Peripheral artery disease (PAD) in the lower extremities is a chronic atherosclerotic disease characterised by stenosis and occlusion of the arteries, covering a clinical spectrum from no symptoms to effort-induced ischaemic muscle discomfort and/or pain and critical ischaemia with tissue loss(Reference Hiatt, Goldstone and Smith1–Reference Patel, Conte and Cutlip3). Symptomatic PAD is associated with functional limitations, diminished quality of life and a high risk for major cardiovascular events and death(Reference Steg, Bhatt and Wilson4, Reference Fowkes, Aboyans and Fowkes5). The global burden of PAD is expected to increase markedly in the near future and identification of factors that may lower disease risk is urged(Reference Fowkes, Rudan and Rudan6).
The plant-derived n-3 fatty acid α-linolenic acid (ALA) is a precursor of long-chain (LC) n-3 PUFA, which may influence inflammatory processes that may be involved in development and progression of atherosclerosis(Reference Calder7–Reference Chang and Deckelbaum9). However, the conversion capacity of ALA into LC n-3 PUFA may be limited in humans(Reference Baker, Miles and Burdge10). ALA may possibly exert health benefits independent of its precursor role(Reference Rajaram11) and has been suggested to be an important nutrient in the Mediterranean diet(Reference de Lorgeril and Salen12), which has ascribed many health benefits including lowering risk of PAD(Reference Estruch, Ros and Salas-Salvadó13) and major cardiovascular events(Reference Ruiz-Canela, Estruch and Corella14).
The majority of previous follow-up studies investigating the association between ALA intake and the risk of atherosclerotic CVD have focused on CHD. Some cohort studies have reported inverse associations between ALA intake and CHD risk(Reference Hu, Stampfer and Manson15–Reference Koh, Pan and Wang20), but the results were not consistent(Reference Vedtofte, Jakobsen and Lauritzen18, Reference Bork, Jakobsen and Lundbye-Christensen21–Reference Vedtofte, Jakobsen and Lauritzen27). Few studies have investigated associations between ALA intake and the risk of ischaemic stroke(Reference de Goede, Verschuren and Boer22, Reference Fretts, Mozaffarian and Siscovick23, Reference Rhee, Kim and Buring28–Reference Bork, Venø and Lundbye-Christensen30), but to our knowledge no previous follow-up studies have investigated the association between ALA intake and the risk of PAD.
The objective of the present study was to investigate the association between intake of ALA and the risk of PAD. We hypothesised that intake of ALA would be inversely associated with the risk of incident PAD.
Methods
Study population and design
This follow-up study was based on data from the Diet, Cancer and Health cohort that was established to investigate the role of diet and lifestyle in relation to cancer and other chronic diseases(Reference Tjønneland, Olsen and Boll31). The recruitment procedures, sample size considerations and collection of data have been described in detail elsewhere(Reference Tjønneland, Olsen and Boll31). Briefly, native citizens aged 50–64 years who were living in and around Copenhagen and Aarhus in Denmark without a previous diagnosis of cancer were invited between 1993 and 1997 to participate in the study. Potential eligible participants were identified through the Danish Civil Registration System in which every citizen living in Denmark is provided with a unique identification number(Reference Tjønneland, Olsen and Boll31).
The Diet, Cancer and Health cohort study was conducted according to the guidelines laid down in the Declaration of Helsinki and approved by the Health Research Ethics, Capital Region of Denmark, and the Danish Data Protection Agency. All participants gave written informed consent at inclusion(Reference Tjønneland, Olsen and Boll31).
In the present follow-up study, participants registered with a diagnosis of cancer not registered in the Danish Cancer Registry at the time of invitation due to processing delay as well as participants registered with a diagnosis of PAD or chronic kidney disease before enrolment were excluded. Also, participants for whom information on exposure and/or covariates was missing were excluded. The current study has been approved by the Danish Data Protection Agency (2008-58-0028-2016-229).
Exposure assessment
Information on habitual diet over the past 12 months was assessed at baseline using a 192-item semi-quantitative FFQ(Reference Overvad, Tjønneland and Haraldsdóttir32). The average consumption of foods and beverages was reported within twelve categories ranging from never to eight or more times per day. The reported intakes were used to estimate daily intake of ALA and total energy based on Danish food composition tables (version 1.3.2) with the use of the software program FoodCalc (Center for Applied Computer Science, University of Copenhagen; www.ibt.ku.dk/jesper/foodcalc). The FFQ used has previously been validated against two 7-d diet weighed records and found useful for categorising individuals according to their intake of energy and polyunsaturated fat(Reference Tjønneland, Overvad and Haraldsdóttir33). The intake of ALA was expressed as energy-adjusted intake in g/d using the residual method(Reference Willett and Stampfer34).
Covariates
Detailed information on social, lifestyle and health aspects such as length of schooling, smoking habits, physical activity, history of hypercholesterolaemia, hypertension and diabetes, and use of lipid-lowering or anti-hypertensive agents and insulin were collected at baseline using a self-administered questionnaire. The questionnaire was processed by optical scanning and checked for reading errors and omissions at the two study centres where anthropometric measurements including height, weight and waist circumference were also obtained at enrolment. Information on alcohol consumption, total energy intake and intake of other nutrients were obtained from the FFQ.
Outcome assessment
Incident cases of PAD were identified through record linkage with the Danish National Patient Register, which includes information on outpatient visits and discharge diagnoses from all hospitals in Denmark(Reference Andersen, Madsen and Jørgensen35, Reference Lynge, Sandegaard and Rebolj36).
Potential cases of PAD included participants registered with either a primary or secondary discharge diagnosis of PAD according to the International Classification of Diseases codes (International Classification of Diseases-8: 44390; 44500; 44509; 44590; 44599; 44020 and 44030, and International Classification of Diseases-10: I70.2, I70.2A and I73.9A-C). Subsequently, all potential cases of PAD were validated by review of medical records(Reference Lasota, Overvad and Eriksen37). A registered diagnosis of PAD was considered as valid in patients with an ankle brachial index < 0·9, a toe brachial index < 0·7 and/or demonstration of radiologically significant stenosis or calcifications in relevant arteries in the lower extremities. Patients with an ankle brachial index > 1·4 together with a history of diabetes mellitus or treatment by chronic renal dialysis were also considered valid PAD cases. Furthermore, patients who experienced a blood pressure drop in the lower extremities of more than 30 mmHg or a 20 % drop of the resting ankle brachial index immediately after a treadmill test and patients who underwent relevant vascular surgery for atherosclerosis were included as cases. Finally, patients who qualified for vascular surgery for atherosclerosis although not performed due to severe comorbidity were also included as cases(Reference Lasota, Overvad and Eriksen37).
Participants were followed up from baseline until first registration of PAD, death, emigration or end of follow-up in December 2009.
Statistics
Hazard ratios were used to describe associations between energy-adjusted ALA intake and the rate of PAD. Hazard ratios with 95 % CI were calculated using Cox proportional hazard regression with age as underlying timescale allowing for separate baseline hazards among men and women.
Dietary intake of ALA was analysed as a continuous exposure variable using restricted cubic splines with three knots with the median intake as reference in order to visualise the shape of the observed associations. The knots were placed at the 10th, 50th and 90th percentile as recommended by Harrell(Reference Harrell38). The spline curves were formally tested against a horizontal line using Wald tests. The spline plots were restricted to the 95 % central range and presented with 95 % CI. Also, analyses with ALA intake divided into quintiles were conducted using the lowest quintile as reference.
We investigated the association between ALA intake and rate of PAD in three different models specified prior to data analysis. In model 1A, we included baseline age (continuous, years) in order to ensure comparison of participants for whom reported exposure information was of the same age. In model 1B, we additionally included information on established risk factors for PAD including length of schooling (≤7, 8–10 or >10 years), smoking (never, former, current 1–14, 15–24 or >24 g/d), physical activity (inactive, moderately inactive, moderately active or active), waist-circumference (continuous, cm), BMI (continuous, kg/m2) and alcohol intake (continuous, g/d). In model 2, we included the following co-morbidities, which may be considered potential intermediate variables: self-reported history of hypercholesterolaemia and/or use of lipid-lowering medication (yes, no or unknown), hypertension and/or use of anti-hypertensive medication (yes, no or unknown) and diabetes mellitus and/or use of insulin.
The proportional hazard assumption was evaluated by plotting the scaled Schoenfeld residuals against age at event.
In sensitivity analyses, we plotted the whole exposure range and examined whether the spline curves were robust when the number and location of the knots were modified. Also, additional adjustment for a history of myocardial infarction, ischaemic stroke or both prior to enrolment was undertaken as sensitivity analyses.
In supplemental analyses, we investigated the association between ALA intake and the rate of PAD in sex-specific analyses. Also, analyses including adjustment for established risk factors (model 1B) and hormone substitution were undertaken. In further supplemental analyses, potential dietary risk factors were added to model 1B including total energy intake without contribution from alcohol intake (continuous, kJ/d), intake of fibre (continuous, g/d), glycaemic load (continuous, unit-less), and intakes of SFA, MUFA, linoleic acid and marine LC n-3 PUFA (continuous, g/d) (model 3). All continuous covariates were entered into the models using restricted cubic splines with five knots.
Data were analysed using Stata statistical software (version 15; StataCorp LP), and a P value <0·05 was considered statistically significant.
Results
A total of 160 725 men and women were invited to participate in the Diet Cancer and Health cohort study and 57 053 accepted. We excluded 1805 participants because they had a diagnosis of cancer (n 569), PAD (n 330) or chronic kidney disease (n 31) before enrolment, or for whom information on exposure or other covariates was missing (n 960) (Fig. 1). Among the remaining 55 248 participants, we identified 950 participants who developed PAD during a median of 13·6 (95 % central range: 4·3–15·3) years of follow-up. The incidence rate of PAD was 1·32 per 1000 person years.
Baseline characteristics of the participants with complete information on covariates in the cohort and participants who developed PAD during follow-up are presented in Table 1.
Table 1. Baseline characteristics (Medians and 2·5th, 97·5th percentiles; percentages)
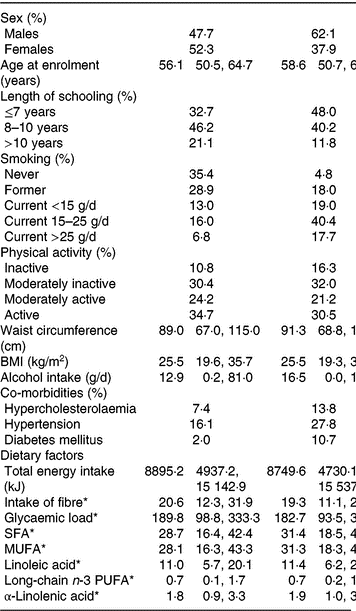
PAD, peripheral artery disease.
* Energy-adjusted intake.
The median energy-adjusted dietary ALA intake in the cohort was 1·76 g/d (95 % central range: 0·94–3·28).
In spline analyses including adjustment for sex and age (model 1A), we found a positive association between ALA intake and the rate of PAD (online Supplementary Fig. S1). However, in multivariable analyses including additional adjustment for established risk factors for PAD (model 1B) a weak statistically non-significant inverse U-shaped association between ALA intake and the rate of PAD was observed (P = 0·339) (Fig. 2). Additional adjustment for co-morbidities (model 2) also showed a weak inverse U-shaped association between ALA intake and the rate of PAD that was not statistically significant (P = 0·338) (online Supplementary Fig. S2). In supplemental analyses, with additional adjustment for dietary risk factors (model 3), we found a weak inverse association to PAD above the median ALA intake, but the overall association was not statistically significant different from a horizontal line (P = 0·314) (online Supplementary Fig. S3).
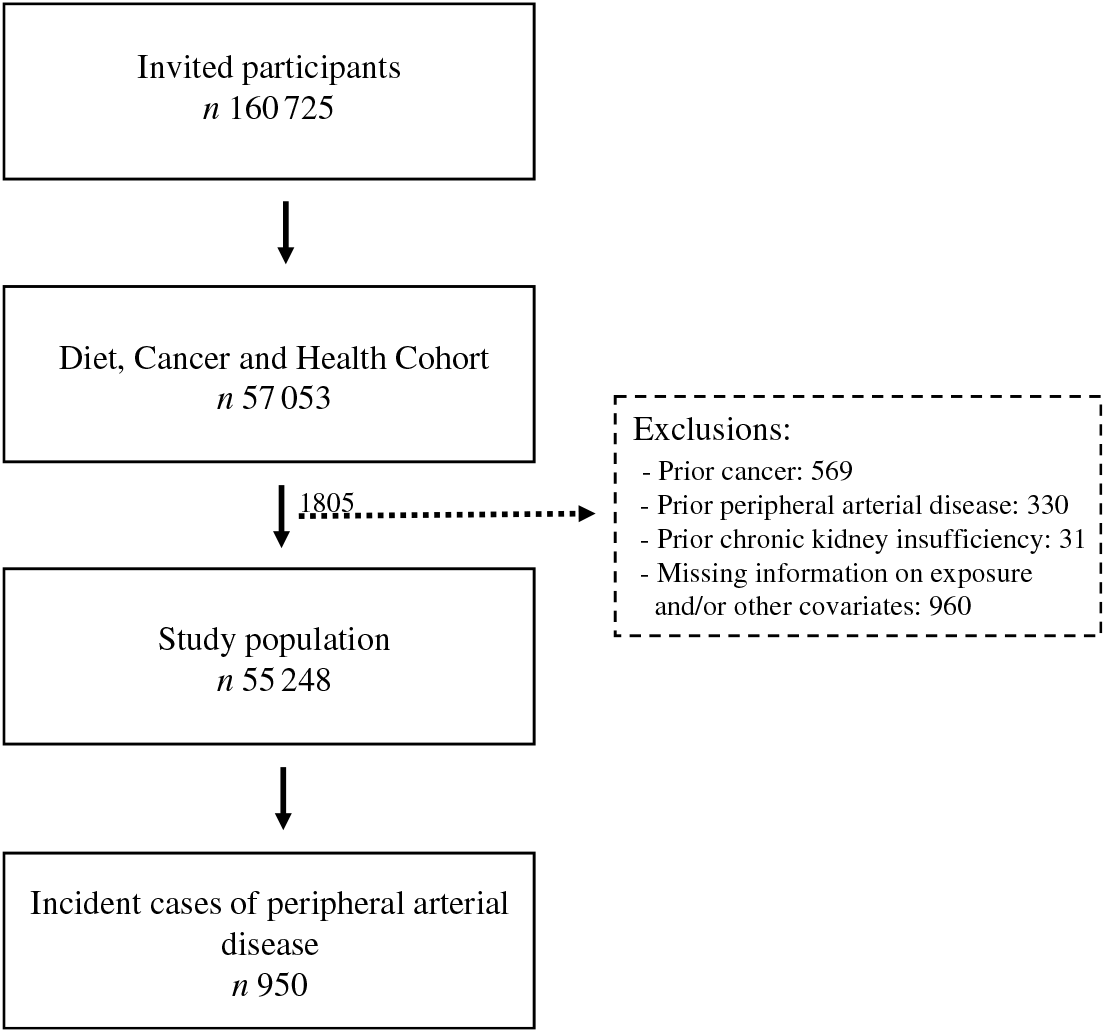
Fig. 1. Flowchart of participants in the Diet, Cancer and Health cohort and incident cases of peripheral artery disease identified during follow-up.
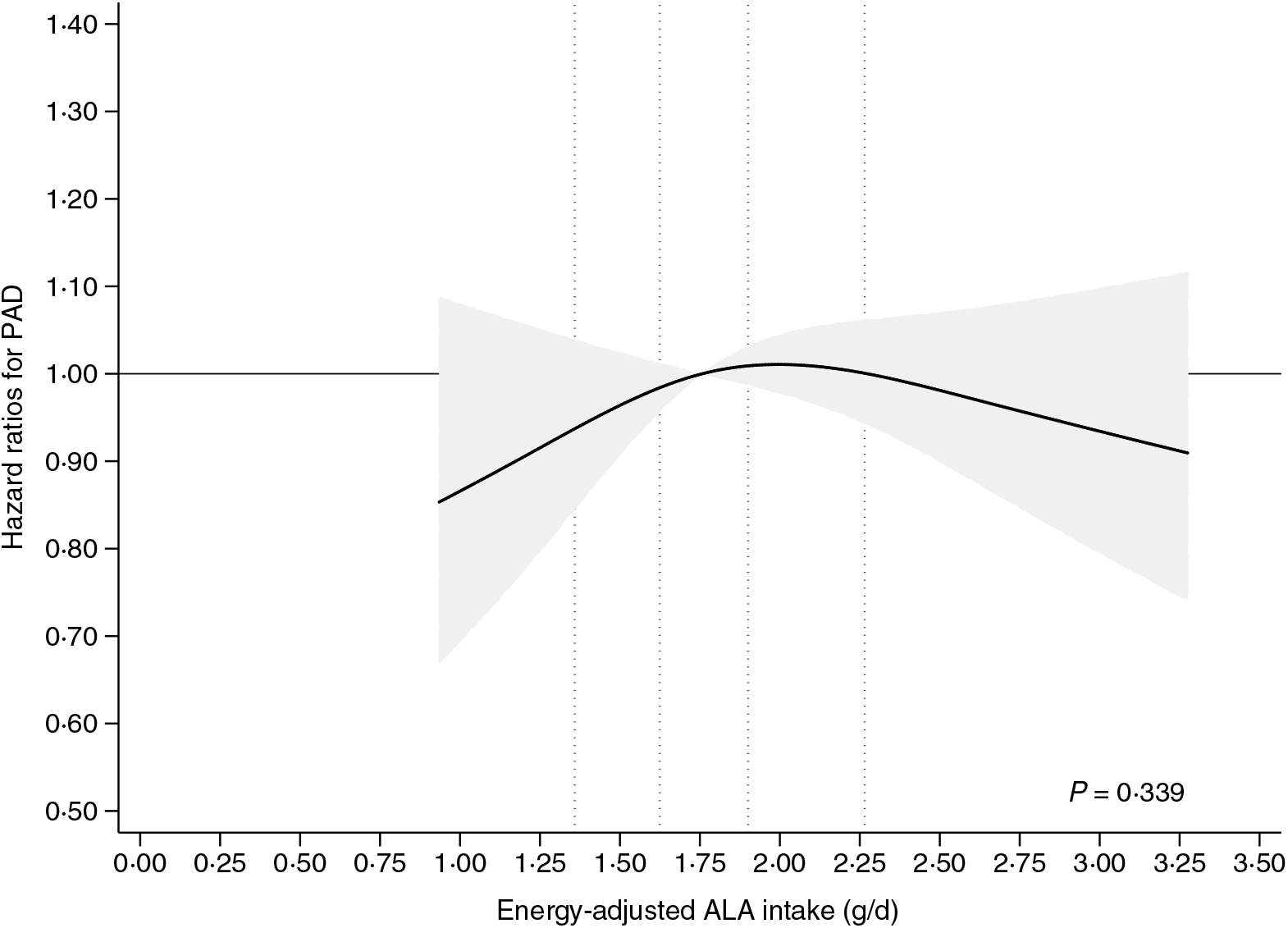
Fig. 2. Intake of α-linolenic acid (ALA) and the risk of incident peripheral artery disease (PAD). The multivariable analyses were conducted using Cox proportional hazard regression including adjustment for established PAD risk factors (model 1B) with the median intake of ALA as reference. The 20th, 40th, 60th and 80th percentiles of ALA intake are shown with dotted lines. The shaded grey area indicates the 95 % CI of hazard ratios of PAD ___. The spline plot is shown for the 2·5–97·5 percentiles of ALA intake.
Sensitivity analyses indicated that models with ALA intake modelled as restricted cubic splines were robust when the location and number of knots for the exposure of interest were modified.
Categorical analyses of the association between ALA intake in quintiles and the rate of PAD are presented in Table 2. We found similar patterns of associations in analyses of ALA intake in quintiles and the rate of PAD as in the spline analyses. The individual hazards in the second to fifth quintile were not statistically significantly different from the reference in the first quintile in either of the multivariable adjusted models. In supplemental analyses, additional adjustment for myocardial infarction and/or ischaemic stroke before enrolment did not influence the observed associations. Also, additional adjustment for use of hormone substitution did not influence the observed associations (data not shown).
Table 2. Quintiles of energy-adjusted α-linolenic acid (ALA) intake and risk for peripheral artery disease* (Hazard ratios (HR) and 95 % confidence intervals)

* Statistical analyses were conducted using Cox proportional hazard regression. All models were adjusted for sex by allowing baseline hazards among men and women to differ.
† Model 1A included baseline age.
‡ Model 1B included the variables of model 1A and the following risk factors for peripheral artery disease: length of schooling, smoking, physical activity, waist circumference, BMI and alcohol intake.
§ Model 2 included the variables of model 1B and the following potential intermediate variables: self-reported history of hypercholesterolaemia and/or use of lipid-lowering medication, hypertension and/or use of antihypertensive medication and diabetes mellitus and/or use of insulin.
‖ Model 3 included the variables of model 1B and the following potential dietary risk factors: total energy intake, intake of fibre, glycaemic load, and intake of SFA, MUFA, linoleic acid and long-chain n-3 PUFA.
Similar patterns of associations between ALA intake and the rate of PAD were observed when the analyses were conducted separately among men and women (online Supplementary Table S2).
No evidence of a departure from the proportionality assumption was observed in either of the models (data not shown).
Discussion
In this large follow-up study, we found indications of a weak inverse U-shaped association between ALA intake and the rate of PAD in analyses including adjustment for established risk factors and indications of a weak inverse association between ALA intake and the rate of PAD above the median intake in analyses including adjustment for established risk factors and dietary risk factors. However, none of these associations was statistically significant. Given the relatively weak and statistically non-significant associations, the present study suggests that dietary intake of ALA is not appreciably associated with the risk of PAD within this population of middle-aged Danish men and women. It should be stressed that the present study did not investigate the potential effect of a Mediterranean diet on PAD risk, but the results may indicate that the possible protective effect provided by the Mediterranean dietary pattern on PAD and major cardiovascular events is unlikely to be ascribed to ALA intake.
The present study had some limitations that should be mentioned. Participants were followed by linkage with nationwide registries with very limited loss to follow-up, which limits the potential of selection bias. Information on ALA intake was obtained using a self-administered FFQ and measurement error is inevitable, but because of the temporality in a follow-up study, exposure measurement error probably occurred at random, which generally leads to an underestimation of the true association and loss of statistical power. The FFQ used in the present study was not specifically developed to assess ALA intake. Further, information on diet was only available at baseline and changes in dietary habits during follow-up may have occurred. Thus, repeated dietary measurements would have been preferable to limit random measurement error and to capture potential changes in dietary habits over time. However, diets of individuals tend to be relatively consistent over intervals of several years(Reference Rothman, Greenland and Lash39). Information bias is unlikely to have influenced the observed associations significantly because diagnoses of PAD were established and validated independent of the dietary assessment.
Identification of PAD cases in the present study relied on registered discharge diagnosis of PAD and the vast majority of identified cases were symptomatic patients. PAD may be underdiagnosed in the general population(Reference Hirsch, Criqui and Treat-Jacobson40) and cases either asymptomatic or not referred to hospitals were not included in the present study. However, random misclassification of a diagnosis of PAD may bias associations towards no association, but because PAD risk in general was relatively low, such potential bias was probably minor. We included detailed information on established risk factors for PAD in the analyses, but residual confounding from known or unknown PAD risk factors may still be of importance for the observed associations. The observed association between ALA intake and the rate of PAD (model 1A) was weakened after adjustment for established risk factors for PAD (model 1B). However, additional adjustment for history of hypercholesterolaemia, hypertension and diabetes (model 2) showed a similar pattern of association compared with model 1B, which may indicate that potential residual confounding from these co-morbidities was not of major importance. Notably, these co-morbidities may also be considered intermediates and conditioning for these covariates could potentially introduce collider stratification bias(Reference Rothman, Greenland and Lash39), which may bias associations in either direction. In analyses including adjustment for established PAD risk factors and dietary factors (model 3) that may influence PAD risk, the observed association between ALA intake and the rate of PAD was slightly lower compared with model 1B at higher ALA intakes and residual confounding from dietary factors cannot be excluded. However, adjustment for dietary factors may introduce restrictions in the underlying dietary pattern that are not comparable with the ordinary dietary pattern and analyses with and without dietary covariates should not be directly compared. Therefore, given the interpretational challenges of models 2 and 3, we consider model 1B as the most appropriate for interpretation.
The Diet, Cancer and Health cohort only included native Danish participants from selected areas in Denmark who had survived until enrolment into the study without a previous diagnosis of cancer, chronic kidney disease or PAD, which may limit the generalisability of the study results.
We decided to conduct the main analyses as sex-stratified analyses by allowing baseline hazards among men and women to differ(Reference Fowkes, Aboyans and Fowkes5, Reference Fowkes, Rudan and Rudan6). Thus, the hazard ratios from these analyses should be interpreted as a weighted average of the association in men and women. Previous studies have suggested that the endogenous conversion efficiency of ALA into LC n-3 PUFA may be stimulated by sex hormones and is greater in women(Reference Baker, Miles and Burdge10). However, in sex-specific analyses, we found similar patterns of association between ALA intake and the risk of PAD among men and women. Almost 60 % of the female participants were post-menopausal at baseline(Reference Bork, Jakobsen and Lundbye-Christensen21) and a potential higher conversion efficiency of ALA mediated by female sex hormones may therefore not be of major importance in this cohort. In supplemental analyses, additional adjustment for hormone substitution at baseline also did not influence the observed associations.
A previous cross-sectional study including 422 cases reported that ALA intake was associated with a lower odds of PAD(Reference Lane, Magno and Lane41). Another cross-sectional study including 199 cases reported that the content of ALA in erythrocytes was associated with lower odds of lower limb disease(Reference Leng, Taylor and Lee42), whereas two case-control studies did not find any appreciably nor statistically significant differences between circulating levels of ALA between cases and controls(Reference Leng, Horrobin and Fowkes43, Reference Gautam, Izawa and Shiba44). However, none of these studies included detailed adjustment for risk factors of PAD and the results should be interpreted with caution due to the risk of residual confounding and reverse causation (cross-sectional studies).
We used restricted cubic splines to evaluate the shape of the association between ALA intake and the rate of PAD. ALA can be further metabolised into LC n-3 PUFA and lipid signalling molecules, which occurs in a complex biological pathway by enzymes that may be influenced by a combination of several factors including sex, genetics and background diet(Reference Hodson, Skeaff and Fielding45) that potentially could influence disease risk in a non-linear manner.
Previous studies have suggested that high intakes of the major n-6 PUFA linoleic acid and LC n-3 PUFA may lower the conversion efficiency of ALA into LC n-3 PUFA due to inhibition on shared enzymes. The median intake of LC n-3 PUFA in this cohort was 0·7 g/d, which was higher than that in cohort studies reporting inverse associations between ALA intake and CHD(Reference Hu, Stampfer and Manson15, Reference Ascherio, Rimm and Giovannucci16, Reference Vedtofte, Jakobsen and Lauritzen18–Reference Koh, Pan and Wang20) and this may be of importance for our study findings because a large study has suggested that ALA may reduce CHD risk in particular when intake of LC n-3 PUFA is low(Reference Mozaffarian, Ascherio and Hu17). However, further well-powered studies investigating the role of genetics and intake of LC n-3 and n-6 PUFA on the association between ALA and the risk of atherosclerotic CVD are warranted.
In conclusion, dietary intake of ALA was not consistently associated with the risk of incident PAD among Danish middle-aged men and women.
Acknowledgement
The Danish Cancer Society funded the Diet, Cancer and Health study. The current study has been financially supported by the Danish Heart Foundation (CSB, 17-R115-A7415-22060), Helene and Georg Jensens and Ethel Merethe and Christian Pontoppidan’s Fund. The funding agencies had no role in the design, analysis or writing of this manuscript.
All authors contributed to the conceptualisation of the present study. C. S. B. conducted the statistical analyses, prepared the tables and figures and wrote the first draft of the manuscript. C. S. B., A. N. L., S. L. C., M. U. J., P. C. C., E. B. S. and K. O. contributed to the planning of the statistical analyses, interpretation of the data and writing of the manuscript. S. L. C. supervised the conduct of the statistical analyses. A. T. contributed to the interpretation of the data and writing of the manuscript. All authors have read and approved the final manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000874