The use of fibrolytic enzymes (e.g. cellulases, xylanases) as feed additives for ruminants has been viewed with considerable scepticism for a long time, but in recent years has received increasing interest. Despite the considerable number of studies conducted recently on this topic, the mechanisms by which fibrolytic enzymes improve fibre digestion in the rumen have not been clearly elucidated, and seem to be affected by different factors, such as the type of enzyme used and the nature of the substrateReference Wang, McAllister, Rode, Beauchemin, Morgavi, Nsereko, Iwaasa and Yang1, Reference Colombatto, Morgavi, Furtado and Beauchemin2. Treating forage-based substrates with fibrolytic enzymes has been reported to enhance fibre degradability and production of volatile fatty acids (VFA) in vitro, but also to increase methane productionReference Dong, Bae, McAllister, Mathison and Cheng3–Reference Giraldo, Tejido, Ranilla and Carro5. Because methane represents a significant loss of energy for the host animal and contributes to global warmingReference Moss, Jouany and Newbold6, reducing methane production is an important goal of ruminant nutritionists and a range of chemical compounds such as methane analogues, ionophores and unsaturated fatty acids have been tested as potential feed additives to depress rumen methanogenesis. However, some of these compounds simultaneously produce adverse effects on rumen fermentation, such as a depression of fibre degradation or reduction of microbial growthReference Demeyer and Fievez7. Conversely, fumarate has been shown to produce a decrease in methane production accompanied by an increase in both the production of VFA and diet degradationReference López, Valdés, Newbold and Wallace8–Reference García-Martínez, Ranilla, Tejido and Carro10. Our hypothesis was that fumarate and exogenous fibrolytic enzymes could act synergistically to improve rumen fermentation. The objective of this study was therefore to investigate the effects of mixed fibrolytic enzymes from fungal origin, fumarate and a 1 : 1 mixture of both additives on rumen fermentation of a 0·6 : 0·4 forage:concentrate substrate, methane production and microbial growth in Rusitec fermenters. The enzyme preparation used in this study and the doses of application were selected from previous workReference Giraldo, Tejido, Ranilla and Carro5. The enzymes were shown to increase substrate degradability and production of VFA and methane in batch cultures of mixed rumen microorganisms fermenting high-forage substrates. The experimental procedure was designed to investigate some of the mechanisms of action proposed for exogenous fibrolytic enzymes.
Materials and methods
Apparatus and experimental treatments
The unit of RusitecReference Czerkawski and Breckenridge11 consisted of eight fermenters with an effective volume of 600 ml each. Solid and liquid fermentation inocula were collected from four rumen-cannulated sheep immediately before feeding and transferred to the fermenters within 30 min as previously describedReference Carro, Lebzien and Rohr12. Sheep were fed the same diet fed to fermenters and managed according to the protocols approved by the León University Institutional Animal Care and Use Committee. The flow through the fermenters was maintained by continuous infusion of artificial salivaReference McDougall13 (pH 8·4) at a rate of 580 ml/d (dilution rate 4·03 % per h).
Each fermenter received daily 20 g DM substrate fed into nylon bags (100 μm pore size). The substrate consisted of grass hay and concentrate (600 and 400 g/kg DM, respectively), and contained 939 g organic matter, 159 g crude protein, 496 g neutral detergent fibre (NDF) and 271 g acid detergent fibre (ADF)/kg DM. Concentrate was based on barley, maize grain, soyabean meal and mineral/vitamin premix in the proportions of 500:310:160:30 (air-dry basis). Grass hay was chopped to about 0·5 cm pieces and concentrate was ground through a 4 mm sieve. Both feed components were weighed independently and carefully mixed before applying the experimental treatments.
The four experimental treatments were: no additive (control; CON), mixed fibrolytic enzymes produced by Trichoderma longibrachiatum (FE), disodium fumarate (FUM; Sigma-Aldrich Química, S.A., Madrid, Spain) and treatment with both additives (MIX). The enzyme preparation was a powdered preparation commercialised by Fluka Chemicals (Seelze, Germany) and was previously characterisedReference Giraldo, Tejido, Ranilla and Carro5. At pH 6·5 and 39°C, 1 mg enzyme liberated 1·72 μmol xylose/min from oat spelt xylan, and 2·40, 0·040 and 0·385 μmol glucose/min from carboxymethylcellulose, Avicel PH-101 and soluble starch, respectively. One enzymatic unit was defined as the amount of enzyme required to release 1 μmol/min reducing xylose or glucose from the corresponding substrate at 39°C and pH 6·5. Each fermenter received 286 mg enzyme daily, which corresponded to 34·3, 0·57, 24·7 and 5·51 endoglucanase, exoglucanase, xylanase and amylase units, respectively, per g substrate DM. The enzyme preparation was dissolved daily in a sodium phosphate buffer solution (1 mm; pH 6·5) and carefully applied directly onto the substrate (1 ml/g DM) using a manual sprayer. After spraying, the substrate was kept at room temperature (21–23°C) for 24 h before being placed into nylon bags and fed to fermenters. This pre-treatment of substrate with the enzyme preparation was selected because previous studiesReference Wang, McAllister, Rode, Beauchemin, Morgavi, Nsereko, Iwaasa and Yang1, Reference Giraldo, Ranilla, Tejido and Carro14 showed that an enzyme–feed interaction before incubation with rumen fluid can enhance the beneficial effects of enzymes on rumen fermentation. Substrate for CON and FUM fermenters was sprayed with the corresponding amount of buffer solution without added enzyme preparation. Fermenters belonging to FUM and MIX treatments were supplemented daily with 30 mg fumarate/g substrate DM (600 mg fumarate per fermenter). Fumarate was weighed and carefully mixed with the substrate immediately before this was placed into the nylon bags and fed to fermenters.
Experimental procedure and sampling
Two 18-d identical incubation runs were carried out independently, and experimental treatments were assigned randomly within each experimental run so that two fermenters received each of the treatments; each treatment was, therefore, conducted in quadruplicate. After 10 d adaptation, on days 11, 12, 13 and 14 samples for gas, VFA and ammonia-N determination were collected following the procedures described previouslyReference Carro and Miller15. One nylon bag from each fermenter was collected daily, washed twice with 40 ml fermenter's fluid, and then washed in the cold rinse cycle (20 min) of a washing machine. The DM apparent disappearance (DMD) after 48 h incubation was calculated from the loss in weight after oven drying at 60°C for 48 h, and the residues were analyzed for NDF and ADF to estimate NDF and ADF disappearance (NDFD and ADFD, respectively).
On day 12, a dose of 2·04 mg 15N (98 % enriched 15NH4Cl; Tracer S.A., Madrid, Spain) was added into each fermenter to instantaneously label the ammonia-N pool. A solution of 15NH4Cl was then added to the artificial saliva at a rate of 4·00 mg 15N/g substrate N. Microbial growth was measured on days 15 and 16 following the procedures described previouslyReference Gómez, Tejido and Carro16. Briefly, for each fermenter, the total effluent for days 15 and 16 were mixed and homogenised. One portion (300 g) was frozen and lyophilised for determination of DM, non-ammonia N (NAN) and 15N enrichment; about 100 ml were frozen for determination of 15N enrichment in ammonia-N, and the rest of the mix was used to isolate liquid-associated microbial pellets (LAM). The contents of the nylon bags removed on days 15 and 16 were used to determine the growth of solid-associated micro-organisms (SAM)Reference Ranilla and Carro17. Microbial pellets were isolated by differential centrifugationReference Carro and Miller15. The substrate was also analysed for their natural 15N content, and this value was used for background correction before 15N infusion.
On days 17 and 18, the substrate administered to each fermenter was distributed into three nylon bags, one containing 18 g DM, and two containing 4 g DM each. The two bags containing 4 g were removed after 6 h incubation, whereas the 18 g DM bag remained for 48 h in the fermenters. One 4 g bag was washed as described above, and residues were analysed for NDF and ADF to estimate NDFD and ADFD. The other 4 g bag was washed, its content emptied, weighed and lyophilised to determine DM, NAN and 15N enrichment. On these days, 4 ml of each fermenter fluid were taken both immediately before feeding and after 6 h incubation for VFA determination.
On day 17, samples (4 ml) of each fermenter fluid before feeding and of the liquid obtained from the first washing of the 6 h incubated bags were taken and immediately frozen at − 80°C for determination of enzymatic activities. After removing and washing the 6 h incubated bags, 1 ml of each fermenter's fluid was diluted through a series of tubes containing 9·0 ml anaerobic dilution solutionReference Dehority18. Using the 10− 6 through 10− 11 dilution tubes, 1 ml was placed in each of three tubes containing the Most Probable Number mediaReference Dehority, Tirabasso and Grifo19. Total and cellulolytic bacteria concentrations were determined according to the Most Probable Number procedureReference Dehority, Tirabasso and Grifo19.
Adaptative changes in the microbial population of fermenters to each treatment were studied using each fermenters' fluid as inoculum for batch cultures and measuring the response in the production of gas and VFAReference Gómez, Tejido and Carro16. The fermentative activity of the fluid contained in each fermenter was tested against four pure substrates (Sigma-Aldrich Química S.A., Madrid, Spain): cellulose, oat spelt xylan, pectin from citrus peel and a mixture of starch (40 % wheat, 40 % barley and 20 % potato starch). On the last day of each incubation run, the two nylon bags present in each fermenter were removed, their content emptied and mixed with the effluent. The mixture was homogenised for 30 s with a blender and filtered through two layers of nylon cloth (40 μm pore size); then, 440 ml filtrate were mixed with 110 ml artificial saliva (enriched with 472 mg NH4Cl and 791 mg trypticase per litre saliva), and 30 ml of the final mixture were anaerobically dispensed to 120 ml serum bottles containing 300 mg of one of the substrates described earlier. Ten bottles (two bottles for each substrate and two bottles without substrate) were incubated per each fermenter. The bottles were capped and incubated at 39° C for 9 h for cellulose and for 6 h for the rest of substrates. The amount of gas produced was measured, the bottles were then opened and samples for VFA determination were taken.
Effects of mixed enzymes on fibre content of substrate
In order to investigate the effects of the 24 h pre-treatment with the enzyme preparation on NDF and ADF content of substrate, samples of substrate (500 mg) were weighed into artificial fibre bags (#F57 bags; 50 × 40 mm; 25 ± 10 μm pore size; ANKOM Technology Corporation, Macedon, NY, USA) and 1 ml buffer solution (CON) or 7·15 mg of enzyme preparation was added into each bag. Bags were heat sealed, and kept at room temperature (21–23°C) for 24 h before NDF and ADF analyses were conducted. This procedure was repeated five times.
Analytical procedures
Procedures for determination of DM, ash, N, NDF, ADF, VFA and ammonia-N have been reported previouslyReference Carro and Miller15. An ANKOM220 Fiber Analyzer unit (ANKOM Technology Corporation, Fairport, USA) was used for NDF and ADF analyses. The volume of gas produced was measured with a drum-type gas meter (model TG1; Ritter Apparatebaum GmbH, Bochum, Germany) and the concentration of methane was analyzed by gas chromatographyReference Carro and Miller15. Samples were prepared for 15N analysisReference Carro and Miller15 and analyses of 15N enrichment were performed by isotope ratio mass spectrometry (VG Prism II, Middlewich, UK) connected in series to a DUMAS-style N analyzer (Model 1108, Carlo Erba Instruments, Milan, Italy).
For determination of enzymatic activities in rumen fluid samples, cells were lysed using a Mini-BeadbeaterTM (BioSpec Products, Inc., Bartlesville, OK, USA) to release intracellular enzymes. The treatment consisted of three 60-s pulses at 4°C using 0·1 mm zirconia beads. Unbroken cell material was removed by centrifugation (10 000 g, 10 min, 4°C) and the supernatant was used to analyze enzymatic activities (endoglucanase, exoglucanase, amylase and xylanase)Reference Colombatto and Beauchemin20.
Calculations and statistical analyses
The proportion of digesta NAN (liquid or solid) of microbial origin was estimated for each fermenter by dividing the 15N enrichment (atoms % in excess) of the NAN portion of digesta by the enrichment of the corresponding microbial pellets (LAM or SAM). Daily microbial N production (mg/d; LAM or SAM) was estimated by multiplying total NAN production in the corresponding digesta (liquid or solid) by the proportion attributed to the microbes. Total daily microbial production was calculated as the sum of the flows of LAM and SAM. The amount of organic matter apparently fermented was estimated from net productions of acetate, propionate and butyrateReference Demeyer and Jouany21. This value was used to estimate the efficiency of microbial growth (mg microbial N/g organic matter apparently fermented). The volume of gas produced in the fermenters (litres/d) was corrected for temperature (0°C) and pressure (1·013 × 105 Pa) conditions, and the amount of methane produced (mmol) was calculated by multiplying the gas produced by the methane concentration in the analysed sample.
The amounts of VFA produced in the batch cultures were obtained by subtracting the amounts present initially in the incubation medium from those determined at the end of the incubation period. Values of gas production in the batch cultures were corrected for the amount of gas produced in the bottles without substrate inoculated with the fluid from the corresponding fermenters.
Data relative to fermentation parameters were analysed as a split-plot design with additive treatment as the main-plot treatment and day of sampling as the subplot treatment. The model included additive treatment, incubation run, fermenter nested within additive treatment and day of sampling. Significance of additive treatment effects were tested using the variance between fermenters within treatment as the error term. Effects of other factors were tested against the residual error. In the analysis of data relative to microbial growth, microbial counts and enzymatic activities in the fermenters, and production of gas and VFA in batch cultures, day of sampling was excluded from the model. When a significant effect of additive treatment (P ≤ 0·05) was detected, differences between means were assessed by LSD test. All statistical analyses were conducted using the GLM procedure of the Statistical Analysis Systems statistical package version 8.02 (SAS Institute, Cary, NC, USA).
Results
Effects of mixed enzymes on fibre content of substrate
Compared to buffer-treated substrate, the treatment with FE reduced the NDF content (496 and 448 g/kg DM, respectively; P = 0·001; sem 6·2), although ADF content was unaffected (271 and 263 g/kg DM; P = 0·266; sem 4·9).
Effects of additives on rumen fermentation and microbial growth in Rusitec fermenters
The experimental treatments did not affect either the daily amount of effluent (586, 581, 591 and 578 ml/d for CON, FE, FUM and MIX, respectively; P = 0·951) or the pH of fermenters' contents before feeding (6·56, 6·58, 6·61 and 6·61; P = 0·881). The effects of additives on substrate apparent disappearance and daily production of VFA and methane are shown in Table 1. Compared to CON, both FE and MIX treatments increased (P < 0·05) DMD after 6 h of incubation by 21 %, but the increase was reduced to 6·3 and 6·1 %, respectively, after 48 h of incubation (P < 0·05). Disappearance of NDF and ADF followed a similar pattern, since FE and MIX treatments increased (P < 0·05) NDFD at 6 h by 22 and 16 %, compared to CON, but the increase was reduced to 12 % after 48 h incubation for both treatments. On the contrary, there was no effect (P>0·05) of FUM on DMD, NDFD and ADFD at any incubation time.
Table 1 Effect of experimental treatments on apparent disappearance of substrate dry matter (DMD), neutral-detergent fibre (NDFD) and acid-detergent fibre (ADFD) after 6 and 48 h incubation and daily production of VFA and methane in Rusitec fermenters*
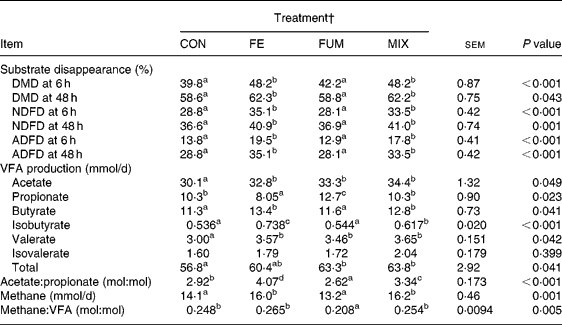
a,b,c Mean values within a row with unlike superscript letter were significantly different (P < 0·05).
* Values are the mean of two daily observations in each of four fermenters for substrate disappearance after 6 h incubation (n 8) and of four daily observations in each of four fermenters (n 16) for the rest of the variables. Substrate was composed of grass hay and concentrate (600 and 400 g/kg DM, respectively).
† CON: control (no additive); FE: 34·3, 0·57, 24·7 and 5·51 endoglucanase, exoglucanase, xylanase and amylase units, respectively, per g substrate DM; FUM: 30 mg disodium fumarate/g substrate DM; MIX: FE treatment plus 30 mg disodium fumarate/g substrate DM. Each fermenter received daily 20 g substrate DM.
All experimental treatments increased daily production of acetate (P = 0·049) and valerate (P = 0·042). Compared to CON, FUM increased (P < 0·05) and FE reduced (P < 0·05) the production of propionate, but no differences (P>0·05) were found for MIX. Butyrate production was augmented (P < 0·05) by 2·1 and 1·5 mmol/d for FE and MIX treatments, respectively, but no effect (P>0·05) was detected for FUM. As a consequence of these changes, FE, FUM and MIX treatments increased total VFA production by 3·6, 6·5 and 7·0 mmol/d, relative to CON, but differences between FE and CON were not significant (P>0·05). The acetate:propionate ratio was lowest (P < 0·05) for FUM and greatest (P < 0·05) for FE, with CON and MIX having intermediate values. Compared to CON, the treatment of substrate with FE and MIX increased (P < 0·05) the production of methane by 1·9 and 2·1 mmol/d, whereas supplementing with FUM decreased it by 0·9 mmol/d (P>0·05).
Both FE and MIX treatments increased the daily flow of ammonia-N (P < 0·001), relative to CON (Table 2), but no differences (P>0·05) between CON and FUM were found. The daily flow of total NAN was not affected (P = 0·339) by any experimental treatment, but microbial N flow was greater (P = 0·05) in FE and MIX fermenters, with no differences (P>0·05) between CON and FUM. Whereas no differences (P = 0·969) between treatments were detected for SAM flow, both FE and MIX increased (P < 0·05) the flow of LAM compared to CON and FUM. Efficiency of microbial synthesis (mg microbial N/g organic matter fermented) was not affected (P = 0·206) by any additive treatment.
Table 2 Effect of the treatment with different additives on daily production of ammonia-N and non-ammonia-N (NAN), daily N flow of liquid-associated (LAM) and solid-associated microorganisms (SAM), efficiency of microbial synthesis (EMS) and microbial numbers in Rusitec fermenters*
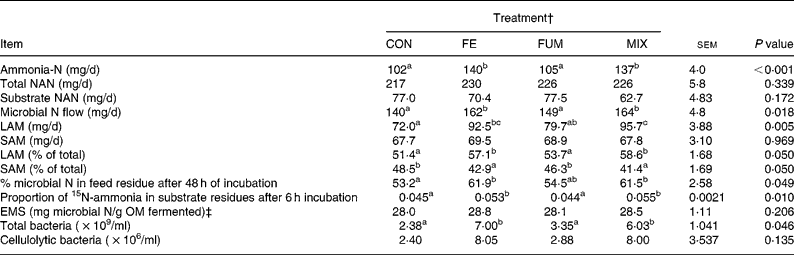
om, organic matter.
a,b Mean values within a row with unlike superscripts letter were significantly different (P < 0·05).
* Values are the mean of four daily observations in each of four fermenters (n 16) for ammonia-N and the mean of one observation in each of four fermenters (n 4) for the rest of the variables. Substrate was composed of grass hay and concentrate (600 and 400 g/kg DM, respectively).
† CON: control (no additive); FE: 34·3, 0·57, 24·7 and 5·51 endoglucanase, exoglucanase, xylanase and amylase units, respectively, per g substrate DM; FUM: 30 mg of disodium fumarate/g substrate DM; MIX: FE treatment plus 30 mg of disodium fumarate/g substrate DM. Each fermenter received daily 20 g substrate DM.
‡ Organic matter (OM) apparently fermented was estimated from net production of acetate, propionate and butyrate as described by Demeyer 21.
The proportion of microbial N in the substrate residue after 48 h incubation was greater (P < 0·05) for FE and MIX than for CON, but no difference (P>0·05) was detected between CON and FUM (Table 2). In agreement with these results, the proportion of 15N-ammonia incorporated in substrate residues after 6 h of incubation was greater (P < 0·05) for FE and MIX than for CON and FUM. Both FE and MIX treatments resulted in a significant (P < 0·05) increase in total bacteria numbers, but cellulolytic bacteria were unaffected (P = 0·135).
As shown in Table 3, greater (P < 0·05) endoglucanase, exoglucanase, amylase and xylanase activities were detected both in the fermenters' liquid content and in the liquid obtained from washing the 6-h incubated bags for FE and MIX fermenters, relative to CON ones, but no differences (P>0·05) were found between CON and FUM treatments. Compared to CON, all additive treatments produced greater (P < 0·001) increases in total VFA concentrations during the first 6 h after feeding.
Table 3 Effect of the treatment with different additives on enzymatic activities and on volatile fatty acid (VFA) concentrations after 6 h incubation in Rusitec fermenters

a,b,c Mean values within a row with unlike superscripts letter were significantly different (P < 0·05).
* CON: control (no additive); FE: 34·3, 0·57, 24·7 and 5·51 endoglucanase, exoglucanase, xylanase and amylase units, respectively, per g substrate DM; FUM: 30 mg disodium fumarate/g substrate DM; MIX: FE treatment plus 30 mg disodium fumarate/g substrate DM. Each fermenter received daily 20 g substrate DM.
† Endoglucanase, exoglucanase and amylase activities are expressed as nanomol glucose liberated per min and ml of sample at 39°C and pH 6·5 using carboxymethylcellulose, Avicel PH-101 and soluble starch as substrate, respectively. Xylanase activity is expressed as nanomol xylose liberated from oat spelt xylan per min and ml of sample at 39°C and pH 6·5. Rumen fluid was sampled before feeding and the washing liquid was obtained from washing the 6-h incubated bags into the fermenters. Values are the mean of four observations.
‡ Calculated for each fermenter as the VFA concentration in rumen fluid at 6 h after feeding minus the VFA concentration measured immediately before feeding.
The results of the in vitro incubations with batch cultures are shown in Table 4. Batch cultures inoculated with rumen fluid from fermenters fed the enzyme-treated substrate (FE and MIX) produced greater (P < 0·01) amounts of both gas and VFA with cellulose, and greater (P = 0·012) amounts of VFA with xylan. Compared to CON cultures, MIX treatment produced greater (P < 0·05) amounts of gas and VFA with pectin as substrate. On the contrary, there were no effects of additive treatments on production of VFA (P = 0·208) and gas (P = 0·120) for starch.
Table 4 Production of gas and volatile fatty acids (VFA) in batch cultures containing 300 mg different substrates (cellulose, xylan, pectin and starch) inoculated with fluid from Rusitec fermenters fed a grass hay:concentrate substrate (600 and 400 g/kg DM, respectively) after being treated with different additives (Mean values of eight fermentations)*
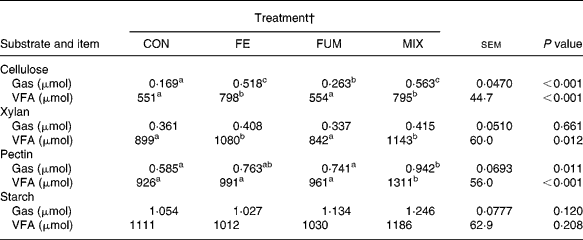
a,b,c Mean values within a row with unlike superscripts letter were significantly different (P < 0·05).
* Cellulose was incubated for 9 h, and the rest of the substrates for 6 h.
† CON: control (no additive); FE: 34·3, 0·57, 24·7 and 5·51 endoglucanase, exoglucanase, xylanase and amylase units, respectively, per g substrate DM; FUM: 30 mg disodium fumarate/g substrate DM; MIX: FE treatment plus 30 mg disodium fumarate/g substrate DM. Each fermenter received daily 20 g substrate DM.
Discussion
In agreement with results from other studiesReference Nsereko, Morgavi, Rode, Beauchemin and McAllister22, Reference Colombatto, Mould, Bhat, Morgavi, Beauchemin and Owen23, the treatment with the enzyme preparation stimulated the initial phases of substrate degradation, but the effects were reduced as incubation time increased. It has been suggestedReference Nsereko, Morgavi, Rode, Beauchemin and McAllister22, Reference Wallace, Wallace, McKain, Nsereko and Hartnell24 that exogenous enzymes could increase fibre degradation through a hydrolytic action prior to feeding or in vitro incubation with rumen micro-organisms. In the present experiment, the treatment of substrate with FE reduced its NDF content, and similar effects have been previously reported for other fibre-degrading enzymesReference Nsereko, Morgavi, Rode, Beauchemin and McAllister22, Reference Colombatto, Hervás, Yang and Beauchemin25. The treatment of substrate with the enzyme preparation increased significantly the disappearance of substrate after 48 h incubation, which contrasts with the general agreement that enzymes increased the rate, but not the extent, of feed degradation in the rumenReference Beauchemin, Morgavi, McAllister, Yang, Rode, Garnsworthy and Wiseman26. As pointed out by Colombatto and co-workersReference Colombatto, Morgavi, Furtado and Beauchemin2, 48 h incubation cannot be considered as an end point for some substrates. In a later trial conducted at our laboratory, the in situ degradation of substrate in the rumen of sheep was measured, and substrate DMD after 48 h incubation in Rusitec fermenters (58·6 %) represented about 71 % of its potential DM degradability (82·5 %; LA Giraldo et al., unpublished results).
The increase in substrate degradability produced by FE and MIX treatments is in accordance with the greater VFA production observed in the fermenters fed the enzyme-treated substrate. After 6 h incubation, the increase in total VFA concentration in rumen fluid was, relative to that in CON fermenters, 14·6, 11·9 and 15·1 mmol/l greater in FE, FUM and MIX fermenters, respectively. Since all fermenters had similar liquid volumes and dilution rates, these results would indicate that all additives stimulated VFA production during the first 6 h of incubation. The increased VFA production in FE and MIX fermenters was probably due to the enhanced substrate degradability, since DMD at 6 h was increased by 8·4 percentage units, relative to CON. In contrast, no significant effects of FUM on DMD at 6 h incubation were found, although DMD in FUM was 2·4 percentage units greater. This would suggest that the observed increase in VFA concentrations was mainly due to fermentation of fumarate itself, since fumarate can be converted into propionate and acetate by different rumen micro-organismsReference Asanuma, Iwamoto and Hino27. If a mean liquid volume of 500 ml for each fermenter is assumed (volumes were measured on the last day of each incubation run), the increase in total VFA from 0 to 6 h after feeding would be 6·0 mmol greater in FUM than in CON fermenters. Since on days 17 and 18 each fermenter received daily 4·88 mmol fumarate, the results would indicate that all fumarate was fermented during the first 6 h incubation. In agreement with this hypothesis, it has been reported that 192 mg fumarate were completely consumed by mixed rumen micro-organisms in batch cultures (60 ml volume) after 6 h incubationReference Asanuma, Iwamoto and Hino27.
When comparing the increases in VFA concentrations after 6 h incubation, it was observed that there were no differences between FE, FUM and MIX treatments (39·2, 36·5 and 39·7 mmol/l, respectively). Since MIX fermenters were treated with both additives, it would be expected that the increases in VFA concentrations were greater in these fermenters than in FE or FUM ones. The reasons for the lack of differences are unknown, but the results would indicate that FE and FUM did not act synergistically to increase VFA production. The observed increases in propionate concentration at 6 h incubation in FUM and MIX fermenters were similar, but were greater than those in FE fermenters (9·61, 10·2 and 5·77 mmol/l, respectively). This would again indicate that fumarate was rapidly fermented, since FE treatment decreased significantly the daily production of propionate compared to the rest of experimental treatments.
Treating the substrate with FE increased the daily production of acetate and butyrate, but decreased propionate production, thus indicating a change in fermentation pattern. Similar changes have been reported by treating the substrate fed to continuous culture fermenters with a commercial enzyme produced by T. longibrachiatum Reference Yang, Beauchemin and Vedres28, and it has been suggested that changes in fermentation pattern may reflect a shift in the species profile of colonising bacteria in response to pre-treatment of feed with exogenous enzymesReference Wang, McAllister, Rode, Beauchemin, Morgavi, Nsereko, Iwaasa and Yang1. The change in VFA pattern in our study is consistent with the greater methane production and fibre degradation observed in the enzyme-treated fermenters, since acetate and butyrate production is associated with the release of H2 which can be used by methanogens to form methaneReference Stewart, Flint, Bryant, Hobson and Stewart29. All these effects would indicate a greater activity of fibre-degrading bacteria in FE fermenters, which is supported by the greater xylanase, endoglucanase and exoglucanase activities observed in their rumen fluid. In addition, numbers of cellulolytic bacteria were 3·3 times greater in FE than in CON fermenters, although differences were not significant (P>0·05). Since only the fermenters' fluid was used to inoculate the Most Probable Number culture tubes, any effect of FE on SAM population could not have been detected. Both LAM and the non-adherent micro-organisms washed out of the substrate (nylon bags were washed twice with 40 ml of fermenters' fluid and the washing liquid was returned to the fermenters before using the fluid as inoculum for the Most Probable Number cultures) should have been present in the fermenters' fluid as inoculum, but not SAM. In contrast, the inoculum used for batch cultures should have included partially SAM, since it was prepared by mixing the rumen fluid from each fermenter with the corresponding substrate residues, followed by homogenising and filtering. Homogenising has been used as a method to detach SAMReference Carro and Miller30, and a greater activity of SAM in the inoculum from FE and MIX fermenters could explain the enhanced VFA production observed in the batch cultures with cellulose and xylan as substrates and inoculated with rumen fluid from these fermenters, relative to CON ones.
A mechanism proposed as a possible mode of action of fibrolytic enzymes in the rumen is to stimulate the attachment of rumen micro-organisms to feed particlesReference Newbold31. To evaluate this possibility, nylon bags with substrate were incubated for 6 h and the 15N enrichment in substrate residues was determined. Since substrate residues were washed, dried and treated with NaOH (pH>10) to eliminate ammonia-NReference Firkins, Berger, Merchen, Fahey and Mulvaney32, 15N incorporation should be exclusively from SAM origin. The greater incorporation of 15N in substrate residues (calculated by dividing the 15N enrichment in substrate residues by the 15N enrichment of ammonia-N) in FE and MIX fermenters, compared to CON ones, would indicate that enzyme treatment stimulated the initial phases of microbial colonisation. In addition, the proportion of microbial N in substrate residues after 48 h incubation was greater for FE and MIX treatments than for CON, thus indicating a greater colonisation of feed particles after a long incubation period. In agreement with these results, it has been reported that treating a 0·5:0·5 alfalfa hay:barley grain substrate with exogenous xylanase increased 15N incorporation into SAM-N after 24 and 48 h incubation in a Rusitec systemReference Wang, McAllister, Rode, Beauchemin, Morgavi, Nsereko, Iwaasa and Yang1.
Although the proportion of microbial N in substrate residues after 48 h incubation was greater for FE and MIX than for CON fermenters, the daily flow of SAM did not differ between treatments. This was due to the lower amount of substrate residues recovered for FE and MIX after 48 h incubation, as a consequence of increased substrate degradation. On the contrary, the treatment of substrate with enzyme increased LAM flow. This microbial fraction is composed of micro-organisms located in free suspension or loosely associated with feed particles, and therefore they are not expected to ferment structural carbohydrates. It is possible that the treatment with the enzyme preparation produced a greater amount of secondary products derived from structural carbohydrates, which entered the liquid pool and stimulated LAM growth. In agreement with these results, it has been reported that treating the diet of dairy cows with a commercial product from T. longibrachiatum increased the numbers of rumen bacteria that utilise hemicellulose or secondary products of cellulose digestionReference Nsereko, Beauchemin, Morgavi, Rode, Furtado, McAllister, Iwaasa, Wang and Wang33. The greater LAM growth in our study is in accordance with the observed increase in enzymatic activities in the rumen fluid from FE and MIX fermenters. The enzyme preparation used in this experiment presented mainly endoglucanase and xylanase activities, but it seems unlikely that the observed increase in these enzymatic activities in rumen fluid was due to a direct effect of the enzyme preparation. Since the enzyme preparation was applied onto the substrate 24 h before being placed into the fermenters, probably little intact enzymes remained at the start of fermentation. In addition, enzymatic activities in rumen fluid were measured in samples taken 24 h after feeding, and after this incubation time possibly the enzymes had been completely fermented by the rumen micro-organisms. Moreover, enhanced cellulase and xylanase activities in rumen fluid produced by the treatment of feed with exogenous fibrolytic enzymes have been reported in in vitro Reference Wang, McAllister, Rode, Beauchemin, Morgavi, Nsereko, Iwaasa and Yang1, Reference Colombatto, Hervás, Yang and Beauchemin25 and in vivo studiesReference Yang, Beauchemin and Rode34, Reference Hristov, McAllister and Cheng35, and Morgavi et al. Reference Morgavi, Beauchemin, Nsereko, Rode, Iwaasa, Yang, McAllister and Wang36 demonstrated synergism between exogenous enzymes produced by T. longibrachiatum and those produced by rumen micro-organisms such that the net combined hydrolytic effect in the rumen was much greater than that estimated from the individual activities.
Methane:VFA ratios were in the range of values previously reported for fermentation of similar diets in a Rusitec systemReference Czerkawski and Breckenridge37, and were not affected by FE and MIX treatments, but were decreased by FUM. In contrast to the marked effects of FE treatment on rumen fermentation, the treatment with FUM produced only few changes on rumen variables. Compared to CON, FUM treatment increased the production of acetate, propionate and total VFA, but failed to increase substrate disappearance or to reduce methane production, as has been reported in previous studiesReference López, Valdés, Newbold and Wallace8–Reference García-Martínez, Ranilla, Tejido and Carro10. The reasons for the lack of effects of FUM may be related to the dose of fumarate and the nature of the incubated substrateReference García-Martínez, Ranilla, Tejido and Carro10, Reference Newbold, López, Nelson, Ouda, Wallace and Moss38. López et al. Reference López, Valdés, Newbold and Wallace8 reported that methane production decreased by 17 % (1·3 mmol/d; P = 0·017) and acetate and propionate production augmented by 3·5 (P = 0·182) and 4·9 (P = 0·003) mmol/d, respectively, when Rusitec fermenters fed a 0·5 : 0·5 grass hay:concentrate substrate were supplemented daily with 6·25 mmol fumarate. In the present study each fermenter received daily 3·75 mmol fumarate, and methane production was decreased by 6·4 % (0·9 mmol/d; P>0·05) and acetate and propionate production augmented by 3·2 and 2·4 mmol/d, respectively (P < 0·05). Since in both studies fermenters were supplied daily with 20 g substrate, in the study of López et al. Reference López, Valdés, Newbold and Wallace8 fumarate represented 5 % of substrate, compared with 3 % in the present study. The decision to supplement the fermenters with a lower dose of fumarate was taken based on previous results, since García-Martínez et al. Reference García-Martínez, Ranilla, Tejido and Carro10 did not find differences between the effects of 4 and 8 mm fumarate on fermentation of three different substrates in batch cultures of mixed rumen micro-organisms (3·75 mmol/d represented a final concentration of 6·25 mm in our fermenters). Comparison of our results to those of López et al. Reference López, Valdés, Newbold and Wallace8 seems to indicate that a greater amount of fumarate than that used in our study would be necessary to modify rumen populations, and thus, substrate fermentation in Rusitec fermenters. In our study FUM treatment did not affect bacterial numbers, whereas López et al. Reference López, Valdés, Newbold and Wallace8 reported a significant increase in both total and cellulolytic bacteria by supplementing with fumarate.
The results of the present study indicate that the use of FE as feed additive had a stimulatory effect on rumen fermentative activity. Treating the substrate with the enzyme preparation altered the fibre structure of substrate and increased its microbial colonisation, resulting in enhanced fibre degradation, VFA and methane production, and growth and enzymatic activities of rumen micro-organisms. Supplementing with fumarate increased VFA production and reduced slightly methane production, but these effects seem to be mainly due to fumarate fermentation itself. Finally, the lack of differences between FE and MIX treatments in most of the measured variables would indicate that at the dose used in this study, fumarate did not further improve rumen fermentation, compared to the use of FE alone. Studies with greater amounts of fumarate are necessary to confirm the hypothesis that fibrolytic enzymes and fumarate could act synergistically to improve rumen fermentation, since observed effects of both additives seem to be complementary.
Acknowledgements
The authors acknowledge the financial support received from the CICYT (Project AGL2001-0130) and Junta de Castilla y León (Project LE040A05). L. A. Giraldo gratefully acknowledges receipt of a grant from the Fundación Carolina.






