Fe is important for physically active women, including military women, due to its essential role in maintaining physical and neuropsychological performance( Reference McClung and Murray-Kolb 1 ). Premenopausal women are particularly at risk of poor Fe status due to inadequate dietary intake of Fe and menstrual blood loss( Reference Heath, Skeaff and Williams 2 , Reference Harvey, Armah and Dainty 3 ). Exercise may further contribute to negative Fe balance( Reference Peeling, Dawson and Goodman 4 , Reference Shephard 5 ).
The prevalence of both Fe-deficiency non-anaemia (IDNA) and Fe-deficiency anaemia (IDA) is widely reported to be higher in physically active women and competitive athletes when compared to the general population( Reference DellaValle and Haas 6 – Reference Sinclair and Hinton 10 ). Following initial military training, the prevalence of IDNA has been shown to increase in female army recruits( Reference McClung, Marchitelli and Friedl 11 , Reference McClung, Karl and Cable 12 ). The effect of IDA on reduced aerobic performance is well documented( Reference Celsing, Blomstrand and Werner 13 – Reference Tufts, Haas and Beard 15 ). Although the effect of IDNA is not well described, it has been reported to impair aerobic adaptation and endurance capacity in women( Reference Brownlie, Utermohlen and Hinton 16 – Reference McClung, Karl and Cable 18 ).
Whilst all military personnel are exposed to cognitive and physical challenges, the changes in Fe status that women typically incur during intensive periods of physical exertion could impact their response to these challenges( Reference McClung, Marchitelli and Friedl 11 ). Basic combat training (BCT) provides a unique setting to investigate Fe status. Not only do all recruits perform the same form and intensity of physical training, but the environment is controlled for variables such as sleep, dietary intake, sedentary behaviour, altitude and access to health care( Reference Jones, Hauret and Dye 19 ). Previous studies investigating the Fe status of female recruits have been limited to 10 weeks and measured running performance as a marker of physical fitness during training( Reference McClung, Marchitelli and Friedl 11 , Reference McClung, Karl and Cable 12 , Reference McClung, Karl and Cable 18 , Reference Karl, Lieberman and Cable 20 ). As these studies were all based in the USA, it is important to confirm these findings in other populations that may differ due to age, ethnicity, dietary intake, physical fitness and variations in the military training conducted.
The New Zealand Defence Force (NZDF) comprises 17 % women in the Regular Force, with a goal to achieve 25 % by 2025( 21 ). This is consistent with a global drive from international defence forces to enhance roles and opportunities for women in the military. Identifying the effects of diminished Fe status on physical performance, particularly with more subtle changes in Fe status that fall outside of the clinical diagnostic parameters, has been considered as an important area for future research( Reference McClung and Murray-Kolb 1 , Reference Anderson and Frazer 22 ). Therefore, the aim of this study was to characterise Fe status in female New Zealand Army recruits during BCT and associations with operationally relevant indicators of physical performance.
Methods
Participants
All female recruits who enlisted in the New Zealand Army from February 2014 to March 2016 were eligible and invited to participate in this longitudinal cohort study. The study was conducted at Waiouru Military Camp located at 792 m above sea level in the central North Island of New Zealand. It is the only location for the 16-week BCT course for all New Zealand Army recruits. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Massey University Human Ethics Committee: Southern A (Application – 13/85). Investigators also adhered to Defence Force Order 3, which prescribes the NZDF policy relating to conduct and approval of personnel research. All participants provided informed and voluntary consent.
Study procedures
Anthropometric measurements and blood collection for Fe status indicators were conducted in a fasted state between 06.00 and 07.30 hours during week 1 (baseline) and week 16 (end of BCT) for all participants, with instructions not to consume water before analysis. No exercise was performed for at least 8 h before these assessments. Demographic data (age and ethnicity) were collected at baseline using a questionnaire. Physical performance was assessed at baseline and during week 8 (mid). A timed 2·4 km run was conducted as a measure of aerobic fitness followed by maximum press-ups as a measure of muscular endurance of the upper body.
Basic combat training
BCT involves aerobic activities such as prolonged standing and marching in formation; tactical marching with load carriage; distance running and obstacle courses; as well as muscle strength training that includes own-body weight exercises, lifting, load carriage and climbing. Organised physical training sessions are conducted three to four times per week for a period of approximately 1 h. Other military activities involve the fundamentals of weapons training, first aid, navigation, classroom instruction and military field training. The 16-week course is residential and recruits live in shared barrack accommodation. Recruits consume three meals per d from a range of dining options, including self-selected meals in a dining hall facility to bag lunches and operational ration packs. Additional snacks are provided and recruits are not permitted to consume any dietary supplements unless prescribed by a medical doctor. All menus for dining halls and packed rations meet the Nutrient Reference Values for Australia and New Zealand( 23 ).
Ethnicity
This study used the concept of total response ethnicity( 24 ) which classifies participants in all ethnic groups they identify with. Therefore, participants may appear in more than one ethnic group and the sum of the ethnic groups may be greater than the number of participants.
Anthropometrics
Participants were asked to wear light shorts and a T-shirt and remove any metal such as jewellery. Anthropometric measures were determined at baseline and end, with the exception of height, which was measured at baseline only using a SECA 213 Portable Stadiometer (German Healthcare Export Group). Height was measured by a trained anthropometrist using the International Society for the Advancement of Kinanthropometry protocols( Reference Stewart and Marfell-Jones 25 ). Body mass was measured via bioelectrical impedance analysis using the InBody230 (Biospace Co. Ltd). BMI was calculated as mass (kg)/height (m)2.
Blood sampling and analysis
A venepuncture blood sample of 18 ml in total was collected at both baseline and end. Hb and erythrocyte distribution width (RDW) were analysed using a Sysmex XT-2000i automated haematology analyser (Sysmex Corporation). The Cobas® 6000 (Roche Diagnostics) was used to analyse serum ferritin (sFer) (electrochemiluminescence immunoassay, e601); serum Fe (sFe) (two-point end method with a colourimetric assay, c501); and transferrin (Tf) and C-reactive protein (CRP) (both using the two-point end method with an immunoturbidimetric assay, c501). For sFer, at control levels of 1·1, 12·3, 20·5 and 392 µg/l the within-assay CV were 12·4, 3·8, 4·1 and 2·1 %, respectively, and the between-assay CV were 23·4, 6·4, 8·1 and 4·3 %, respectively. For sFe at 11·3 and 54·5 µmol/l, the within-assay CV were 1·3 and 0·8 %, respectively, whilst the between-assay CV at 11·8 and 55·1 µmol/l were 1·8 and 1·3 %. For Tf at 1·27 and 2·63 g/l, the within-assay CV were 1·2 and 1·5 %, respectively; whilst the between-assay CV at 2·14 and 2·96 g/l were 0·06 and 0·08 %. Total Fe binding capacity (TIBC) was calculated by multiplying Tf by 22·8. Transferrin saturation (TS) was calculated by dividing sFe by TIBC and multiplying by 100. Soluble transferrin receptor (sTfR) was analysed using a Siemens BN™ II System (Siemens Healthcare Diagnostics Inc.) using N-point nephelometry with monoclonal antibodies. At control levels of 0·71 and 1·47 mg/l for sTfR, the within-assay CV were 1·4 and 1·5 %, respectively, and the between-assay CV were 4·2 and 3·8 %, respectively. For the Siemens BN™ II System, Fe deficiency for sTfR is indicated when values exceed 1·76 mg/l.
The sTfR was analysed at Canterbury Health Laboratories, while all other biomarkers were analysed at Medlab Whanganui. All assays were run with the appropriate standards and both internal and external quality control processes. In the absence of an external QC programme for sTfR, samples were exchanged with another New Zealand laboratory to analyse precision. All laboratories are accredited with International Accreditation New Zealand.
Participants were excluded if they had IDNA (sFer <12 µg/l and Hb ≥120 g/l) or IDA (sFer <12 µg/l and Hb <120 g/l) at baseline; CRP >10 mg/l at baseline or end; or if they were prescribed Fe supplementation during the study period. Participants who had received Fe supplementation before the study period, but not during, were included in the analysis.
Physical performance
A timed 2·4 km (1·5-mile) run, followed by maximum press-ups and curl-ups are the three components of the base physical fitness standard for New Zealand Army personnel. All recruits must qualify at the minimum standard during BCT. For women aged 16–24 years, this is completion of the run in 12 min and 20 s, fourteen press-ups and fifty curl-ups. The run time and number of press-ups were analysed at baseline (week 1) and the midpoint (week 8).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp). The Kolmogorov–Smirnov and Shapiro–Wilk tests and box plots were used to assess the data for normality. Descriptive statistics are presented as either mean values and standard deviations or median values with 25th and 75th percentiles. Changes in Fe status indicators and anthropometric measures were investigated using paired t tests. Independent t tests investigated any difference in Fe status indicators or anthropometric measures between those participants that completed BCT and participants that did not. Associations between Fe status indicators and physical performance were evaluated using Pearson correlation coefficients. A P value <0·05 was considered statistically significant.
As the sample size was determined by the feasibility of recruitment, effect sizes are reported as r and classified as small=0·1 (accounts for 1 % of total variance), medium=0·3 (accounts for 9 % of total variance) and large=0·5 (accounts for 25 % of total variance). Effect sizes are calculated as r=√t 2/(t 2+df), where t is the test statistic( Reference Field 26 ).
Results
Of the 108 female recruits invited to take part in this study, 106 volunteered to participate. Data for sixteen were excluded: seven had IDNA and three had IDA at baseline; and four participants at baseline and two at the end had a CRP >10 mg/l. A further fourteen participants did not complete BCT: ten were removed from their initial course due to lower limb injuries, including four stress fractures; one due to mental health; and three participants self-withdrew from BCT. Table 1 shows age, ethnicity and anthropometric measures for the remaining seventy-six participants. There was no significant difference in body mass, BMI or Fe status indicators at baseline between those participants that completed BCT and the fourteen participants that did not.
Table 1 Characteristics of study participants at baseline and end of basic combat training (Mean values and standard deviations; medians, 25th and 75th percentiles)
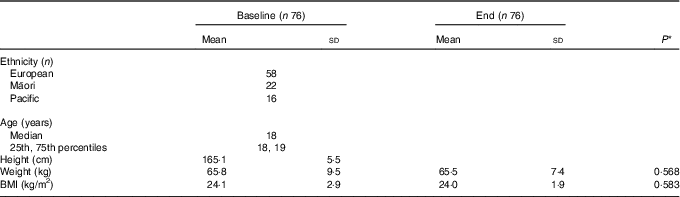
* P value determined using paired t tests.
Nearly all Fe indicators suggest a decline in Fe status at the end of BCT (Table 2). sFer and TS decreased (effect size, r 0·67, P<0·001 and r 0·28, P=0·014, respectively), while sTfR (r 0·63) and RDW (r 0·62) increased (P<0·001), all indicating reduced Fe status. Hb increased (r 0·30, P=0·009) during BCT. The mean sFer was reduced by 32·2 % from baseline, whilst the mean sTfR and TS changed by 14·9 and 11·3 %, respectively, over the same 16-week period. At the end of BCT, 11·8 % of participants had an sTfR level greater than the clinical cut-off of 1·76 mg/l compared with 3·9 % at baseline. Nine percent participants with normal Fe status at baseline had IDNA at the end and a further 3 % had IDA.
Table 2 Changes in iron status indicators during basic combat training (Mean values and standard deviations)

sFer, serum ferritin; sTfR, serum transferrin receptor; TS, transferrin saturation; RDW, erythrocyte distribution width.
* P value determined using paired t tests.
† sTfR is typically reported to two decimal points for clinical significance.
Due to injuries, only sixty-nine of seventy-six participants completed the 2·4 km run and press-ups at baseline. All seventy-six completed the 2·4 km run and press-ups at the midpoint. The range for run times was 10min 0 s to 14min 18s at baseline and 9min 20s to 13min 11s at the midpoint. Pearson’s correlation coefficient was used to analyse the association between Fe status indicators and physical performance during BCT. At the end point, sTfR was positively associated (r 0·29, P=0·012) and TS inversely associated (r –0·32, P=0·005) with the 2·4 km run time at the midpoint. There was no significant correlation between the Fe status indicators and the number of press-ups performed.
Discussion
This is the first study to investigate the Fe status of women in the New Zealand Army and associations with operationally relevant functional outcomes. Fe indicators (excluding Hb) suggested a decline in Fe status during BCT for female recruits. Secondary findings were that reduced Fe status, as indicated by increased sTfR and decreased TS, were associated with impaired running performance; and there was no significant correlation between Fe indicators and press-ups. These findings are important in the military training environment because of the associations between aerobic fitness and injury risk( Reference Jones, Hauret and Dye 19 ) and the ability to carry out physically demanding occupational tasks( Reference Hauschild, DeGroot and Hall 27 ). Female soldiers will continue to be exposed to strenuous tasks during their careers, including the period immediately after BCT when soldiers will complete an advanced training course.
The significant changes in Fe indicators observed during BCT reflect those found in previous studies of women undergoing initial military training( Reference McClung, Marchitelli and Friedl 11 , Reference McClung, Karl and Cable 12 , Reference McClung, Karl and Cable 18 , Reference Karl, Lieberman and Cable 20 ). The 32 % reduction in sFer from baseline is similar to the 34 % decrease in the US Army female recruits during 8 weeks of BCT( Reference McClung, Karl and Cable 18 ) and the 33 % decrease observed in elite female weight-bearing athletes during the first 4–6 weeks of a training season( Reference Ashenden, Martin and Dobson 28 ). sFer was the largest biomarker change during the present study and may indicate that Fe stores are most sensitive to declines in Fe status due to military training. This finding supports the importance of sFer as a biomarker for the early identification of Fe deficiency, as despite the longer duration of the present study, decreases in sFer of a greater magnitude were not seen.
The significant elevation in Hb observed in the present study has been reported previously for female recruits following BCT over 8–9 weeks( Reference McClung, Karl and Cable 12 , Reference McClung, Karl and Cable 18 , Reference Karl, Lieberman and Cable 20 ). Increased Hb levels coupled with reduced sFer levels may indicate the mobilisation of Fe away from storage proteins (ferritin) for the production of erythrocytes and maintenance of oxygen delivery( Reference McClung, Karl and Cable 12 ). Alongside instructions not to consume water before blood sampling, the use of a dynamic panel of Fe biomarkers provides a further control for hydration state, indicating true biological changes in Fe status. The present study did not extend beyond the 120- d lifespan of erythrocytes to help understand what may happen to the Fe status of female recruits over that duration. This is of interest, as upon completion of BCT, soldiers will likely proceed immediately on to advanced training.
Significant changes in sTfR and TS from baseline to end indicated a reduction in Fe status in the present study and were the only indicators associated with impaired aerobic fitness. Our finding that sTfR was correlated with 2·4 km running performance at the midpoint supports the findings of McClung et al. ( Reference McClung, Karl and Cable 18 ) who demonstrated that the change in sTfR during BCT was correlated with 3·2 km run time. Work in previously untrained premenopausal women also identified a relationship between sTfR and aerobic performance during time trials on a cycle ergometer( Reference Brownlie, Utermohlen and Hinton 16 , Reference Brownlie, Utermohlen and Hinton 17 , Reference Hinton, Giordano and Brownlie 29 ). Decreases in sTfR following Fe supplementation were significantly associated with improvements in relative maximal aerobic capacity (VO2max)( Reference Brownlie, Utermohlen and Hinton 16 ) and %VO2max used during work( Reference Brownlie, Utermohlen and Hinton 17 , Reference Hinton, Giordano and Brownlie 29 ). TS was correlated with run time at the midpoint in the present study. Brownlie et al. ( Reference Brownlie, Utermohlen and Hinton 16 ) also found an association with TS and aerobic capacity, particularly in subjects who had elevated sTfR levels. Brownlie et al. ( Reference Brownlie, Utermohlen and Hinton 16 ) and Hinton et al. ( Reference Hinton, Giordano and Brownlie 29 ) demonstrated that improvements in Fe status following supplementation can increase oxidative capacity at the tissue level to improve maximal work capacity and endurance performance, at least when the sTfR levels approach the cut-off values used for Fe deficiency. These findings suggest that indicators of tissue-Fe deficiency, such as sTfR and TS, are more closely associated with aerobic fitness, compared with sFer (stored Fe). Brownlie et al. ( Reference Brownlie, Utermohlen and Hinton 17 ) proposed that tissue-Fe deficiency impairs the ability of mitochondria and myoglobin to adapt in response to aerobic training. sTfR and TS can therefore be used to distinguish between Fe depletion and functional Fe deficiency, which impairs the adaptation in endurance capacity as a result of aerobic training. In the present study, reduced tissue-Fe status likely impaired the ability of female recruits to carry out typical military tasks, which are often aerobically demanding( Reference Hauschild, DeGroot and Hall 27 ).
The association between poor Fe status and reduced aerobic performance is typically strongest in those with IDA( Reference Celsing, Blomstrand and Werner 13 – Reference Tufts, Haas and Beard 15 ); whilst IDNA has been reported to impair aerobic adaptation and endurance capacity in women( Reference Brownlie, Utermohlen and Hinton 16 – Reference McClung, Karl and Cable 18 ). After excluding participants with IDNA and IDA at baseline (but not at the end), the present study indicated a linear relationship between declining Fe status and slower run times in physically active women who mostly had clinically normal Fe status.
Run times are a validated measure of aerobic fitness( Reference Hauschild, DeGroot and Hall 27 ), and slower run times indicate lower aerobic fitness, which has been strongly and consistently associated with higher risk of injuries among male and female recruits in both the US and British Armies( Reference Jones, Hauret and Dye 19 , Reference Blacker, Wilkinson and Bilzon 30 – Reference Nindl, Jones and Van Arsdale 33 ). Press-ups are a measure of muscular endurance. Whilst a recent systematic review demonstrated a strong correlation between press-ups and performance of common military tasks, press-ups have not been shown to be as strongly or as consistently associated with risk of injury as aerobic fitness measures( Reference Lisman, de la Motte and Gribbin 34 ). As hypothesised in the present study, there was no significant correlation between Fe status indicators and the number of press-ups performed. Press-ups predominantly use the anaerobic energy system, and Peeling et al. ( Reference Peeling, Dawson and Goodman 4 ) suggested that anaerobic-based tests are not sensitive or specific enough to demonstrate the effects of Fe status on physical performance.
Of the 100 female recruits who volunteered to participate in the present study and did not have elevated CRP levels, 7 % had IDNA and 3 % had IDA at baseline. This is lower than the 10·6 and 5·2 % for IDNA and IDA, respectively, in females aged 15–18 years living in New Zealand( 35 ). The prevalence of Fe deficiency at baseline was also lower than that reported previously in female army recruits in the USA( Reference McClung, Marchitelli and Friedl 11 , Reference McClung, Karl and Cable 12 , Reference Karl, Lieberman and Cable 20 ) and Israel( Reference Dubnov, Foldes and Mann 36 , Reference Israeli, Merkel and Constantini 37 ). Whilst all studies used a sFer cut-off of 12 µg/l, the US-based studies used a three-variable model to identify participants with IDNA or IDA. Participants were categorised with Fe deficiency if they presented with at least two of the following three abnormal Fe status indicators: sFer <12 µg/l, TS <16 % or RDW >15 %. The participants were then classified as IDNA if Hb ≥120 g/l or IDA if Hb <120 g/l( Reference McClung, Marchitelli and Friedl 11 , Reference McClung, Karl and Cable 12 , Reference McClung, Karl and Cable 18 , Reference Karl, Lieberman and Cable 20 ). Although the present study used sFer as a single indicator for IDNA, all subjects with a CRP >10 mg/l at baseline or end were excluded from the analysis( Reference Zimmermann and Hurrell 38 ) to mitigate against sFer elevations in response to inflammation or infection( 39 ). Whilst not reported, all participants with IDNA and IDA at the end of BCT had at least two and four diminished Fe biomarkers, respectively.
A number of factors are likely to contribute to the decrements in Fe status demonstrated in the present study. Increased Fe demand may result from higher rates of erythrocyte production and whole-body Fe turnover during periods of more intense physical activity( Reference Beard and Tobin 40 ). The development of lean body mass is a potentially Fe demanding process that may contribute to depletion of Fe status. There was an 8·9 % increase (P<0·001) and 22·5 % decrease (P<0·001) in skeletal muscle mass and fat mass, respectively, from baseline to end. These body composition changes are in the expected direction following BCT. Increased Fe losses, over and above menstrual bleeding, have been well described in athletes; particularly those involved in exercise that is of high impact, weight-bearing and of long duration( Reference Telford, Sly and Hahn 41 ), as encountered during BCT. These exercise-induced losses include gastrointestinal bleeding, haemolysis due to repetitive foot strike, haematuria and sweat( Reference Peeling, Dawson and Goodman 4 , Reference Shephard 5 ).
Physical training may also reduce Fe status through the action of the liver-derived peptide, hepcidin, to inhibit Fe absorption( Reference Park, Valore and Waring 42 , Reference Nemeth, Tuttle and Powelson 43 ). Physical training stimulates the production of pro-inflammatory cytokines, including IL-6, increasing hepcidin expression( Reference Roecker, Meier-Buttermilch and Brechtel 44 ). Hepcidin levels have been shown to peak 3–6 h post-exercise, typically following a peak in IL-6 activity( Reference Peeling, Dawson and Goodman 45 – Reference Peeling, McKay and Pyne 49 ). It has therefore been proposed that during this transient elevation in hepcidin expression post-exercise, dietary Fe absorption and Fe recycling from macrophages may temporarily be reduced( Reference Peeling 50 ). Consequently, during periods of heavy training, with regular and successive inflammatory responses, consistent with the nature of BCT, this reduction in Fe absorption and recycling during hepcidin expression may further compromise the individual’s Fe status. Fe deficiency will likely occur if dietary Fe intake is not sufficiently increased to match the Fe demand, Fe losses or blockage of Fe absorption.
Duration is a strength of the present study. Fe status indicators were measured 15 weeks apart, while no previous studies in military women undergoing training have investigated Fe status beyond 10 weeks. The findings therefore reflect changes in Fe status that are much closer to the 120- d lifespan of erythrocytes( Reference Dallman 51 ). A further strength is the measurement of the inflammatory biomarker, CRP, and exclusion of participants who had a CRP >10 mg/l at either baseline or the end( Reference Zimmermann and Hurrell 38 ). Of the 108 participants available for this study, 106 volunteered to participate. The results therefore accurately reflect the characteristics of female recruits joining the New Zealand Army during the study period.
A limitation of this study is that the follow-up physical performance tests were not conducted at the same time as the Fe status biomarkers, creating challenges in interpreting and comparing the results. The volunteers who presented with Fe deficiency at baseline were provided Fe supplementation and excluded from further investigation. This ruled out the ability to investigate whether Fe status indicators in the clinically deficient range at baseline were associated with completion of BCT. The study was also limited by no analysis of the contribution of dietary intake or blood loss (e.g. menstrual bleeding) towards understanding the cause of diminished Fe status during BCT. However, all food is provided to recruits during BCT and a previous assessment of New Zealand Army male and female recruits’ nutrition intake in garrison (NM Martin and RJM Smeele, unpublished results) indicated that recruits consume on average 16 000 kJ, 150 g protein and 22 mg Fe/d, suggesting adequate intake.
Future studies should attempt to investigate the dietary intake and menstrual cycle of female recruits in conjunction with Fe status indicators, including inflammatory biomarkers in an effort to identify the mechanism by which Fe status declines during BCT. Fe status in military women should be monitored after BCT and throughout advanced training in order to understand the impact on Hb beyond 120 d and any further consequences for physical performance. Establishing a consensus amongst clinicians and researchers for Fe status indicators and their cut-off values that infer a negative effect on aerobic performance should be prioritised. In physically active premenopausal women, including military women, consideration should be given to expected declines in Fe status during intensive training periods.
In conclusion, Fe status indicators (excluding Hb) were diminished during BCT, and poor Fe status was associated with slower run times, indicating impaired aerobic fitness. In contrast, reduced Fe status did not appear to affect press-ups, a predominantly anaerobic-based test. These findings suggest that tissue-Fe status in particular is reduced in female recruits during BCT and may impair aerobic fitness, which has been strongly associated with the risk of injuries in military recruits and is fundamental to carrying out mission critical tasks. Maintaining or improving Fe status may help optimise the physical performance of military women. Initiatives for future consideration include Fe screening at appropriate times throughout a military woman’s career, Fe supplementation as appropriate, nutrition education regarding dietary intake and timing of bioavailable Fe, and ensuring that military dining halls provide foods that enhance Fe absorption.
Acknowledgements
The authors would like to thank the female recruits who volunteered to participate in the present study; the Command staff and instructors at the Army Depot; and the medical staff at the Waiouru Defence Health Centre, Waiouru Military Camp. The authors would like to acknowledge the following New Zealand Army Medical Officers for their professional support during the data collection, Drs Ali Riniker, Malcolm Joblin, Katia Hayes and Kate Stanbridge. The authors would also like to thank Catherine Rollo and Drs Peter Elder and John Lewis from Canterbury Health Laboratories and Darrell Monk and Jane Kerridge from Medlab Whanganui for their support in the writing of the methodology.
The present study was funded by the New Zealand Army, with a financial contribution towards the conduct of the study from the School of Sport, Exercise and Nutrition, Massey University.
The authors’ contributions are as follows: N. M. M. was the principal investigator and wrote the manuscript; N. M. M., K. L. B., C. A. C., P. R. v. H. and J. P. M. contributed to the study design; N. M. M., R. J. M. S. and O. A. R. M. contributed to the conduct of the study, including participant briefings and data collection; N. M. M. and K. L. B. completed the data analyses; and N. M. M., K. L. B., C. A. C., P. R. v. H. and J. P. M. contributed to the interpretation of the findings. All authors read, revised and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.





