, McCabe and Northrop-Clewes 9 ) which applies specific cutoffs for elevated levels of serum CRP and AGP, irrespective of the magnitude of inflammation.
In this study we aimed to: (a) characterise biomarkers for Fe, Zn, vitamin A, and Se status for a cohort of infants aged 6, 9 and 12 months of age from Sumedang District, Indonesia after adjustment for inflammation using the new standardised BRINDA approach; (b) determine the prevalence of micronutrient deficiencies after the BRINDA adjustment for inflammation, (c) determine trends in biomarkers and prevalence of deficiencies between the ages of 6 months to 12 months, and (d) present these prevalence estimates at each age with those generated using the earlier Thurnham inflammation adjustment method. Prevalence estimates for Fe deficiency were based on serum ferritin or soluble transferrin receptor (sTfR), whereas for deficiencies of vitamin A, Zn and Se, serum RBP, Zn and Se, respectively were used.
Methods
Study design and participants
The study was a prospective, longitudinal design to evaluate micronutrient status and growth of breast-fed Indonesian infants. Infants were enrolled at 6 months and followed-up at 9 and 12 months of age from August 2014 to August 2015. Infants were randomly selected from all thirty villages in three sub-districts of Sumedang district, West Java, using local birth registry data. Only infants who were not premature (>37 weeks gestation), ≥1500 g at birth, and apparently healthy with no evidence of chronic disease or acute malnutrition were recruited in the study( Reference Diana, Mallard and Haszard 23 ). Pre-tested interviewer-administered questionnaires were used to collect information on the socio-economic, health and morbidity status of the infants. The study was conducted according to the Declaration of Helsinki guidelines for the ethical conduct of medical research involving children( Reference Hull 24 ) and all procedures involving human respondents were approved by the Human Ethics Committees of Padjadjaran University, Indonesia and the University of Otago, New Zealand. Written informed consent was obtained from all parents of respondents.
Blood collection and processing
Experienced phlebotomists collected morning non-fasting venepuncture blood samples from the infants. Blood was drawn into a trace-element-(TE)-free evacuated tube following the International Zinc Nutrition Consultative Group (IZiNCG) procedures( Reference Brown and Rivera 25 ) and one containing EDTA as an anticoagulant (Becton Dickinson). Symptoms of infection were also recorded. Time of blood collection and time of the last meal were recorded before immediate transfer of the blood samples to a chilled container (−4°C) for transport to the base laboratory. Here serum was separated using TE-free techniques( Reference Brown and Rivera 25 ), and aliquots frozen at −20°C before shipment on dry ice to the Department of Human Nutrition, University of Otago, New Zealand and to the laboratory of Dr J Erhardt in Germany for analysis.
Biomarker analyses
Hb was determined by means of a complete blood count using an automated counter (Sysmex XN 1000; Sysmex Corporation). Serum was analysed in duplicate in the laboratory of Dr J Erhardt for ferritin, sTfR, RBP, CRP and AGP by a combined sandwich ELISA technique( Reference Erhardt, Estes and Pfeiffer 26 ). Inter-assay CV of a control sample (n 26) were 3·0 % for ferritin, 3·3 % for sTfR, 3·3 % for RBP, 6·7 % for CRP and 9·2 % for AGP.
Serum Zn and Se were analysed by inductively coupled plasma MS (ICP-MS) (Agilent 7500ce ICP-MS; Agilent Technologies) at the University of Otago. The CV for a pooled sample (n 38) and control (UTAK, Utak Laboratories Inc.; n 41) for both Zn and Se were <3 %, with the mean results for the UTAK control both within 3 % of certified values.
Thresholds for defining suboptimal biomarkers were as follows: Hb<110 g/l( 27 ); serum ferritin<12 µg/l( 28 ); sTfR>8·3 mg/l( Reference Erhardt, Estes and Pfeiffer 26 ); RBP<0·83 µmol/l( Reference Engle-Stone, Haskell and Ndjebayi 29 ); serum Zn<9·9 µmol/l( Reference Hess, Peerson and King 30 ); and serum Se≤0·82 µmol/l( Reference Thomson 31 ). Inflammation was assessed by serum CRP>5 mg/l and AGP>1 g/l, respectively( Reference Thurnham, McCabe and Haldar 13 ). Values for ferritin, sTfR, RBP, Zn and Se were adjusted for inflammation using both CRP and/or AGP.
Statistical analyses
The sample size of 200 healthy breast-fed infants was calculated to be able to estimate the prevalence of stunting (length-for-age z score<−2 sd) with a 95 % CI precision of at most 7 %. This sample size also allowed us to conduct regression analyses involving several variables with at least a minimum of ten respondents per variable in the model( Reference Schumacker and Lomax 32 ).
Descriptive statistics were calculated for selected health, morbidity, and biomarker variables at aged 6, 9 and 12 months. Serum ferritin and RBP values were adjusted for sub-clinical inflammation using three approaches: (1) internal correction factors (ICF) calculated from the study population data using a four-level model of inflammation proposed by Thurnham et al.( Reference Thurnham, McCabe and Haldar 13 , Reference Thurnham, Northrop-Clewes and Knowles 33 ); (2) external correction factors (ECF) derived from a meta-analysis by Thurnham et al.( Reference Thurnham, McCabe and Northrop-Clewes 9 , Reference Thurnham, McCabe and Haldar 13 ); and (3) the recent BRINDA regression modelling( Reference Suchdev, Namaste and Aaron 18 , Reference Larson, Addo and Sandalinas 34 ). Values for serum sTfR, Se and Zn were adjusted for inflammation using both ICF( Reference Thurnham, McCabe and Haldar 13 ) and the BRINDA regression approach( Reference Suchdev, Namaste and Aaron 18 ). Adjustments for serum Zn for time of day and time since the last meal before the blood collection were made before adjusting for inflammation( Reference Arsenault, Wuehler and de Romana 35 ). The non-transformed AGP and CRP values were used to define the four-level model of inflammation. All continuous biomarker variables were plotted and visually assessed for normality. Ferritin, sTfR, RBP, Zn, Se, CRP and AGP were log transformed to reflect better regression diagnostics. Means and standard deviations or geometric means (GM) and 95 % CI were calculated, where appropriate, for Hb and unadjusted and adjusted concentrations for serum ferritin, sTfR, RBP, Zn and Se at each age.
Adjustment by the BRINDA method began by running a linear regression model with each biomarker as the dependent variable; and CRP and AGP as the independent variables. The slope (regression coefficient) of CRP (β 1) and AGP (β 2) was then used to adjust for the effect of inflammation as follows: exp(unadjusted ln biomarkers–β 1 (CRPobserved−maximum of lowest decile for CRP)−β 2 (AGPobserved−maximum of lowest decile for AGP)). A reference concentration (maximum of lowest decile) for serum CRP and AGP was used to avoid over-adjusting the micronutrient biomarkers among individuals with low levels of inflammation. All models were checked to ensure all assumptions were met by examining the plot of residuals, homogeneity of variance and normality.
Multi-level mixed modelling was used to detect changes in biomarker concentrations by age, whereas generalised estimating equations were used to detect changes in the prevalence of micronutrient deficiencies at 6, 9 to 12 months. The paired t test and the McNemar test were used to detect differences in biomarker concentrations and the prevalence of deficiencies before and after adjustment using the regression approach, respectively. Sex differences in the proportion with micronutrient deficiencies were examined using the chi-square test. Statistical analyses were conducted using Stata version 12 (Stata Corporation). A P value of less than 0·05 (two-sided) indicated statistical significance.
Results
Health and morbidity characteristics
There were 230, 202 and 190 respondents at 6, 9 and 12 months. Blood was collected from all respondents, however there was insufficient volume for the determination of Hb at 6 and 9 months for two and three respondents, respectively. For sTfR, ferritin, RBP, CRP and AGP examinations, there were sixteen, six and five respondents at 6, 9 and 12 months (respectively) who also had insufficient blood sample volume; and a further reduction of thirty-six respondents at all age groups who had insufficient blood volume for Zn and Se examinations. The proportion of male and female in this study is quite constant at all age groups, with 45–46 % male participants (Table 1).
Table 1 Health and morbidity characteristics (Numbers and percentages)
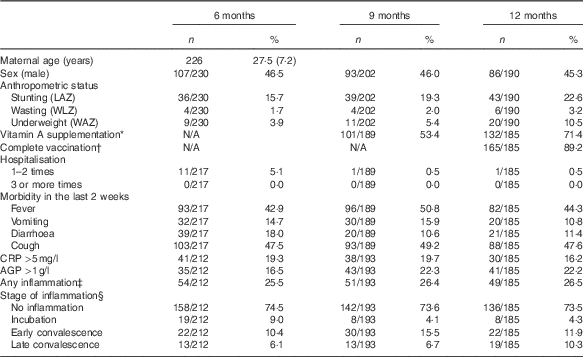
LAZ, length-for-age z score; WLZ, weight-for-length z score; WAZ, weight-for-age z score; CRP, C-reactive protein; AGP, α-1-glycoprotein; BCG, Bacillus Calmette–Guérin; DPT, Diphtheria, Pertussis, Tetanus.
* In the last 9 months and since the last examination (for 12 months).
† At least one BCG, one polio, one DPT, one hepatitis B and one measles (taken from immunisation card).
‡ Any inflammation (CRP>5 mg/l or AGP >1 g/l).
§ Stage of inflammation: no inflammation (CRP<5 mg/l and AGP<1 g/l); incubation (CRP>5 mg/l and AGP<1 g/l); early convalescence (CRP>5 mg/l and AGP>1 g/l); late convalescence (CRP≤5 mg/l and AGP>1 g/l).
More than two-thirds of the infants at 12 months had received vitamin A supplements since the last visit, and almost 90 % had been completely vaccinated. The proportion who had ever been hospitalised was low, irrespective of age. In contrast, those with fever and/or cough (based on maternal self-reports) at each study visit was much higher (43–51 %); vomiting and diarrhoea were lower (11–18 %). CRP and AGP levels ranged from 0·01–65·48 mg/l and 0·18–2·79 g/l, respectively, at 6 months; 0·01–78·10 mg/l CRP and 0·24–3·60 g/l AGP at 9 months; and 0·01–99·99 mg/l CRP and 0·22–3·44 g/l AGP at 12 months. The prevalence of any inflammation was about 25 % at each age, with more infants classified in the early convalescence stage (i.e. CRP >5 mg/l and AGP >1 g/l) at all three ages (Table 1).
Micronutrient biomarkers unadjusted and adjusted for inflammation
GM concentrations for Hb were similar at all ages approximately 111 g/l, whereas those for serum sTfR, ferritin, RBP and Se (but not Zn) were dependent on age (P<0·001), irrespective of the adjustment method applied (Table 2). All adjusted GM values for serum ferritin and sTfR were lower, and RBP and Zn higher, than unadjusted values (P<0·001). At all ages the regression approach generated the lowest GM values for serum ferritin and STfR, but the highest values for serum RBP and Zn. For serum Se, only the regression approach produced adjusted GM values that were lower at 6 and 9 months, but higher at 12 months compared with unadjusted values (P<0·001) (Table 2).
Table 2 Hb and micronutrient biomarkers (Geometric means and 95 % confidence intervals)
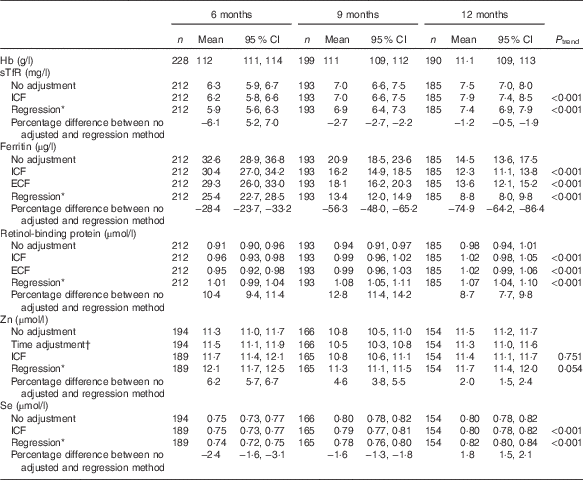
sTfR, soluble transferrin receptor; ICF, study generated/internal correction factors; ECF, Thurnham external correction factors; CRP, C-reactive protein; AGP, α-1-glycoprotein.
* Adjusted for inflammation=exp (unadjusted ln biomarkers−(regression coefficient for CRP)×(CRP−(maximum of lowest decile for CRP))−(regression coefficient for AGP)×(AGP−(maximum of lowest decile for AGP))).
† Adjusted for time of the day and interval since the last meal=exp (unadjusted ln biomarkers+(regression coefficient for time of day×(time of day 8)−(regression coefficient for interval since previous meal×interval since previous meal))).
Anaemia (unadjusted Hb<110 g/l) ranged from 33 to 42 %, with the highest prevalence at 9 months (42 %; 95 % CI 55, 69 %). For Fe deficiency, the prevalence varied depending on the biomarker, adjustment applied, and age group. Compared with unadjusted values, the regression approach across all ages generated the highest prevalence of Fe deficiency based on low serum ferritin, but the lowest prevalence based on elevated sTfR levels. For both biomarkers, the prevalence of Fe deficiency was lowest at aged 6 months and highest at 12 months (Table 3).
Table 3 Proportion of micronutrient deficiencies with and without adjustments (Numbers and percentages)
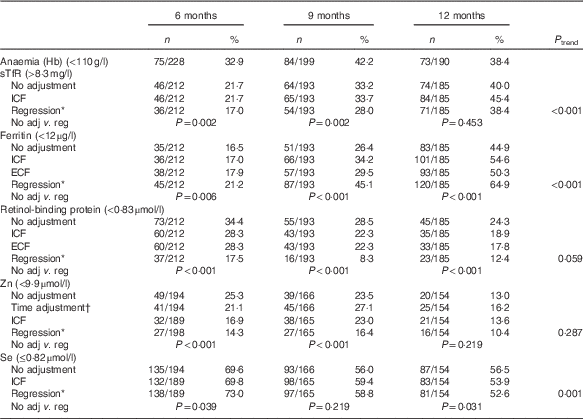
sTfR, soluble transferrin receptor; ICF, study generated/internal correction factors; ECF, Thurnham external correction factors; CRP, C-reactive protein; AGP, α-1-glycoprotein.
* Adjusted for inflammation=exp(unadjusted ln biomarkers−(regression coefficient for CRP)×(CRP−(maximum of lowest decile for CRP))−(regression coefficient for AGP)×(AGP−(maximum of lowest decile for AGP))).
† Adjusted for time of the day and interval since the last meal=exp [unadjusted ln biomarkers−(regression coefficient for CRP)×(CRP−(maximum of lowest decile for CRP))−(regression coefficient for AGP)×(AGP−(maximum of lowest decile for AGP)+(regression coefficient for time of day)×(time of day 8)+(regression coefficient for interval since previous meal)×(interval since previous meal))).
The regression adjustment produced the lowest prevalence of both vitamin A and Zn deficiency across all ages compared with unadjusted values, with no significant trend across the three age groups. In contrast, changes in the prevalence of Se deficiency, although significant across the three age groups after the regression adjustment, were inconsistent, increasing at 6 (P=0·039) and 9 months (P=0·219), but decreasing at 12 months (P=0·031) (Table 3).
Boys had a higher prevalence than girls (P<0·05) for Fe deficiency at 6 months of age irrespective of the biomarker or inflammation adjusted approach. No sex-related difference existed for vitamin A, Zn or Se (Table 4).
Table 4 Proportion of micronutrient deficiencies with adjustments stratified by sex (Numbers and percentages)
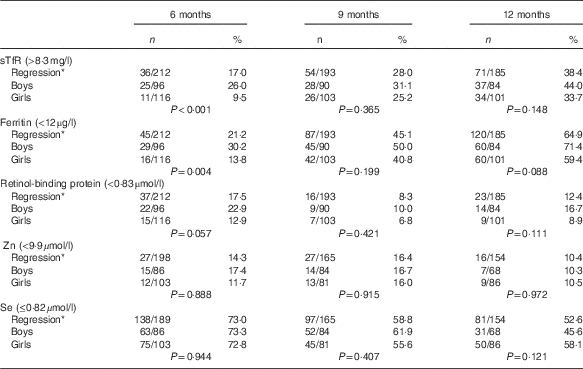
sTfR, soluble transferrin receptor; CRP, C-reactive protein; AGP, α-1-glycoprotein.
* Adjusted for inflammation=exp(unadjusted ln biomarkers−(regression coefficient for CRP)×(CRP−(maximum of lowest decile for CRP))−(regression coefficient for AGP)×(AGP−(maximum of lowest decile for AGP))).
Discussion
Our findings highlight a much higher prevalence of Fe and Se deficiency among these Indonesian infants than vitamin A and Zn deficiency, especially for Fe at 12 months of age. Correcting the biomarkers of Fe, vitamin A and Zn for inflammation, irrespective of the adjustment method used, markedly changed the corresponding prevalence estimates for deficiency at all three ages. The adjustments increased the prevalence of Fe deficiency based on adjusted low serum ferritin, whereas simultaneously decreasing the prevalence of vitamin A and Zn deficiency, with the new regression approach generating the greatest changes compared with unadjusted prevalence estimates. Regression adjustment also affected the prevalence of Se deficiency, although the direction and magnitude of the effect was inconsistent across the three age groups.
Iron status and iron deficiency
GM values of serum ferritin among these Indonesian infants at 6 and 9 months (even after the regression adjustment) were much higher than those reported earlier for infants in Indonesia( Reference Wieringa, Dijkhuizen and West 36 , Reference Fahmida, Rumawas and Utomo 37 ) and elsewhere in Asia( Reference Lander, Enkhjargal and Batjargal 38 , Reference Wieringa, Berger and Dijkhuizen 39 ). This discrepancy probably reflected their high intake of Fe fortified infant foods at 6 months of age( Reference Diana, Mallard and Haszard 40 ). However, by 12 months of age, serum ferritin had declined and sTfR had increased significantly (P<0·001) (Table 2), an age-related trend described by other investigators( Reference Oski 41 , Reference Domellof, Braegger and Campoy 42 ) and attributed to a gradual depletion of Fe stores for growth. In this study consumption of Fe fortified infant foods declined markedly after 6 months of age, which did not appear to be compensated by the consumption of wheat flour products fortified with multi-micronutrients including Fe during later infancy. Indonesia has a national wheat flour fortification programme and all flour products are fortified with 50 mg Fe/100 g, although the form of the Fe fortificant is uncertain. It is possible that poor availability of the fortificant Fe in the wheat flour products exacerbated the rise in the prevalence of Fe deficiency by 12 months (Table 3). Not surprisingly, the prevalence of Fe deficiency based on sTfR was lower (17–38 %) than that based on ferritin (21–65 %) as serum ferritin declines as body Fe stores fall, whereas sTfR only changes after the Fe stores are depleted( Reference Gibson 4 ).
Vitamin A status and deficiency
In the study presented here, serum RBP was used as a proxy for serum retinol to identify vitamin A deficiency. Serum RBP is recommended because it is more stable than retinol( Reference Fujita, Brindle and Rocha 43 ), the assay is cheaper and easier and RBP concentrations correlate closely with serum retinol provided individuals are not obese and have normal kidney function( Reference Tanumihardjo, Russell and Stephensen 44 ). RBP is not consistently 100 % saturated with retinol, and thus, the RBP-retinol molar ratio is not always approximately 1:1. We did not measure serum retinol in a subset of our population to establish a specific cutoff for serum RBP as recommended by Tanumihardjo et al.( Reference Tanumihardjo, Russell and Stephensen 44 ). Instead we adopted a cutoff of <0·83 µmol RBP/l which was reported to correspond to serum retinol concentrations of <0·70 µmol/l in a study of Cameroon young children( Reference Engle-Stone, Haskell and Ndjebayi 29 ).
The prevalence of vitamin A deficiency adjusted for inflammation was lower than that reported in an earlier Indonesian study( Reference Dijkhuizen, Wieringa and West 45 ), and at ages 9 and 12 months no longer of public health concern (i.e. >20 %)( 46 ). This trend arose because 71 % of the infants at 12 months and some of the lactating mothers received vitamin A supplements through the national vitamin A supplementation programme initiated in Indonesia in 1978. Breast milk vitamin A concentrations are influenced by maternal vitamin A status( Reference Allen 47 ).
Zinc status and deficiency
The low prevalence of Zn deficiency reported here is not unexpected, as comparable findings have been reported among Indonesian infants earlier( Reference Wieringa, Dijkhuizen and West 36 , Reference Fahmida, Rumawas and Utomo 37 ). Only at 9 months of age was the prevalence of Zn deficiency among the infants of public health concern (i.e. > 20 %)( Reference Hotz 48 ). Several factors may have played a role in these findings, including the finding that rice grown in the high Zn soils of Indonesia and consumed by the infants is likely to have a high Zn concentration (i.e. approximately 24 μg Zn/g)( Reference Herawati, Suzuki and Hayashi 49 ), unlike rice grown in low Zn soils (i.e. 0·38 uμg Zn/g) which has much lower Zn concentrations (i.e. 11 ug Zn/g)( Reference Sunanda, Sumathi and Venkatasubbaiah 50 ). In addition, at 6 months many of the Fe fortified infant foods consumed were also fortified with Zn, and at aged 12 months were replaced by the consumption of wheat flour products also fortified with Zn as well as Fe. Moreover, the Zn fortificant level in wheat flour in Indonesia is higher than other countries with mandatory fortification programmes (30 μg Zn/g v.15 or 16 μg Zn/g)( Reference Kimura 51 ) and hence may have contributed to the relatively low prevalence of Zn deficiency observed here. Nonetheless, few studies have shown any impact of Zn fortification on serum Zn or Zn-related health outcomes( Reference Hess and Brown 52 ), despite evidence of absorption of the Zn fortificant in wheat flour products( Reference Lopez de Romana, Lonnerdal and Brown 53 ), perhaps because the Zn fortificant levels are lower than those used in Indonesia.
Selenium status and deficiency
Despite the increasing concern about Se deficiency, to our knowledge there are no data on the Se status of infants in Indonesia. Of the infants, 72·5 % (95 % CI 66·6, 79·4 %) at 6 months of age had Se deficiency, falling progressively to 52·6 % (95 % CI 44·6, 60·6 %) at aged 12 months. Such low Se status is probably associated, at least in part, with the very low Se concentrations of rice grown in Sumedang district (0·011 μg Se/g)( Reference Holik, Bianti and Mutakin 54 ) where soils are low in Se. Lactating Sumedang mothers who consume low-Se-rice as their major staple without other sources of Se-rich foods are likely to be at risk of low Se status, and like vitamin A, breast milk Se concentrations are strongly affected by maternal Se status( Reference Allen 47 ). As a result, low breast milk Se levels probably also contributed to the high prevalence of Se deficiency among the Sumedang breast-fed infants, especially at 6 months of age. The later decline in the prevalence may have been associated with a gradual increase in the consumption of Se-rich foods such as fish and chicken liver in place of breast milk(40).
Influence of inflammation on the interpretation of the micronutrient biomarkers
In this study about 25 % of the infants across all age groups had elevated CRP and/or AGP concentrations indicative of inflammation. Therefore, exclusion of these children from the analyses would reduce the sample size and may bias the results, as reported in other studies( Reference Larson, Addo and Sandalinas 34 , Reference Grant, Suchdev and Flores-Ayala 55 ). Conducting the survey in a season with low rates of inflammation, as suggested by WHO( 28 ) was not feasible because the prevalence of inflammation was reasonably constant in Sumedang district during the one year study period (Table 1).
During the acute phase response, concentrations of serum ferritin markedly increase independent of Fe status, resulting in an under-estimate of the prevalence of Fe deficiency when no adjustment for inflammation or infection is performed, as noted earlier( Reference Tomkins 56 – Reference Northrop-Clewes 58 ). This discrepancy arises because proinflammatory cytokines stimulate an increase in circulating hepcidin, which reduces absorption of Fe and its release from body stores( Reference Ganz and Nemeth 59 , Reference Wessling-Resnick 60 ). As a consequence, serum ferritin rises whereas serum Fe falls limiting access to Fe by invading pathological microorganisms for growth and reproduction, as well as reducing free radical production( Reference Galloway, McMillan and Sattar 57 ). However, unlike ferritin, sTfR is not an APP, and is less affected by the acute phase response( Reference Thurnham and McCabe 61 ), perhaps because erythropoietin production is reduced and erythropoeisis suppressed by cytokines( Reference Northrop-Clewes 58 , Reference Beguin 62 ). Hence, it is not surprising that inflammation was responsible for only slight changes in GM serum sTfR and the corresponding prevalence estimates for Fe deficiency, after adjustment.
Over-estimates for the prevalence of Zn and Se deficiency in response to inflammation, irrespective of their true burden of deficiency, arise from the redistribution of Zn and Se from the circulating plasma to the sites of inflammation( Reference King, Brown and Gibson 6 , Reference Galloway, McMillan and Sattar 57 ), whereas over-estimates for vitamin A deficiency result from a decrease in hepatic synthesis of RBP mRNA, which interrupts the release of retinol-RBP from the liver, lowering serum RBP concentrations( Reference Rosales, Ritter and Zolfaghari 63 , Reference Stephensen 64 ).
In this study we used the two WHO recommended inflammatory biomarkers – CRP and AGP – in combination( 12 ), irrespective of the adjustment method applied, to capture the three phases of inflammation as described by Thurnham et al.( Reference Thurnham, Mburu and Mwaniki 65 ) in our infant population as well as the healthy state. In general prevalence estimates for deficiencies of Fe and vitamin A were similar, irrespective of whether internal or ECF for Thurnham’s method were applied to adjust serum ferritin and RBP for inflammation, as reported earlier( Reference Larson, Addo and Sandalinas 34 , Reference Grant, Suchdev and Flores-Ayala 55 ).
We only applied ICF to adjust serum Zn and Se for inflammation; prevalence estimates were consistently lower for Zn after adjustment for all three ages than unadjusted estimates as noted earlier, although for Se, the adjustment effect for inflammation was inconsistent, varying with age, as reported earlier( Reference Galloway, McMillan and Sattar 57 , Reference Ghashut, McMillan and Kinsella 66 , Reference Duncan, Talwar and McMillan 67 ).
Thurnham’s approach uses fixed cutoffs for CRP (>5 mg/l) and AGP (>1 g/l) irrespective of the magnitude and stage of the inflammatory response. Hence some individuals classified in the subgroup without inflammation may have sub-clinical inflammation resulting in an elevated GM for serum ferritin and a lower GM for serum RBP in the seemingly ‘healthy’ reference subgroups so the true prevalence of Fe and vitamin A deficiency may still be under- or over-estimated, respectively( Reference Bui, Stein and DiGirolamo 14 ).
In an effort to overcome this limitation, some investigators have suggested using very low cutoffs for CRP and AGP( Reference Bui, Stein and DiGirolamo 14 , Reference Beard, Murray-Kolb and Rosales 16 ). The BRINDA regression method uses an alternative approach to avoid over-adjusting the micronutrient biomarkers for individuals with low levels of inflammation. Here the reference cutoffs for CRP- and AGP-adjustment were each defined by the maximum of the lowest decile for their distribution in the study population, so it is not surprising that the regression approach generated the greatest changes compared with unadjusted prevalence estimates for Fe, vitamin A and Zn deficiency, as noted earlier. Se was an exception, because the prevalence of deficiency decreased significantly (P<0·01) at 12 months but increased at 6 (P<0·05) and 9 months compared with the corresponding unadjusted prevalence estimates; reasons for this inconsistency are unclear.
Strengths and limitation of the study
Our data are based on a longitudinal study designed to describe trends in micronutrient biomarkers of infants at age 6, 9 and 12 months. Our study was restricted to infants from Sumedang district and was not designed to evaluate the micronutrient status of a representative sample of infants from West Java, Indonesia. Nonetheless, the prevalence of stunting in our Sumedang infants at 9 months was comparable with that reported for 6–11-month-old infants in the West Java Province (19·3 v. 21·5 %), the most populous province in Indonesia. However our Sumedang infants had a higher prevalence of anaemia based on a cutoff of 110 g/l across all three age groups than national prevalence data reported for infants at 12–59 months (33–42 v. 28·1 %)( 68 ). There is some concern that this WHO recommended cutoff may be too high for infants( Reference Domellof, Dewey and Lonnerdal 69 ) resulting in an overestimate of the anaemia prevalence in our study population.
We measured four micronutrient biomarkers known to be influenced by inflammation and infection to varying degrees, and applied the new BRINDA regression approach to adjust all four biomarkers for inflammation using both serum CRP and AGP as recommended by WHO( 12 ). Our results have highlighted marked changes in the prevalence of deficiency of these micronutrients by age, and in some cases sex, after adjustment for inflammation, confirming the importance of taking age, sex and inflammation into account when comparing such prevalence estimates across countries. In addition, serum Zn was adjusted for time of day, time since the last meal, and inflammation to reduce the variance, as recommended by IZiNCG( Reference King, Brown and Gibson 6 ).
Nonetheless our prevalence estimates were based on cutoffs for the micronutrient biomarkers, some of which are ill defined during infancy, and inconsistent across studies making comparisons difficult( Reference Raghavan, Ashour and Bailey 70 ). In addition, even the BRINDA regression cutoffs for the reference values for CRP and AGP may have failed to exclude only infants without inflammation. Finally, because micronutrient biomarker concentrations such as serum Zn and ferritin are subject to day-to-day variation, theoretically more than one independent measurement is required to obtain an accurate value at each age( Reference Borel, Smith and Derr 71 ), although during infancy taking more than one blood sample at each age is not appropriate.
In conclusion, our results emphasize the importance of adjusting for inflammation when interpreting biomarkers of Fe, Zn, vitamin A and possibly Se status; however, further work is needed to validate the proposed approaches with a particular focus on assessing the influence of varying degrees of inflammation (i.e. recurrent acute infections and low-grade chronic inflammation) on each affected nutrient biomarker. Nonetheless, our study demonstrates that without such adjustments, prevalence estimates for these micronutrient deficiencies will be incorrect in settings with a high burden of inflammation. Fe deficiency may be grossly under-estimated, and vitamin A and Zn deficiency over-estimated, and possibly at levels of public health concern that require urgent intervention, even though the true prevalence estimates are much lower.
Acknowledgements
Special thanks to Professor Anna Alisjahbana, Frontiers for Health (F2H), the people of the communities, and our team of dedicated research assistants.
This work was supported by Meat and Livestock Australia (MLA) (L. H., R. S. G.) and a University of Otago Research Grant (UORG) (R. S. G.). MLA and UORG had no role in the design, analysis or writing of this article.
A. D. and R. S. G. wrote the manuscript with input from other authors. L. H., A. D. and R. S. G. developed the study hypothesis and secured the funding. G. I. N. facilitated introduction to the community and A. D. co-ordinated the field work assisted by D. M. P, I. N., D. E. L. and S. R. and J. E. analysed and interpreted biomarker results. The following participated in the analyses and/or interpretation of the data: A. D., J. H., R. S. G. and L. H. All the authors reviewed and approved the final manuscript.
The authors declare that there are no conflicts of interest.






