Iodine is essential for the production of thyroid hormones, which are crucial for neurodevelopment in utero, in infancy and beyond. Iodine deficiency is a major public health problem affecting 1·9 billion globally, and the most preventable cause of intellectual disability( 1 ). This issue is not limited to developing countries, with mild maternal iodine insufficiency (classification based on median urinary iodine concentration being 50–99 μg/l, according to the WHO criteria( 1 )) recently shown to affect the cognitive function of the offspring, in the Avon Longitudinal Study of Parents and Children (ALSPAC) study( Reference Bath, Steer and Golding 2 ) and in the Gestational Iodine Cohort study( Reference Hynes, Otahal and Hay 3 ).
In the UK, the Reference Nutrient Intake for adults is 140 μg/d, without any proposed increment for pregnant and lactating women. Meanwhile, the WHO/United Nations Children's Fund (UNICEF)/International Council for the Control of Iodine Deficiency Disorders (ICCIDD) recommended daily intake for adults is 150 μg/d, increased to 250 μg/d for pregnant women( 1 ). The European Food Safety Authority (EFSA)( 4 ) recently proposed a new reference value of adequate intake for pregnant women of 200 μg/d. This increase is necessary to ensure that mothers have sufficient iodine to allow for increased thyroid hormone production, fetal needs and increased maternal renal clearance during this time( Reference Andersson and Zimmerman 5 – Reference Ritchie, King, Lammi-Keefe, Couch and Philipson 7 ). Principal dietary sources of iodine include seafood and dairy products (mainly due to the iodine fortification of cattle feeds and the use of iodophores for sanitation during milking), and in some countries, iodine fortified salt or bread( Reference Zimmermann 8 – Reference Combet and Lean 10 ). The unborn or the breastfed infant is entirely reliant on the mother for iodine supply. Young infants and pregnant or lactating mothers are the most vulnerable groups of the population because of their special requirements during these critical periods( Reference Delange 11 , Reference Ordookhani, Pearce and Hedayati 12 ). At present, there is no recommendation for routine iodine supplementation in the UK, unlike folic acid and vitamin D, or routine testing in pregnancy that would reflect iodine levels, unlike Fe. Instead, women rely on recommendations for a nutritionally balanced diet in pregnancy, such as those disseminated by the National Health Service (NHS, Ready Steady Baby book) and the Food Standard Agency (Eating while you are pregnant)( 13 ).
While mild iodine insufficiency has been shown in UK females of childbearing age( Reference Vanderpump, Lazarus and Smyth 14 ), as well as pregnant women( Reference Bath, Walter and Taylor 15 ), there is no iodine prophylaxis in place, and no pregnancy-specific recommended iodine intake. According to international guidelines, mothers need to meet the recommended iodine intake, an increase of 100 μg/d over that of an adult, via dietary choices alone. However, general nutritional knowledge of western populations has been shown to be poor( Reference Fowles 16 ), especially when it comes to iodine-specific needs: this was true in Australia before the mandatory iodine fortification of salt and bread( Reference Charlton, Gemming and Yeatman 17 ), and remained so even after the introduction of the measure despite a slight improvement of knowledge and intake of iodine( Reference Charlton, Yeatman and Lucas 18 ). A notable exception is New Zealand, where iodine fortification and awareness measures are more common than in the UK, the US or Australia( Reference Nithiananthan, Carroll and Krebs 19 ), as the problem in those countries has been noticed earlier and has been the centre of attention for research.
In the absence of prophylaxis for iodine in the UK and the need of mothers' empowerment, the aims of this study were to (1) define the dietary iodine intake of this population using a validated iodine-specific questionnaire( Reference Combet and Lean 10 ) and (2) to examine the levels of knowledge and awareness of mothers, either pregnant or with a child aged up to 36 months, regarding pregnancy-specific dietary recommendations, as well as iodine-specific requirements.
Experimental methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Glasgow ethics committee. Consent was informed, implied and confirmed by both ticking on the questionnaire and submitting the completed questionnaire. A sample size calculation based on an estimated 33 % prevalence of provision of iodine-related information during pregnancy( Reference Williamson, Lean and Combet 20 ), a 95 % CI, a design effect of two and an absolute precision of 5 % resulted in a sample size of at least 680 women. To allow for subgroup comparisons, a sample size of at least n 823 was required (α = 0·05, 1 − β = 0·8), based on the prevalence of iodine-related information cited above, and to detect a difference in prevalence of low iodine intake of 15 % between groups( Reference Gorstein, Sullivan and Parvanta 21 ).
Study design and participants
The study was cross-sectional, and the participants were recruited (1) using convenient sampling in Greater Glasgow, and (2) via distribution of an online version of the questionnaire in the UK, between July 2011 and February 2012. In greater Glasgow, both public and private locations were used for recruitment, including mothers and toddler groups, pregnancy classes, shops, libraries, community playgroups, public parks, playgrounds and indoor soft play areas. Recruitment posters were displayed with the study team contact detail as well as a link to the electronic form of the survey. In parallel, women were approached as they entered or exited the facility/shop. The online version of the questionnaire was posted on parenting websites and forums (mumsnet.com, netmums.com, bounty.com, pregnancyforum.org.uk and Gurgle.com) as well as regional Gumtree groups throughout the UK, parenting Facebook groups (Single Mums UK, West of Scotland Birth Support Group, Teenage Mums, Young Mums, British Mum and Toddler Group and North London Mums) and via Twitter. To be eligible, participants had to be women residing in the UK (England, Scotland, Northern Ireland or Wales) who were either pregnant or mother to a child aged from 0 to 36 months.
Questionnaire design and data collection
Data were collected anonymously through questionnaires employing qualitative and semi-quantitative measures. The questionnaire was validated before use for face value and content (content validation was carried out independently by three subject experts, assessing the representativeness of the items, while face validation was carried out by five lay contributors, assessing presentation, phrasing and clarity of instructions). The same questionnaire was used locally and online. It consisted of four sections.
The first section focused on participants' socio-demographic characteristics (age, ethnicity and education), details of specific dietary habits (veganism, vegetarianism, lactose intolerance and other specific requirements) and existence of thyroid condition and lifestyle habits during pregnancy (alcohol and tobacco consumption). It also included questions regarding the participant's current/most recent pregnancy (due date, parity, age of last child and whether the pregnancy was planned) and breastfeeding intentions.
The second part of the questionnaire assessed participants' awareness of pregnancy-specific dietary recommendations, their usual source of information and level of understanding of these recommendations. A 7-point Likert scale assessed how closely participants followed dietary recommendations in pregnancy and the likelihood of ceasing the consumption of dairy and seafood in case of any doubt regarding the safety of consuming the item.
The third section of the questionnaire included a validated iodine-specific FFQ( Reference Combet and Lean 10 ). A question focused on pregnancy vitamins and supplements, evaluating frequency of intake (every day or some days) and pregnancy period when supplements were taken (first trimester (T1) only, or throughout pregnancy), along with brand names.
Multiple choice and open questions investigated the reasons motivating any dietary changes during pregnancy, as well as awareness about micronutrient requirements and confidence about the level of information received to achieve requirements.
The final section of the questionnaire assessed knowledge about iodine (food sources, and consequences of maternal deficiency on the offspring via closed and multiple choice questions) and confidence in how to achieve the recommended iodine intake (7-point Likert scale).
Data analysis
Data were entered manually in a database, or downloaded from the University of Glasgow server hosting the electronic survey. The overall database was cleaned, and descriptive statistics were calculated for all outcome variables. Open text questions were reviewed for key themes. Results were expressed as: mean and standard deviation for parametric continuous data; median and interquartile range (IQR) for non-parametric continuous data; mode and frequencies for categorical data. The FFQ was analysed as described by Combet & Lean( Reference Combet and Lean 10 ), and dietary iodine intake was reported as a single value for the whole pregnancy. Supplemental iodine intake was defined according to the brand name, the frequency (‘every day’, or ‘some days’ estimated as every other day). Since supplemental iodine intake was reported either throughout the pregnancy or in T1 only, total iodine intake was calculated for T1, and the second and third trimesters (T2 and T3) separately.
The chosen cut-off for adequate iodine intake was the WHO recommendation for pregnant women (250 μg/d)( 1 ) and not that of the UK Reference Nutrient Intake( 22 ); this choice was motivated by the fact that the UK Reference Nutrient Intake has not been revised since 1991, despite evidence that iodine requirement increases during pregnancy( 1 ). Comparison between groups was carried out using the χ2 test for categorical data, the Student's t test for parametric continuous data, or the Mann–Whitney U test for continuous non-parametric data.
A multinomial logistic regression analysis was performed for the prediction of dietary iodine intake, total iodine intake (dietary intake plus supplements) during T1 and during T2 and T3. The categories for iodine intake were defined as: less than 140 μg/d, which is the cut-off for adequacy in adults; 140–250 μg/d which is between the cut-off of adequacy for adults and the recommended intake for pregnant women; and >250 μg/d( 1 ). Because iodine intake might be affected by a range of factors (socio-economic and existing knowledge of the iodine importance), relevant independent variables were included in the model. These were age, education level (school, college or university), ethnicity (British, other white groups, other ethnic groups), smoking status, having received any information on iodine or Ca, and being aware of the importance of iodine for healthy development of the unborn baby. The statistical software SPSS version 21.0 (IBM Corporation) was used.
Results
Participants' characteristics
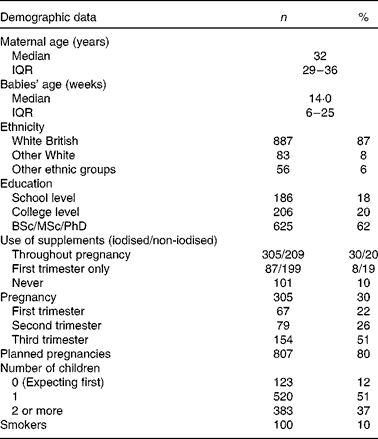
A total of 1026 women took part in the survey, 30 % of whom were pregnant at the time of the study. The median age of participants was 32 (IQR 29–36) years with their youngest child of a median age of 14 (IQR 6–25) weeks. Most had one child (51 %), 37 % had two or more children while 12 % were pregnant with first child. Most pregnant women were in T3 of pregnancy (51 %). Most pregnancies (80 %) were planned and discovered at 4 (IQR 4–6) weeks. The majority of participants were White British (87 %), and had degree level education or more (62 %) (Table 1).
Table 1 Basic characteristics of participants (Number of participants and percentages; median and interquartile ranges (IQR))
General nutritional awareness during pregnancy
The majority of women (96 %) reported awareness of dietary and lifestyle recommendations specific for pregnancy, such as those currently provided in the Ready Steady Baby NHS book or websites on pregnancy diet (such as the Food Standard Agency ‘Eating while you are Pregnant’)( 13 ). The main sources of information were the internet (65 %) and books and magazines (62 %), followed by written and oral advice from their doctor (59 and 52 %, respectively), and family and friends (43 %). Only 16 % received information during antenatal classes. The majority (90 %) were aware of recommendations about smoking, alcohol and caffeine. However, a third (34 %) of smokers continued smoking during pregnancy, and 14 % of the total population did not stop or limit alcohol consumption.
Most of the respondents found the dietary recommendations easy to understand (92 %), and easy to follow (83 %). There was a high level of awareness for most dietary recommendations for pregnancy with the exception of the recommendation for vitamin A intake (Table 2). Confusion over the recommendations was reported by 47 % (n 482) of the overall population, with 41 % (n 419) seeking clarification or further information. The internet was the main source of complementary information (82 %), followed by Health Care Professionals (18 %), books (15 %), and family and friends (8 %).
Table 2 Pregnancy dietary recommendations* – self-reported awareness and confusion

N/A, not applicable.
* Recommendations available in the National Health Service Ready Steady Baby book, and the Food Standard Agency ‘Eating while you are pregnant’( 13 ).
† Folic acid, cheese, eggs, nuts, vitamin D.
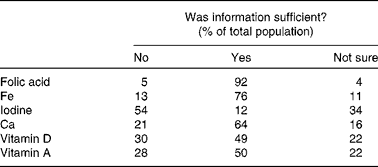
Information provision about specific nutrients (folic acid, Fe, iodine, Ca and vitamins A and D) varied. All the participants (100 %) had heard about folic acid, and most had heard about Fe (96 %) in pregnancy. However, 64 % of the mothers had never received information about iodine, and only 11 % had heard about iodine from a health care professional (Table 3). Only 12 % reported that information received was sufficient for iodine, followed by 50 and 49 % for vitamin D and vitamin A (Table 4).
Table 3 Sources of information for specific nutrients (respondents could select several options).
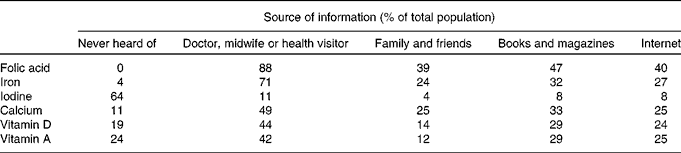
Table 4 Perceived sufficiency of the information received regarding specific nutrients in order to make decisions on dietary modification to achieve adequate intake/levels in pregnancy
Changes in dietary habits during pregnancy
Only a minority of the participants were vegetarian (9 %), vegan (1 %) or lactose intolerant (1 %), and 6 % of the mothers reported an existing thyroid condition. Salt was added to food by 48 % of women (at the table or during cooking).
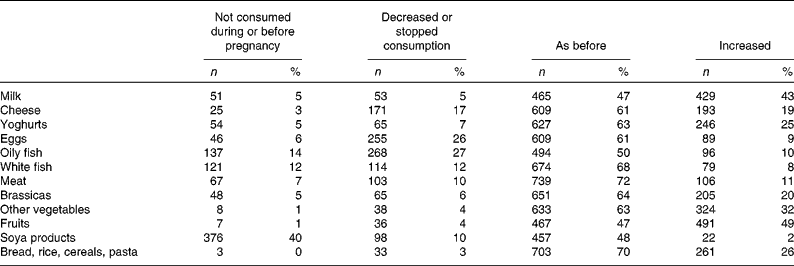
Most reported increased fruit consumption during pregnancy (49 %). The majority reported similar levels of intake for milk, cheese, yoghurts, eggs, oily fish, white fish, meat, brassicas, other vegetables, soy products and grains, cereals and pasta during pregnancy, compared to intake levels before pregnancy (Table 6). Increased intakes for milk, cheese and yoghurt were reported by 43, 19 and 25 %, respectively (Table 5).
Table 5 Reported changes in the intake of specific foods during pregnancy
Nearly half (44 %) of changes in dietary patterns were motivated by recommendations and advice specific for food and diet during pregnancy. Foods most often mentioned were fruit (8 %), fish (9 %), cheese (6 %) and milk (6 %). Morning sickness was the reason motivating 30 % of the dietary changes reported, mostly meat, fish and milk. Other reasons were heartburn (21 %) and change in taste (7 %).
High adherence to dietary recommendation was reported (mode 6, frequency 33 % on a 7-point Likert scale), which was reflected in choices to withdraw from consuming a particular type of food, if the safety of consuming the item could not be confirmed (cheese: mode 7, frequency 25 %; fish: mode 7, frequency 31 %).
Iodine intake during pregnancy
Without taking supplements into consideration, median dietary iodine intake in pregnancy was estimated at 190 (IQR 144–256) μg/d, with 74 % consuming below the recommended 250 μg iodine/d, and 55 % below the proposed 200 μg/d threshold (Fig. 1). The highest contributors to iodine intake were milk (40 %, contributing toward 75 (IQR 42—113) μg/d, followed by other dairy products (31 %) and fish (24 %).

Fig. 1 Iodine intake in pregnancy in 1026 women, recruited in the UK, August 2011–February 2012, according to set levels of adequacy from WHO/United Nations Children's Fund/International Council for the Control of Iodine Deficiency Disorders(
1
) (>250 μg/d) and European Food Safety Authority panel on Dietetic Products, Nutrition and Allergies(
4
) (>200 μg/d). Supplement use for each sector of iodine intake is depicted on the inside circle. Only 26 % during first trimester (T1) and 25 % during second (T2) and third trimester (T3) reached the 250 μg threshold through diet only (regardless whether they consumed iodised supplements). The new proposed level of adequate intake (200 μg/d) was reached by 63 % during T1 (26 % thanks to supplements) and 58 % during T2 and T3 (21 % thanks to supplements). ■, With iodised supplements; □, without iodised supplements; ![]() , dietary intake sufficient, and also took iodised supplements.
, dietary intake sufficient, and also took iodised supplements.
Daily iodine intake from foods was not different between women taking supplements or not (P= 0·36). However, taking supplements significantly increased total iodine intake, both during T1 (237 (IQR 163–320) v. 190 (IQR 144–256) μg/d, non-supplemented) and during T2 and T3 (223 (IQR 157–309) v. 190 (IQR 144–256) μg/d, non-supplemented) (P< 0·001).
Knowledge of iodine-rich foods and awareness of its role during pregnancy
Knowledge of iodine-rich foods was low, with 56 % unable to identify any iodine-rich food and a majority wrongfully believing that dark green vegetables (54 %) and table salt (21 %) (which is not fortified in the UK) are iodine-rich foods. Milk and yoghurt were only recognised as iodine-rich sources by 9 and 6 % of the population, respectively, with fish faring slightly better (33 % for oily fish and 14 % for white fish).
While 84 % were unaware that, during pregnancy, iodine from the diet is important for healthy development of the unborn baby, most mothers (85 %) agreed or strongly agreed they would attempt to increase their iodine intake if made aware of the impact of iodine deficiency (7-point Likert scale). Mothers (72 %) however, disagreed or strongly disagreed that they were confident on how to achieve an adequate iodine intake in pregnancy (7-point Likert scale).
Impact of dietary advice and awareness on iodine intake
Receiving any advice on iodine (n 371, 36 %) had no impact on iodine intake from food only (P= 0·218), or intake from food and supplements in the T1 of pregnancy (P= 0·106). However, intakes were marginally higher in T2 and T3 for those who had received information (P= 0·049) (Table 6). Those who perceived the advice to be sufficient (n 112, 12 %) had no higher iodine intake from either food only during the whole pregnancy (P= 0·974), or from food and supplements during T1 (P= 0·402) or during T2 and T3 (P= 0·530).
Table 6 Iodine intake with and without supplements in the whole group, in women having received advice on iodine, advice on calcium and in women who were aware of iodine importance during pregnancy† (Medians and interquartile ranges (IQR))

T1, first trimester; T2, second trimester; T3, third trimester.
* Iodine intake values were significantly different from the rest of the group (P< 0·05; Mann–Whitney U test).
† Advice received could be from any source (doctors, midwives or health visitors, family and friends, books and magazines or from the internet).
‡ Total indicates iodine from food and supplement sources.
Receiving any advice on Ca (n 911, 89 %) increased iodine intake from food only (P= 0·009), intake from food and supplement in T1 of pregnancy (P= 0·001), and intake in T2 and T3 (P= 0·001) (Table 6). Participants who perceived the Ca advice to be sufficient (n 610, 64 %), had a higher intake of iodine from food only (P= 0·014), and food and supplement in T1 (P< 0·001), as well as T2 and T3 (P< 0·001).
Awareness of the impact of low iodine during pregnancy on healthy development of the unborn baby (n 165, 16 %) did not lead to significantly higher iodine intake, with or without supplementation (food only P= 0·782, from food and supplement in T1 P= 0·905, or T2 and T3 P= 0·660).
Other factors affecting iodine intake in pregnancy
Planned pregnancies, salt usage and smoking did not impact on iodine intake (with or without supplement). However, education level had an impact on dietary iodine intake (P= 0·009) and total iodine intake in T2 and T3 (P= 0·010), with higher intake generally associated with higher education levels. Total iodine intakes in T2 and T3 were also higher in older women compared to younger (P= 0·036).
The multinomial logistic regression models (Table 7) for iodine intake from the diet alone, total iodine intake in T1, and T2 and T3 had an improved fit, compared to the empty models (models with no predictor variables) (P= 0·05, 0·02 and 0·01, respectively), with low pseudo R 2 values (below 0·05). Receiving information about Ca significantly lowered the odds of having a low iodine intake ( < 140 μg compared to >250 μg/d) at any stage of pregnancy, from diet alone, or taking supplements in consideration. It also decreased the odd of having a total iodine intake between 140–250μg compared to >250μg, in T1, and T2 and T3. Those who had ever been informed about iodine had surprisingly higher odds of having a low total iodine intake ( < 140 μg compared to >250 μg/d) in T1, T2 and T3. Being aware of the importance of iodine had no predictive value in the models. Education did not consistently predict iodine intake, with achieving school education predicting higher odds of lower total iodine intake ( < 140 μg compared to >250 μg/d) in T2 and T3.
Table 7 Logistic regression analysis for predictors of dietary iodine intake (throughout pregnancy), total iodine intake in the first trimester (T1) and total iodine intake in trimesters 2 (T2) and 3 (T3)† (Odds ratios and 95 % confidence intervals)

* P< 0·05.
** P< 0·01.
† Total indicates iodine from food and supplement sources.
Discussion
Principal study findings
Dietary iodine intake in pregnancy was lower than the WHO's recommendation of 250 μg/d, even when supplemental iodine was taken in consideration. This is consistent with recent findings in pregnant women in the South East of the UK( Reference Bath, Walter and Taylor 15 ). Despite generally high self-reported awareness of existing dietary and lifestyle recommendations for pregnancy, iodine awareness and knowledge were low among the UK mothers (pregnant or with children aged 0–36 months). This is in agreement with those of mothers in other countries, where a large gap still exists for iodine awareness and knowledge( Reference Fowles 16 , Reference Charlton, Gemming and Yeatman 17 , Reference Moraveji, Farmanbar and Soleimannezhad 23 ).
Iodine is crucial during pregnancy and the first few months of child life, to ensure adequate brain development, driven by the thyroid hormones. Mild insufficiency has been linked to measurable cognitive decline in school performance and cognition( Reference Bath, Steer and Golding 2 , Reference Bougma, Aboud and Harding 24 ). A recent meta-analysis of twenty-four studies on iodine and intellectual disability in young children concluded that iodine insufficiency leads to mental impairment, and this is apparent when comparing the intelligence quotient score of children of iodine-deficient mothers, which is 7·4 points lower compared to that of children of iodine-replete mothers( Reference Bougma, Aboud and Harding 24 ). Although many of the studies included in the review suffered from poor design, with only a few randomised controlled trials, there is some evidence that iodine deficiency impacts on cognitive function. In particular, supplementation with 300 μg iodine during T1 of pregnancy led to improved cognitive development in the offspring( Reference Velasco, Carreira and Santiago 25 ). It is therefore a major concern that the majority of (recently) pregnant women in the UK are unaware of the importance of iodine, when the evidence points toward inadequate iodine status in women( Reference Vanderpump, Lazarus and Smyth 14 , Reference Bath, Sleeth and McKenna 26 , Reference Lampropoulou, Lean and Combet 27 ) in a country with no iodine prophylaxis.
Awareness is a means of empowerment in any case of choice. While the messages about folic acid and Fe were heard (from various sources) and perceived to be sufficient, mothers were not confident about their iodine intake, in terms of dietary sources or how to meet the adequate levels for pregnancy, in accordance with recent Australian findings( Reference Charlton, Gemming and Yeatman 17 ).
The UK has been listed as the top eighth iodine-deficient country in the world( Reference Andersson, Karumbunathan and Zimmermann 28 ). Participants of the present study had an average daily iodine intake of 190 μg/d from food only, which is lower than the recommended 250 μg/d during pregnancy( 1 ), and the newly proposed 200 μg/d EFSA threshold( 4 ). In fact, through their diet, only 26 % of women were able to meet the 250 μg/d recommendation, and 45 % the 200 μg/d mark. Milk and other dairy products were the main contributors to iodine intake, in agreement with the data of 1997–8( Reference Fordyce 29 ).
Taking supplements containing iodine in consideration (consumed by 38 % of the participants), the daily iodine intake was 237 μg/d in T1 and 223 μg/d in T2 and T3. Use of iodised supplements helped women to achieve an adequate iodine intake. It has been shown that nutritional knowledge is strongly associated with the use of supplements during pregnancy( Reference Popa, Niţă and Graur Arhire 30 ). Iodine supplementation given to pregnant women with mild deficiencies appeared to benefit subsequent child development( Reference Melse-Boonstra and Jaiswal 31 ). However, in the present study less than half of the participants were taking iodine containing supplements, in line with previous findings( Reference Bath, Sleeth and McKenna 26 , Reference Kibirige, Hutchison and Owen 32 – Reference Zimmermann and Delange 35 ). This is despite the fact that popular prenatal multivitamin brands do contain iodine; but in practice many women take single folic acid supplement or a formulation not labelled for pregnancy or not containing iodine( Reference Bath, Sleeth and McKenna 26 ).
While healthcare professionals are well placed to impart advice and help effect dietary change, studies indicate that women's dietary patterns change little during pregnancy( Reference Cuco, Fernandez-Ballart and Sala 36 – Reference Inskip, Crozier and Godfrey 38 ). The present results are in agreement with this observation, and consumption levels of milk, dairy and fish were mostly unchanged during pregnancy, despite the increased daily requirement for iodine. Similarly, another UK cohort study examining dietary patterns before and at two points during pregnancy showed that the median weekly consumption of iodine-rich foods did not change significantly( Reference Crozier, Robinson and Godfrey 39 ).
Strengths and weaknesses of the study
Deprivation, defined by income or education, has been shown to be associated with increased risks of insufficient micronutrient intake( Reference Haggarty, Campbell and Duthie 40 ) and poorer quality diet( Reference Berti, Decsi and Dykes 41 ). In the present study, levels of deprivation based on postcodes could not be used, due to differences between the English, Scottish, Northern Irish and Welsh deprivation scoring. Women of higher socioeconomic status (defined by education, income, and/or occupation) are also more likely to consume iodine-rich foods, such as fish and dairy( Reference Darmon and Drewnowski 42 ). In the light of this situation, it appears that women of lower socioeconomic status may be less likely to achieve sufficient iodine intake during pregnancy.
The present study used a FFQ( Reference Combet and Lean 10 ) involving an element of recall, which may have led to a loss of accuracy and overestimation of the nutrient intake( Reference Rasmussen, Ovesen and Bulow 43 ). The convenient sampling method used locally, and the electronic recruitment used nationally have yielded a large sample size. A majority (68 %) of the participants were recruited online. While this was considered to be a limitation in the past( Reference Azar 44 ), the wide access to the internet in the UK renders online recruitment a successful method in health research( Reference Brown and Arnott 45 , Reference Alcalde 46 ). The study participants were generally quite knowledgeable about health, had a high rate of planned pregnancies (80 v. 55 % in the UK population( Reference Wellings, Jones and Mercer 47 )) and were well-educated. This feature appears to be common in survey-based studies, but surprisingly the results show a poor knowledge about iodine even amongst educated women.
Possible mechanisms and implications for clinicians and policy makers
Achieving an intake of 250 μg/d (or 1750 μg/week) is challenging and requires consumption of high amounts of dairy and seafood. For illustrative purpose, 250 μg/d( 1 ) (or 1750 μg/week) would mean consuming all of the following: milk in cereals once a day, milk in drinks (such as tea, coffee) three times a day, two yogurts per day, one dairy-based dish or pudding per day, cheese twice a day, white sea fish twice a week and oily fish once a week (based on the average iodine content of these foods( Reference McCance and Widdowson 48 )). However, receiving information about Ca and iodine during pregnancy, along with a higher education level, predicted sufficient iodine intake; there is hence scope for improved dietary recommendations to address the present iodine insufficiency in this vulnerable group.
Helping pregnant women with resources which ensure causal links between iodine and fetal development may increase the motivation for behavioural change( Reference Skeaff 49 ), as the present study has also observed. The Health Belief Model encompasses this idea. Perceived threats to health can alter behaviour if the individual is confident of carrying out the change; there is an understanding that changed actions will reduce the susceptibility or severity of a health condition, and that motivating factors outweigh the barriers that stand in the way of implementing the behavioural change( Reference Athearn, Kendall and Hillers 50 ).
There is in the UK no guideline on iodine supplementation for mothers during pregnancy or lactation, in contrast to the USA and Canada( Reference Becker, Braverman and Delange 51 ). Such a supplementation during pregnancy and lactation is endorsed by the WHO, the UNICEF and the ICCIDD for iodine-deficient countries without universal salt iodisation, such as the UK( Reference Andersson, de Benoist and Delange 52 ). There is a sustained debate on the ethical implication of a randomised controlled trial of iodine supplementation in pregnancy( Reference Stagnaro-Green, Sullivan and Pearce 53 , Reference Bath, Jolly and Rayman 54 ), in parallel with concerns over the conflicting message that salt iodisation would convey( Reference Bath, Button and Rayman 55 ). A recent review of the Scientific Advisory Committee on Nutrition published their position statement on iodine and health( 56 ), highlighting existing gaps in the evidence base. The present study made an attempt to fill some gaps in evidence on dietary and supplemental intakes of iodine in pregnancy, and underscore a greater understanding of mothers' knowledge and awareness of recommendations relevant to iodine in pregnancy.
Unanswered questions and future research
Many pregnancies remain unplanned in the UK (approximately 55 % planned) and in other high-income countries (France, Spain, Japan and the USA)( Reference Wellings, Jones and Mercer 47 ). Iodine prophylaxis in the peri-conception stage requires further considerations. A stronger evidence-base is required in order to set thresholds for adequacy of iodine intake during and before pregnancy, with careful consideration of iodine uptake and homeostasis as a function of iodine stores.
A recent systematic review concluded that women in developed countries are not nutritionally well-educated, specifically about nutrition during pregnancy( Reference Lucas, Charlton and Yeatman 57 ). The iodine awareness, knowledge and perception of mothers in the UK were high for general recommendations during pregnancy, but low for iodine. Therefore health campaigns, fortification, supplementation and nutrition education should be seriously considered. It is unclear whether any of these strategies will be successful, in the context of the present UK food landscape and dietary habits. We have previously shown that the terminology used to define concepts of nutritional balance is commonly misunderstood by the public( Reference Buckton, Combet and Lean 58 ). In addition to providing causal links, dietary recommendations should be accurate and simple to understand, and give practical advice which is easy to follow. Consistency in the advice provided (by healthcare professionals, websites and books) is essential, to avoid misinterpretations and misleading messages. The current debate on the next required step for iodine prophylaxis in the UK should not ignore the fact that the impact of any intervention will be blunted if the current lack of awareness and knowledge is not tackled first. There are significant differences between awareness of exact requirements at population level, awareness of the need to take supplements (which currently only applies to folic acid in the UK) and awareness of the importance of a nutrient during a crucial stage in the life of women; the present research problem needs further investigations using a more qualitative approach on a global scale.
Acknowledgements
C. O. C. was in receipt of a scholarship from the Rank Prize Funds.
The authors declare there are no conflicts of interest.
The authors' contributions are as follows: E. C. designed and supervised the study, with input from C. O. C. and M. E. J. L; C. O. C., M. B. and B. P. collected the data and contributed to its analysis; E. C. and M. B. prepared the manuscript, with input from all co-authors. All authors raed and approved the final version of the manuscript.











