Glucosinolates have been implicated as potential cancer-protective phytochemicals following the consumption of brassica vegetables in humans and animals(Reference van Poppel, Verhoeven, Verhagen and Goldbohm1). During the preparation and cooking of brassica, glucosinolates are hydrolysed by plant myrosinase (EC 3.2.1.147) into various classes of metabolites, depending on the characteristics of the hydrolysis medium such as temperature, pH and presence of cofactors of myrosinase(Reference Fenwick and Heaney2). Experiments in animal and cellular models have shown that isothiocyanates are one of the main groups of metabolites that may influence carcinogenesis partly by inducing phase 2 xenobiotic metabolising enzymes, which are involved in the detoxification of carcinogens(Reference Keum, Jeong and Kong3, Reference Zhang, Yao and Li4). Brassica vegetables and isolated isothiocyanates have been shown to induce phase 2 detoxification enzymes, such as glutathione-S-transferase (GST) and uridine 5′-diphospho-glucuronosyl transferase (UGT), in cell cultures such as human hepatoma cells and in rodents(Reference Steinkellner, Rabot, Freywald, Nobis, Chabicovsky, Knasmüller and Kassie5).
Cooking denatures plant myrosinase and alters glucosinolate concentrations to variable extents(Reference Verkerk and Dekker6), and may influence the hydrolysis of glucosinolates following the ingestion of cooked vegetables(Reference Rungapamestry, Duncan, Fuller and Ratcliffe7). After consumption of brassica, hydrolysis of glucosinolates in vivo may occur under the action of plant and/or microbial myrosinase. Chewing of raw or cooked vegetables containing plant myrosinase causes glucosinolates to be hydrolysed by plant myrosinase, and their metabolites are then presumably absorbed from the small-intestinal lumen. Intact glucosinolates, reaching the colon after consumption of cooked brassica with low or no plant myrosinase activity, are hydrolysed by resident microbiota, in which myrosinase-like activity has been described(Reference Elfoul, Rabot, Khelifa, Quinsac, Duguay and Rimbault8, Reference Krul, Humblot, Philippe, Vermeulen, van Nuenen, Havenaar and Rabot9).
Following their absorption, isothiocyanates form conjugates with glutathione, undergo consecutive enzyme-catalysed reactions, and are excreted in urine via the mercapturic acid pathway as their corresponding N-acetylcysteine conjugates (NAC)(Reference Brüsewitz, Cameron, Chasseaud, Görler, Hawkins, Koch and Mennicke10). Excretion of NAC following the intake of glucosinolates has been demonstrated in rats(Reference Duncan, Rabot and Nugon-Baudon11) and human subjects(Reference Shapiro, Fahey, Wade, Stephenson and Talalay12) and has been used as a selective biomarker for the formation and absorption of isothiocyanates in the alimentary tract of human subjects and rodents(Reference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung13, Reference Rouzaud, Rabot, Ratcliffe and Duncan14).
The digestive fate of glucosinolates following the consumption of cooked brassica is therefore related to the residual glucosinolate and plant myrosinase content of the cooked vegetables, and the status of the colonic microbiota. Sinigrin, the predominant aliphatic glucosinolate in cabbage, is partly hydrolysed to allyl isothiocyanate (AITC)(Reference Rungapamestry, Duncan, Fuller and Ratcliffe15). Previous work in our laboratory has shown that the formation of AITC in vitro, after the hydrolysis of cooked cabbage, was related to the activity of residual plant myrosinase and its cofactors after cooking(Reference Rungapamestry, Duncan, Fuller and Ratcliffe15). The aim of the present experiment was to determine the extent of excretion of NAC of AITC after hydrolysis of sinigrin in gnotobiotic rats following the ingestion of cabbage, cooked to different extents. Furthermore, we investigated the effect of the cabbage diets on the activity of the phase 2 enzymes GST and UGT.
Materials and methods
Experimental design
The experiment was a 2 × 4 factorial study, involving two colonic microbiota treatments applied to rats (germ-free (GF) and human faecal microbiota-associated (HFM) rats) and four dietary treatments consisting of a control diet or three treatment diets, in which a proportion of the basal diet was substituted with either raw, lightly cooked or fully cooked cabbage. Six rats from each microbiota group received one of the four experimental diets for 14 d and were killed on day 15. The feeding regimens were staggered over six batches of eight rats, each batch comprising four GF and four HFM rats subjected to one of the four experimental diets. Diets were provided ad libitum throughout the experimental period. Urine samples were collected on the last 2 d of feeding and were subjected to analysis of NAC of AITC. Animals were weighed at the beginning of the dietary treatment, on the first day of urine collection and on the day they were killed. Feed intake was recorded daily on the last 4 d of dietary treatment to determine average daily consumption. After the animals were killed, their livers and colons were collected for analysis of phase 2 enzyme activities.
Animals
Forty-eight male F344 rats, born in a GF environment over 1 month and bred in sterile conditions(Reference Coates16) at the Germ-Free Rodent Breeding Facilities of UEPSD (INRA, Jouy-en-Josas, France), were used. The mating of animals was staggered so that all animals were of uniform age at the start of the experiment. Twenty-four of the rats were orally inoculated with a human faecal suspension from a healthy female volunteer, as described by Rouzaud et al.(Reference Rouzaud, Rabot, Ratcliffe and Duncan14), 3 weeks before the start of the experiment to permit establishment of the microbiota. Colonisation of the gastrointestinal tract by microbiota was monitored weekly by microscopic examination of freshly collected faecal pellets. Animals had a mean age of 2·6 (sem 0·04) months and body weight of 284·5 (sem 3·15) g at the beginning of dietary treatment.
Before the start of dietary treatment, GF and HFM rats were maintained on a control diet in common cages within separate sterile isolators (La Calhène, Vélizy, France) to maintain their respective microbiota status. Four of each of GF and HFM rats were transferred at a time to individual cages, within two separate isolators, to be acclimatised to their respective experimental diets for 5 d. They were thereafter moved to individual metabolism cages within the same respective isolators for a further 9 d. Animals were maintained at 21°C under a 12 h light–dark cycle.
All procedures were carried out according to the European guidelines for the care and use of laboratory animals.
Dietary treatment
The control diet was semi-synthetic (Table 1), adapted from Lhoste et al.(Reference Lhoste, Ouriet, Bruel, Flinois, Brezillon, Magdalou, Cheze and Nugon-Baudon17). The cabbage diets were adjusted from the control diet and contained 20 % of freeze-dried cabbage (var. Marathon; Cocklaw Mains, Peterhead, Aberdeenshire, UK) (Table 1). Before freeze-drying and incorporation into rat diets, portions of cabbage, each weighing about 120 g, were subjected to three cooking treatments to yield raw, lightly cooked (microwaved at 750 W for 2 min) or fully cooked (microwaved at 750 W for 5·5 min) cabbage to produce a homogeneous batch for each cooking treatment. Cooking protocols and stabilisation of cooked samples have been described in an earlier study(Reference Rungapamestry, Duncan, Fuller and Ratcliffe15). Diets were pelleted, packed in double-vacuum bags (SAFE, Augy, France) and sterilised by γ radiation at 45 kGy (IBA Mediris, Fleurus, Belgium). The sterilised diets and autoclaved tap water were provided ad libitum throughout the acclimatisation and experimental periods.
Table 1 Composition of control and cabbage-substituted diets (g/kg) given to rats
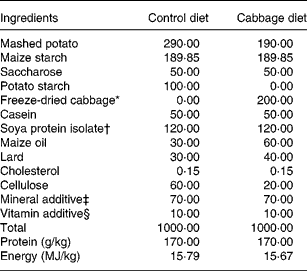
* Consisted of either raw, lightly cooked or fully cooked cabbage.
† Nurish 1500 (DuPont Protein Technologies, Paris, France).
‡ Mineral additive included (per kg diet): Ca, 2·11 g; P, 5·46 g; Na, 2·74 g; K, 3·67 g; Mg, 1·02 g; Fe, 0·10 g; Cu, 0·09 g; Mn, 0·55 g; Zn, 0·31 g; I, 4·3 mg; Co, 0·7 mg.
§ Vitamin additive included (per kg diet): vitamin A, 6 mg (20 000 IU); vitamin D3, 6·25 μg (2500 IU); vitamin E, 117·25 mg (175 IU); menadione, 17·6 mg; thiamin, 20 mg; riboflavin, 15 mg; nicotinic acid, 100 mg; d-pantothenic acid, 6·9 mg; pyridoxine, 10 mg; folic acid, 5 mg; biotin, 0·3 mg; cyanocobalamin, 0·05 mg; l-ascorbic acid, 0·8 mg; choline, 1·36 g; inositol, 150 mg.
Chemicals
All chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) unless stated otherwise.
Sample collection
Metabolism cages were cleaned before the start of urine collection. Urine was collected in two batches over 14 and 10 h in each 24 h period. A volume of 0·2 ml 61·5 mm-sodium azide, dissolved in deionised water, was added to the urine collection receptacles of all metabolism cages before collecting each batch of urine, to prevent bacterial degradation of urine(Reference Rouzaud, Rabot, Ratcliffe and Duncan14). Urine was centrifuged at 8000 g and 4°C for 5 min (model 2K15; Sigma Laborzentrifugen GmBH, Osterode-am-Harz, Germany) to remove particulates, and samples of 0·6 ml of the supernatant fraction were stored at − 20°C until analysis of NAC of AITC.
At the end of dietary treatment, animals were killed by CO2 asphyxiation. The liver and colon were removed after dissection and caecal pH was measured. Rat liver was weighed after removal of the adherent fat. It was then perfused with cold 150 mm-KCl (Prolabo, Paris, France), immediately frozen in liquid N2 and stored at − 80°C until the preparation of its sub-cellular fractions. The colon was gently flushed with 150 mm-KCl to remove the contents. The colon was then cut longitudinally and the mucosal layer was scraped with the edge of a glass slide. Mucosal cells were suspended in 5 ml of homogenising buffer containing 250 mm-sucrose (Euromedex, Souffelweyersheim, France), 140 mm-KCl, 10 mm-EDTA, 10 mm-Trizma HCl, 1 mm-dithiothreitol and 0·25 mm-phenylmethylsulfonyl fluoride, adjusted to pH 7·4. The mucosal suspensions were maintained on ice before the preparation of colonic cytosol on the same day.
Extraction and analysis of biochemical components
Glucosinolate concentration and myrosinase activity in diets
The four types of pelleted diet were finely ground in a coffee grinder and defatted five times in 2 volumes (v/v) of hexane. Glucosinolates were extracted in triplicate from defatted diets, desulfated and analysed by HPLC, as modified from Minchinton et al.(Reference Minchinton, Sang, Burke and Truscott18) and European Union(19). The protocol for the extraction, analysis and quantification of sinigrin has been outlined by Rungapamestry et al.(Reference Rungapamestry, Duncan, Fuller and Ratcliffe15).
Myrosinase activity was analysed in triplicate in 0·10 g freeze-dried raw, lightly cooked or fully cooked cabbage according to Rungapamestry et al.(Reference Rungapamestry, Duncan, Fuller and Ratcliffe15) and its activity was calculated in the respective cabbage diets. A five-point standard curve was prepared using myrosinase from a commercial source (r 2 0·9809 for observed absorbance v. nominal activity of myrosinase standard).
Urinary N-acetylcysteine conjugates
Urine samples collected during each batch were pooled over a 24 h period. N-acetylcysteine conjugates of AITC were determined in duplicate (n 6 for each dietary treatment within each microbiota group) by HPLC according to Rouzaud et al.(Reference Rouzaud, Rabot, Ratcliffe and Duncan14), as adapted from Mennicke et al.(Reference Mennicke, Kral, Krumbiegel and Rittmann20). The dicyclohexylamine salt of NAC of propyl isothiocyanate (N-acetyl-S-(N-propylthiocarbomoyl)-l-cysteine) was synthesised according to Mennicke et al.(Reference Mennicke, Gorler and Krumbiegel21) and 0·1 ml of a 0·2 mm solution was added, as an internal standard, to 0·4 ml of pooled rat urine before extraction. Concentrations of urinary NAC of AITC were calculated from calibration curves (r 2 values of 0·9982–0·9999) of its dicyclohexylamine salt, synthesised according to Mennicke et al.(Reference Mennicke, Gorler and Krumbiegel21). CV of extraction efficiency of NAC of AITC within and between days of analysis were 2·41 and 5·07 % respectively.
Preparation of hepatic and colonic sub-cellular fractions
Samples were kept on ice and buffers were maintained at 4°C throughout preparation. Colonic cytosol was isolated from fresh colonic mucosa on the day of killing by differential centrifugation. Briefly, the suspension of colonic mucosa in homogenising buffer was homogenised using a Eurostar basic tissue homogeniser (IKA Labortechnik Staufen, Germany) and spun at 16 000 g and 4°C for 20 min in a Centrikon T-124 centrifuge (Kronton Instruments, Italy). The resulting supernatant fraction was centrifuged in a Beckman Optima XL-100K ultracentrifuge (Beckman Instruments, Fullerton, CA, USA) at 100 000 g and 4°C for 75 min. The cytosolic fraction recovered in the supernatant fraction was frozen in liquid N2 and stored at − 80°C until analysis of GST by UV-vis spectrophotometry.
Hepatic cytosol was prepared, as modified from Ryan(Reference Ryan, Fleisher and Packer22), within 3 d after killing and frozen storage. Briefly, rat liver was thawed in a buffer of 150 mm-KCl, 10 mm-EDTA, 10 mm-Trizma HCl and 1 mm-dithiothreitol at pH 7·4. The defrosted liver was then homogenised in 15 ml of homogenising buffer at pH 7·4. The homogenate was centrifuged twice, as described for colonic cytosol. Hepatic cytosol contained in the final supernatant fraction was frozen in liquid N2 and stored at − 80°C until analysis of GST by UV-vis spectrophotometry. Hepatic microsomes present in the sediment after the second centrifugation were washed in a buffer consisting of 10 mm-EDTA and 150 mm-KCl at pH 7·4, followed by centrifugation at 100 000 g and 4°C for 75 min. Pelleted microsomes were re-suspended in freezing buffer (250 mm-sucrose, 10 mm-EDTA, 50 mm-Trizma HCl, 1 mm-dithiothreitol and 0·25 mm-phenylmethylsulfonyl fluoride, pH 7·4) and frozen at − 80°C until analysis of UGT by radiometry.
Glutathione-S-transferase and uridine 5′-diphospho-glucuronosyl transferase enzyme assays
Cytosolic and microsomal protein content was determined according to the method of Lowry et al.(Reference Lowry, Rosebrough, Farr and Randall23). Phase 2 enzyme activities were analysed in duplicate (n 6 for each dietary treatment within each microbiota group). Activity of GST in hepatic and colonic cytosol was measured in 0·2 m-phosphate buffer (pH 6·5) at 25°C, as adapted from Habig et al.(Reference Habig, Pabst and Jakoby24) by using 1 mm-1-chloro-2,4-dinitrobenzene as the substrate in the presence of 5 mm-glutathione. The substrate, 1-chloro-2,4-dinitrobenzene, is metabolised by GST-α, -μ and -π(Reference Habig, Pabst and Jakoby24). The change in absorbance was recorded at 340 nm for 3 min (Cary 50; Varian Ltd, Walton-on-Thames, Surrey, UK). Hepatic cytosol was diluted by a factor of 100 to ensure linearity of reaction rates and contained 0·027–0·051 mg protein, while colonic cytosol contained 0·2–1·1 mg of protein. The specific activity of GST was expressed as μmol of S-2,4-dinitrophenylglutathione formed/min per mg cytosolic protein.
Activity of UGT in hepatic microsomes was measured using chloramphenicol (CAP) as the substrate, according to the radiometric method of Young & Lietman(Reference Young and Lietman25), as modified by Rabot et al.(Reference Rabot, Nugon-Baudon and Szylit26). Briefly, the reaction mixture (1·15 ml) contained 225 mm of the Na salt of uridine 5′-diphosphoglucuronic acid, 7·5 mm-MgCl2 and 47 mm-Trizma HCl at pH 8·0, the liver microsomal fraction containing 1·0–2·0 mg protein, and 3·7 MBq (0·1 mCi)/mmol of CAP (prepared with 9 volumes of 225 mm of CAP in ethanol and 1 volume of [14C]CAP (specific activity of 1850 MBq (50 mCi)/mmol; GE Healthcare Europe GmbH, Munich, Germany). The reaction mixture was incubated at 37°C for 2 h. The reaction was stopped by incubating the mixture at 100°C for 2 min. A volume of 3 ml isopentyl acetate (Prolabo, Fontenay-sous-Bois, France) was then added and the free CAP was extracted twice in the organic phase by vortex agitation for 3 min, and removal of the organic phase by centrifugation. The aqueous phase containing the CAP conjugated with uridine 5′-diphosphoglucuronic acid (CAP glucuronide) was collected, and 0·5 ml of this extract was counted for 2 min in 10 ml scintillation fluid (Optiphase ‘Hisafe’ II) using a LKB liquid scintillation counter (model 1209; Perkin-Elmer, Courtaboeuf, France). The specific activity of UGT was reported as pmol CAP glucuronide formed/min per mg microsomal protein.
Statistical analysis
Samples of urine and hepatic and colonic sub-cellular fractions were analysed in duplicate, and results are expressed as mean values with their standard errors of six observations for analysis of NAC of AITC and specific activities of GST and UGT. Rat feed was analysed in triplicate. Statistical analyses were conducted using GenStat software (release 8.1; Lawes Agricultural Trust, Rothamsted Research, Harpenden, Herts, UK(27)). Excretion of NAC of AITC over 24 h was expressed as a proportion of the molar intake of its precursor, sinigrin, in cabbage to ensure that variation in its excretion was not due to differences in sinigrin intake. The effect of dietary treatment and colonic microbiota on the output of NAC of AITC as a proportion of ingested sinigrin, and on the activity of GST and UGT was tested by ANOVA. Since each of the six batches of the feeding regimen consisted of four GF and four HFM rats receiving one of the four dietary treatments, batches of rats were treated as blocks in ANOVA to reduce the effect of temporal variation.
Results
Body weight and feed intake
The glucosinolate profile of the diets given to rats is shown in Table 2. Sinigrin was absent in the control diet but its concentrations were similar in the three cabbage-substituted diets (P = 0·247) and constituted about 40 % (μmol/μmol) of the total glucosinolate concentration. Other major glucosinolates included glucobrassicin and glucoiberin. Mean sinigrin intake/d was significantly higher after consumption of diets containing lightly cooked cabbage as compared with raw (P = 0·04) or fully cooked cabbage (P = 0·01) in GF rats, and fully cooked cabbage (P = 0·02) in HFM rats (Table 3).
Table 2 Glucosinolate profile (μmol/g dry matter) of control and cabbage-substituted diets given to rats
(Mean values and standard deviations for three replicates)
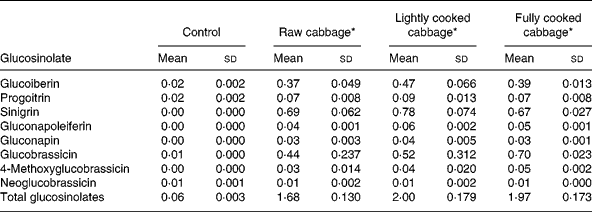
* Control diet substituted with either raw, lightly cooked or fully cooked cabbage.
Table 3 Daily intake of feed (g/d), sinigrin (μmol/d) and plant myrosinase (units/d) by rats given the control or the cabbage-substituted diets*
(Mean values with their standard errors)
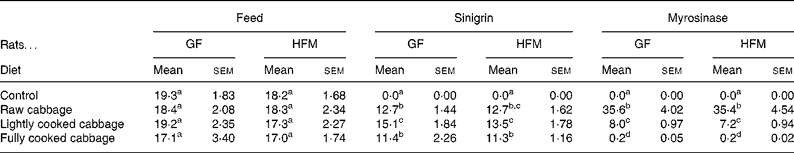
GF, germ-free; HFM, human faecal microbiota-associated.
a,b,c,d Mean values within a column with unlike superscript letters are significantly different at P < 0·05 for feed and sinigrin intakes, and P < 0·001 for myrosinase intake.
* Results are shown as mean intake of six rats, averaged over 4 d, six replicates per group.
Ingestion of plant myrosinase was significantly influenced by dietary treatment (P < 0·001) (Table 3). Mean intake of plant myrosinase by GF and HFM rats per d was 5- and 178-fold higher on the raw cabbage diet than on the lightly cooked or fully cooked cabbage diets respectively.
Daily feed intake varied by up to 30 % over the last 4 d of dietary treatment (P = 0·032), but was not influenced by diet (P = 0·453) (Table 3) or microbiota status (P = 0·298). Rats had a mean weight gain of 4·6 (sem 1·77) g after dietary treatment for 14 d, regardless of microbiota status (P = 0·263) or diet (P = 0·392). Liver weight was not affected by microbiota status (P = 0·587), diet (P = 0·229) or body weight at killing (P = 0·706). Caecal pH was significantly lower in HFM rats (6·8 (sem 0·07)) than in GF rats (7·8 (sem 0·03)) (P < 0·001), but was not affected by dietary treatment (P = 0·381).
Effect of diet and colonic microbiota on excretion of N-acetylcysteine conjugates of allyl isothiocyanate
Total output of NAC of AITC over 24 h, as a proportion of sinigrin intake, was significantly influenced by cooking treatment applied to cabbage before its incorporation into the diets of rats (P < 0·001) (Fig. 1). Excretion of NAC of AITC was significantly higher after consumption of the cabbage diets by HFM as compared with GF rats (P < 0·001) (Fig. 1). As determined by covariate analysis in ANOVA, plant myrosinase activity was positively associated with the effect of cooking treatment on the output of NAC of AITC (P < 0·001). The excretion of NAC of AITC, as a proportion of sinigrin ingested, was higher in HFM than GF rats after the intake of raw (P = 0·005) or lightly cooked (P = 0·006) cabbage, but was similar for the two groups of rats after consumption of fully cooked cabbage (P = 0·363). This resulted in an interaction between the effect of dietary treatment and microbiota status (P = 0·013) on NAC output. Excretion of NAC of AITC varied by up to 50 % between rats consuming similar diets, as determined from the CV.
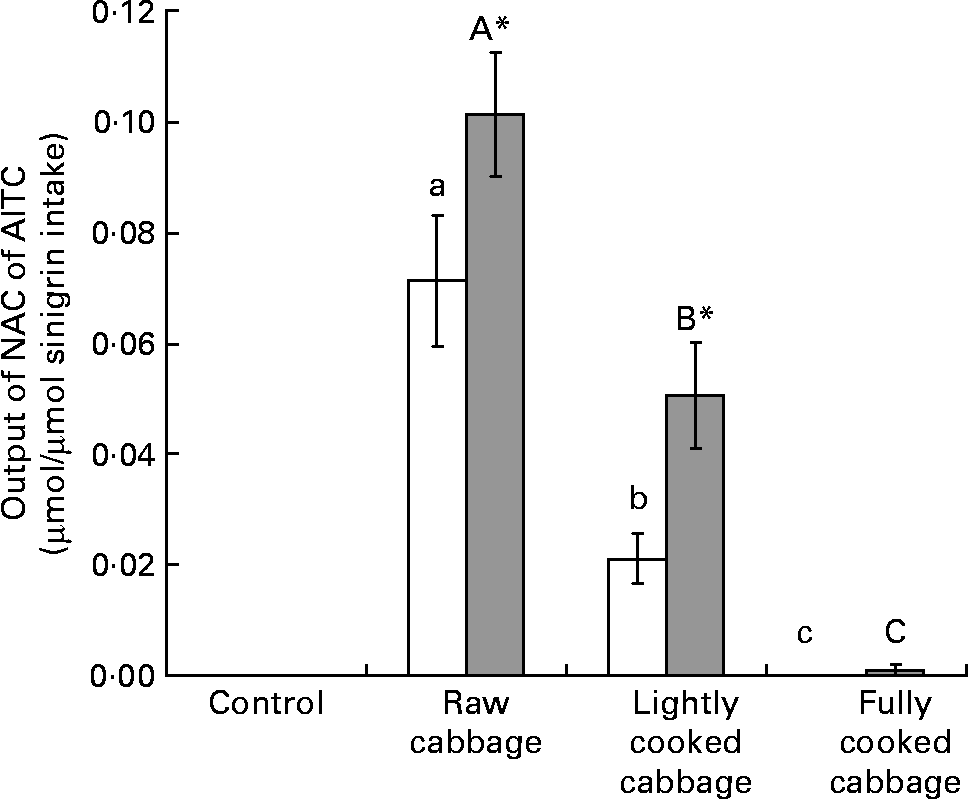
Fig. 1 Excretion of N-acetylcysteine conjugates of allyl isothiocyanate (NAC of AITC) (μmol) over 24 h, expressed as a proportion of sinigrin intake (μmol), after consumption of a control diet or diets containing raw, lightly cooked or fully cooked cabbage by germ-free (GF; □) and human faecal microbiota-associated (HFM; ![]() ) rats. Values are means of six replicates, with standard errors represented by vertical bars. * Mean value was significantly different from that of the GF group consuming the same diet (P < 0·05). a,b,c Mean values for the GF rats with unlike letters were significantly different (P < 0·05). A,B,C Mean values for the HFM rats with unlike letters were significantly different (P < 0·05). Output of NAC of AITC was significantly affected by cooking treatment applied to cabbage (P < 0·001), and microbiota status of rats after raw (P < 0·01) or lightly cooked cabbage (P < 0·01).
) rats. Values are means of six replicates, with standard errors represented by vertical bars. * Mean value was significantly different from that of the GF group consuming the same diet (P < 0·05). a,b,c Mean values for the GF rats with unlike letters were significantly different (P < 0·05). A,B,C Mean values for the HFM rats with unlike letters were significantly different (P < 0·05). Output of NAC of AITC was significantly affected by cooking treatment applied to cabbage (P < 0·001), and microbiota status of rats after raw (P < 0·01) or lightly cooked cabbage (P < 0·01).
Effect of diet and colonic microbiota on phase 2 enzyme activities
In rat liver, the specific activity of GST was not influenced by dietary treatment (P = 0·808) or colonic microbiota (P = 0·113) (Fig. 2 (A)). The effect of dietary treatment on colonic GST (Fig. 2 (B)) and hepatic UGT (Fig. 3) activities was significant only in GF rats (colonic GST, P = 0·041; hepatic UGT, P = 0·003). Orthogonal contrast analysis within ANOVA showed that the consumption of fully cooked cabbage by GF rats led to a slightly higher colonic GST activity (by 1·3 times) than after the intake of the control (P = 0·036) or lightly cooked cabbage (P = 0·012) diet (Fig. 2 (B)). Hepatic UGT activity was higher after the intake of raw (by 1·4 times, P = 0·043), partially cooked (by 1·5 times, P = 0·025), or fully cooked (by 1·8 times, P < 0·001) cabbage, as compared with the control diet, in GF rats. Hepatic UGT activity was not significantly different among the three cabbage-substituted diets in GF rats (P = 0·142) (Fig. 3). The specific activities of colonic GST (Fig. 2 (B)) and hepatic UGT (Fig. 3) were significantly influenced by microbiota status (colonic GST, P = 0·001; hepatic UGT, P = 0·024) since dietary treatment with cabbage altered enzyme activities in GF rats only.
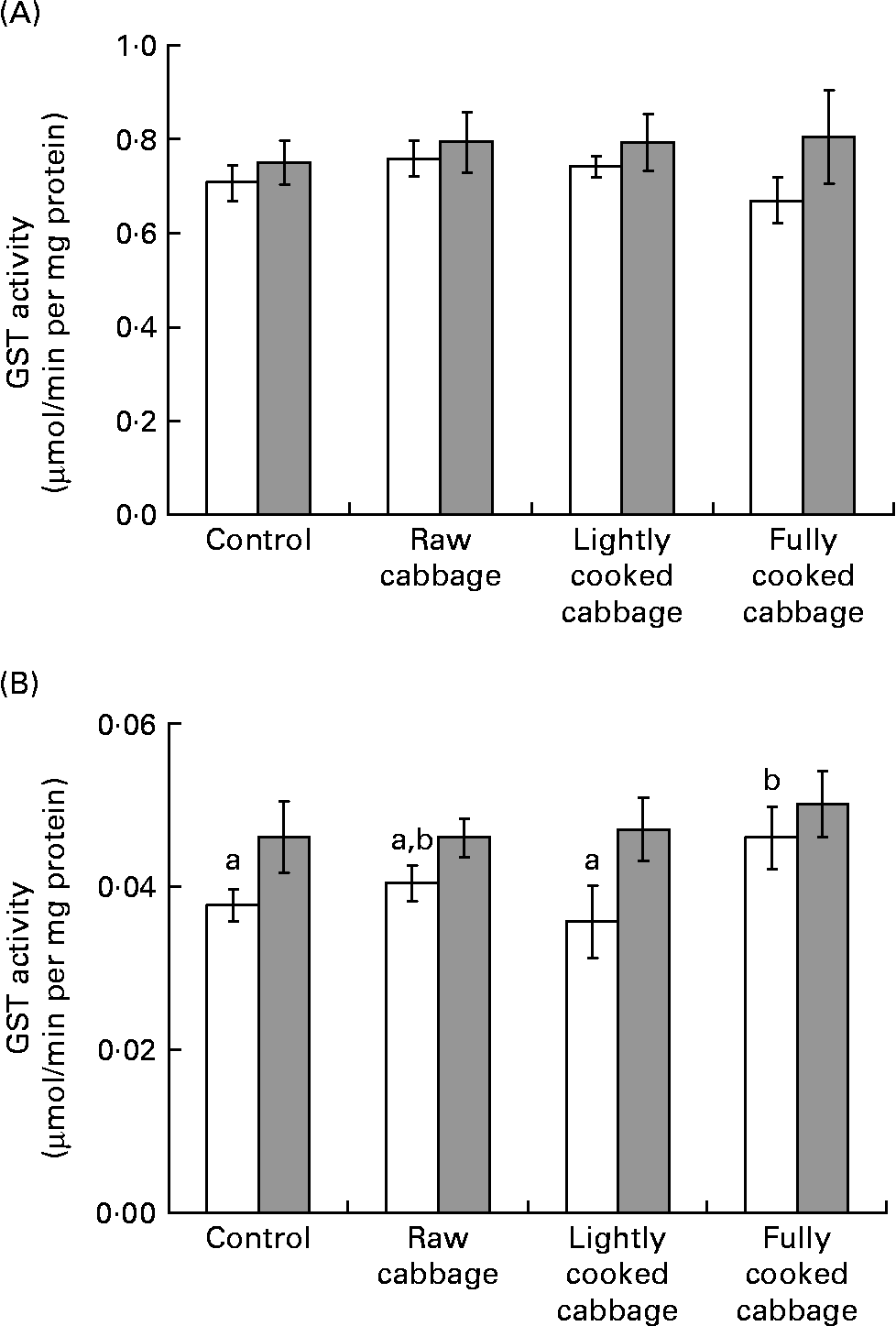
Fig. 2 Specific activity of glutathione-S-transferase (GST) in rat (A) liver and (B) colon, expressed as μmol S-2,4-dinitrophenylglutathione formed/min per mg cytosolic protein, after consumption of a control diet or diets containing raw, lightly cooked or fully cooked cabbage by germ-free (□) and human faecal microbiota-associated (![]() ) rats. Values are means of six replicates, with standard errors represented by vertical bars. a,b Mean values for the GF rats with unlike letters were significantly different (P < 0·05). Liver GST was not influenced by cooking treatment applied to cabbage, or microbiota status. Colonic GST was affected by dietary treatment in germ-free rats only (P < 0·05).
) rats. Values are means of six replicates, with standard errors represented by vertical bars. a,b Mean values for the GF rats with unlike letters were significantly different (P < 0·05). Liver GST was not influenced by cooking treatment applied to cabbage, or microbiota status. Colonic GST was affected by dietary treatment in germ-free rats only (P < 0·05).
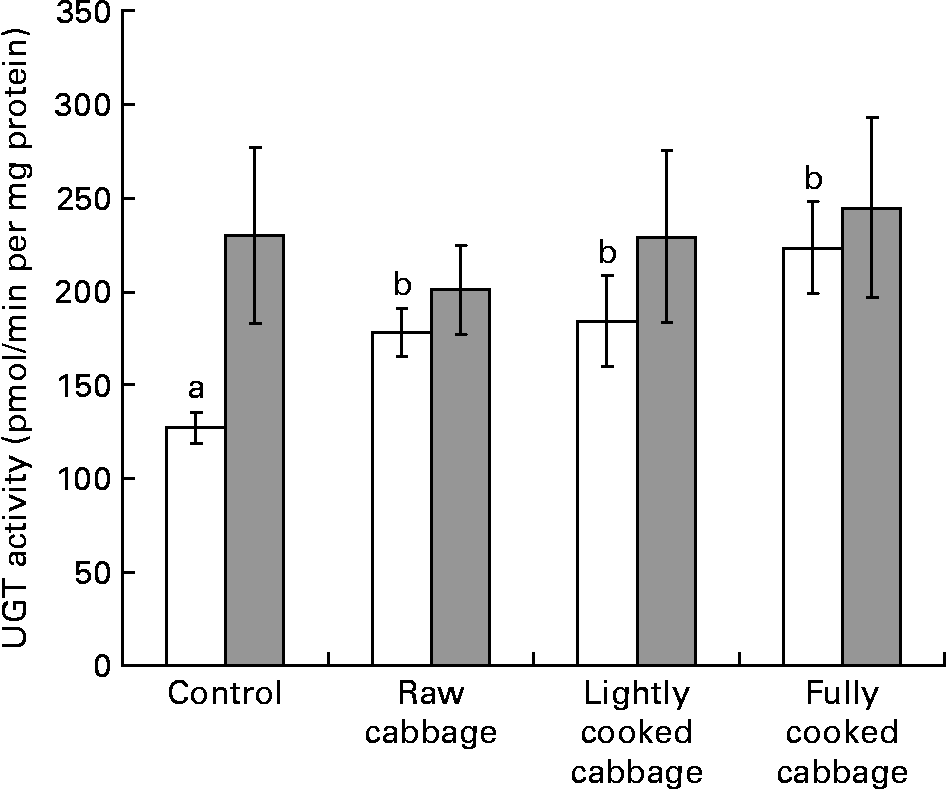
Fig. 3 Specific activity of uridine 5′-diphospho-glucuronosyl transferase (UGT) in rat liver, expressed as pmol chloramphenicol glucuronide formed/min per mg microsomal protein, after consumption of a control diet or diets containing raw, lightly cooked or fully cooked cabbage by germ-free (□) and human faecal microbiota-associated (![]() ) rats. Values are means of six replicates, with standard errors represented by vertical bars. a,b Mean values for the GF rats with unlike letters were significantly different (P < 0·05). UGT was affected by dietary treatment in germ-free rats only (P < 0·05).
) rats. Values are means of six replicates, with standard errors represented by vertical bars. a,b Mean values for the GF rats with unlike letters were significantly different (P < 0·05). UGT was affected by dietary treatment in germ-free rats only (P < 0·05).
Discussion
Previous work has shown that cooking brassica influences the excretion of isothiocyanates in man(Reference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung13, Reference Rouzaud, Young and Duncan28, Reference Rungapamestry, Duncan, Fuller and Ratcliffe29), and that administration of isolated isothiocyanates or consumption of brassica has variable effects on tissue-specific phase 2 enzyme activity in rats(Reference Whitty and Bjeldanes30–Reference Munday and Munday33). The present study is the first to have combined measures of isothiocyanate absorption after consumption of brassica, cooked to different extents, with measurement of phase 2 enzyme activities in a holistic way to investigate the link between the metabolic fate of glucosinolates and their biological effects. Rats share a similar NAC excretion pathway to man but represent a more practical model for the investigation of tissue-specific phase 2 enzyme activities. The use of gnotobiotic rats, as opposed to conventional rats, allowed manipulation of the colonic milieu to mimic that of a healthy human, thus increasing the application of the results to humans. Due to major differences in composition and metabolic activity between the human and rat colonic microbiota(Reference Debure, Colombel, Flourie, Rautureau and Rambaud34), HFM rats have been used as an established model by various authors to study interactions between diet, including glucosinolate intake, and gut microbiota(Reference Rouzaud, Rabot, Ratcliffe and Duncan14, Reference Kassie, Rabot, Kundi, Chabicovsky, Qin and Knasmuller35, Reference Humblot, Lhoste, Knasmuller, Gloux, Bruneau, Bensaada, Durao, Rabot, Andrieux and Kassie36). Both the microbial population composition of the human colonic microbiota, as well as its xenobiotic metabolising enzyme activities, such as hydroxylation of heterocyclic amines and hydrolysis of glucuronides, are reliably preserved in the gut of HFM rats after inoculation(Reference Debure, Colombel, Flourie, Rautureau and Rambaud34, Reference Mallett, Bearne, Rowland, Farthing and Cole37, Reference Rumney and Rowland38).
We have shown that urinary output of AITC conjugate is related to plant myrosinase activity. This observation agrees with previous studies which showed a higher excretion of isothiocyanate conjugates following ingestion of glucosinolate precursors by rodents(Reference Rouzaud, Rabot, Ratcliffe and Duncan14) and human subjects(Reference Rouzaud, Young and Duncan28, Reference Rungapamestry, Duncan, Fuller and Ratcliffe29, Reference Shapiro, Fahey, Wade, Stephenson and Talalay39) when plant myrosinase was active than when denatured by cooking. In our experiment, the action of plant myrosinase was augmented to a similar extent by the colonic microbiota after HFM rats consumed raw or lightly cooked cabbage.
Conversely, when plant myrosinase activity was essentially absent, as occurred after consumption of fully cooked cabbage, the supplementary effect of colonic microbiota on excretion of NAC of AITC was not observed. The output of AITC conjugate in HFM rats was low and similar to that in their GF counterparts. Comparable findings were reported by Rouzaud et al.(Reference Rouzaud, Rabot, Ratcliffe and Duncan14) in HFM rats fed a diet without plant myrosinase. The authors suggest that a proportion of the isothiocyanates released in the colon may be further metabolised by the resident microbiota. However, this cannot explain the different impact of the colonic microbiota on excretion of NAC of AITC after consumption of raw or lightly cooked cabbage, compared with following consumption of fully cooked cabbage, in the present experiment. Incubation of glucosinolates with human faeces has been shown to produce amines instead of isothiocyanates(Reference Combourieu, Elfoul, Delort and Rabot40). It is therefore possible that the AITC produced in the colon of HFM rats, after the intake of fully cooked cabbage in the present experiment, may have been converted to amines and was subsequently not available for excretion as its NAC. Furthermore, the negligible output of AITC conjugate in HFM rats after consumption of fully cooked cabbage, compared with after the intake of raw or lightly cooked cabbage, may be partly due to physico-chemical changes in cabbage after cooking which may have affected the digestive fate of sinigrin.
On the other hand, the very low excretion of AITC conjugate in HFM rats after consumption of fully cooked cabbage in the present experiment does not agree with previous reports of NAC excreted in human subjects after consumption of brassica vegetables essentially free of active plant myrosinase(Reference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung13, Reference Rouzaud, Young and Duncan28, Reference Shapiro, Fahey, Wade, Stephenson and Talalay39). We have recently reported a large inter-individual variation in NAC excretion in human subjects following consumption of cooked brassica(Reference Rungapamestry, Duncan, Fuller and Ratcliffe29). It is possible that the difference in NAC excretion observed between the present study and the above human feeding experiments(Reference Conaway, Getahun, Liebes, Pusateri, Topham, Botero-Omary and Chung13, Reference Rouzaud, Young and Duncan28, Reference Shapiro, Fahey, Wade, Stephenson and Talalay39) may be due to variations in the bacterial profile of the microbiota.
The maximum percentage of sinigrin excreted as NAC of AITC was only 10 %, which is much lower than the 30–50 % reported in rodents administered with isolated glucosinolates alongside a diet containing plant myrosinase or in human subjects consuming raw brassica(Reference Duncan, Rabot and Nugon-Baudon11, Reference Shapiro, Fahey, Wade, Stephenson and Talalay12, Reference Rouzaud, Rabot, Ratcliffe and Duncan14). This may be due to differences in the mode of administration of the glucosinolate precursor, source of plant myrosinase, and metabolic disposal of individual glucosinolates. The excretion of sinigrin as NAC of AITC is a combined function of its hydrolysis to AITC from the gut, the absorption of AITC, and the subsequent metabolism of AITC to its NAC. However, the extent to which glucosinolates are hydrolysed to isothiocyanates in vivo, the relative absorption of isothiocyanates along different parts of the gut and the conversion of isothiocyanates to NAC v. other conjugates, are unknown and may be subject to extensive variation.
Following their release and absorption, isothiocyanates are transported, mostly in a conjugated form, to the liver and colon via the blood. Isothiocyanates produced in the colon may also be directly absorbed through the colonic epithelial cells. In the present experiment, liver GST activity was not affected by dietary treatment with cabbage, in contrast to previous studies. This may be due to differences in species or cultivar of brassica used. A two-fold increase in liver GST activity in rats fed cabbage has been shown, but the glucosinolate profile and cultivar of cabbage used were not reported(Reference Whitty and Bjeldanes30). Glucosinolate concentrations vary extensively within and between cabbage cultivars(Reference Kushad, Cloyd and Babadoost41), and differences attributed to cultivar may influence the activity of xenobiotic metabolising enzymes in the liver of rats fed brassica(Reference Kassie, Uhl, Rabot, Grasl-Kraupp, Verkerk, Kundi, Chabicovsky, Schulte-Hermann and Knasmuller32). An increase in hepatic GST activity of up to two-fold(Reference Bradfield and Bjeldanes42–Reference Wortelboer, Dekruif, Vaniersel, Noordhoek, Blaauboer, Vanbladeren and Falke44) has also been reported in rats fed freeze-dried raw or cooked Brussels sprouts, which besides containing sinigrin, are an abundant source of progoitrin, which may be responsible for their inductive effects on hepatic GST.
Hepatic UGT activity was not modified by brassica intake in HFM rats, in contrast with previous findings reporting an increase in conventional or HFM rats after consumption of a range of raw or cooked brassica(Reference Wortelboer, Dekruif, Vaniersel, Noordhoek, Blaauboer, Vanbladeren and Falke44–Reference Roland, Rabot and Nugon-Baudon46). The effect of brassica consumption on hepatic UGT activity has been shown to be cultivar dependent, as seen following the intake of red cabbage by rats(Reference Kassie, Uhl, Rabot, Grasl-Kraupp, Verkerk, Kundi, Chabicovsky, Schulte-Hermann and Knasmuller32). Conversely, in GF rats, the intake of all cabbage diets increased liver UGT activity by a similar extent, as compared with the control feed, in the present experiment. This stimulation may have been due to other components within the cabbage matrix, rather than isothiocyanates or other metabolites of glucosinolates per se.
In the same way as for the liver, colonic GST activity was not influenced by dietary treatment with cabbage in HFM rats. Likewise, previous studies found that colonic GST activity was unaltered in conventional rats fed freeze-dried raw cabbage(Reference Tan, Lin, Xiao, Kadlubar and Chen47) or Brussels sprouts(Reference Nijhoff, Groen and Peters48) for a similar duration. In the present experiment, sinigrin was the predominant glucosinolate present and this was partly converted to AITC in vivo. In previous work, when rats were dosed with 40 μmol AITC/kg per d for 6 d, no change in colon or liver GST activity was observed(Reference Munday and Munday33). In GF rats in the present experiment, on the other hand, colonic GST activity was altered, as found for hepatic UGT, but only after consumption of fully cooked cabbage. Again, this effect may have been caused by other constituents of cabbage, rather than due to the production of AITC or other metabolites of glucosinolates per se, or may have resulted from the physico-chemical changes due to the more extensive cooking.
In the present experiment, rats ingested about 50 μmol sinigrin/kg per d, of which up to 10 % was excreted as NAC of AITC. Assuming that this percentage of AITC was absorbed, its concentration may have been too low to influence the activities of hepatic GST or UGT, and colonic GST. Furthermore, the reversible conjugation of isothiocyanates with glutathione generates a diverse range of intermediates and forms in which isothiocyanates are transported(Reference Stahl, van den Berg, Arthur, Bast, Dainty, Faulks, Gartner, Haenen, Hollman and Holst49), and it is unclear which of these forms influences phase 2 enzymes most. Moreover, AITC may be a less potent inducer of phase 2 enzymes when ingested within a cabbage matrix, as compared with when it is dosed as a pure compound.
Our findings have highlighted that following a meal substituted with brassica cooked to different extents, excretion of NAC of isothiocyanates may depend on a complex interaction between plant myrosinase, colonic microbiota, and/or possible physico-chemical changes occurring in the vegetable matrix during cooking. The specific activities of hepatic and colonic phase 2 enzymes, GST and UGT, were not modified by cooking cabbage or altering the colonic microbiota, and were not associated with NAC excretion. It was also shown that the role of the colonic microbiota in the metabolic fate of glucosinolates remains controversial in the absence of plant myrosinase.
Acknowledgements
The present study was supported by a grant from the Food Standards Agency (project code T01027; Influence of cooking and processing of brassica vegetables on the release of beneficial and harmful metabolites of glucosinolates in the digestive tract) and a travel grant from the Rank Prize Funds. We thank Mrs Heather Scott (The Robert Gordon University) for her assistance in processing the cabbage used in rat feeds, Mr Anthony Bouclet (INRA) for his valuable help and training in the care of animals, and Mrs Sandrine Bruel (INRA) for conducting the assay for UGT activity.








