The long-standing parental advice to ‘eat your breakfast; it's the most important meal of the day’ has recently acquired scientific support as breakfast skipping is associated with an array of unhealthful outcomes(Reference Keski-Rahkonen, Kaprio, Rissanen, Virkkunen and Rose1) including body weight gain, overweight and obesity(Reference de Castro2–Reference Song, Chun, Obayashi, Cho and Chung5). Several recent studies have shown that men and women consume more total energy throughout the day, especially in the evening, when breakfast is skipped(Reference de Castro2, Reference de Castro6). The types of foods consumed at breakfast may also impact total energy intake. Dietary protein is the most satiating of the macronutrients during energy restriction (ER) and energy balance (EB) conditions(Reference Halton and Hu7). We have previously reported that acute consumption of a higher dietary protein (28 g/meal) breakfast during ER leads to reduced hunger and desire to eat and increased feelings of fullness compared to consumption of a normal amount of dietary protein (17 g/meal)(Reference Leidy, Mattes and Campbell8). Data are limited concerning whether the consumption of additional dietary protein at breakfast leads to a greater differential appetitive response compared to other meal times or when protein is given at each meal. The purpose of the present study was to examine whether increased dietary protein intake at breakfast, lunch, dinner, or dividing it equally across meals influences short-term self-reported fullness during EB and ER.
Materials and methods
Participants
Participants were recruited through newspaper advertisements. Eligibility was based on the following criteria: (1) men ≥ 21 years; (2) BMI between 25 and 39·9 kg/m2; (3) not dieting and no weight loss or gain (>4·5 kg) within the last 6 months; (4) non-smoking; and (5) non-diabetic. Ten men began the study; nine men completed all study procedures (48 (sem 6) years; BMI 32·7 (sem 0·7) kg/m2). Each subject was informed of the purpose, procedures and potential risks of participation in the study before signing an informed consent form approved by the University Biomedical Institutional Review Board.
Experimental design
A randomized cross-over design was utilized in which each subject completed the following five controlled feeding trials: normal protein (NP), higher protein (HP) breakfast (HP-B), lunch (HP-L), dinner (HP-D) and HP equally divided among all meals (HP-E). Each trial consisted of 6 d. During the first 3 d (days 1–3) of each trial, each subject was provided with an EB diet. During the second 3 d (days 4–6), each subject was provided with an individualized ER diet. During day 7, the subjects were asked to follow their habitual energy intake. In each trial, a three meal per day pattern was provided. Appetite was assessed throughout the 15 h period during days 3 and 6.
Diet
The subjects' energy needs were estimated to be 1·5 times their resting energy expenditure calculated using the Harris–Benedict equation for men(Reference Harris and Benedict9). The ER diet was then determined as 3138 kJ/d (750 kcal/d) less than their estimated energy need. The NP-EB diet consisted of 11 % of total energy from protein, 64 % of total energy from carbohydrate and 25 % of total energy from fat, whereas the NP-ER diet consisted of 14 % of total energy from protein, 61 % from carbohydrate and 25 % from fat. Both NP diets contained 0·8 g protein/kg per d equally divided among breakfast, lunch and dinner and was void of eggs and all striated tissue, including pork. The HP-EB diet was composed of 18 % of total energy from protein, 57 % from carbohydrate and 25 % from fat, whereas the HP-ER diet was 25 % of total energy from protein, 50 % from carbohydrate and 25 % from fat. Both HP diets contained 1·4 g protein/kg per d, with 15 and 25 % of total protein provided from egg and pork products, respectively. The additional 0·6 g protein/kg per d was provided as animal protein from eggs and pork and was consumed at breakfast, lunch, dinner or equally divided among all meals (0·2 g protein/meal). For the breakfast, lunch and dinner trials, the meals not containing additional protein were identical to the NP meals. Additional meal nutrient content information is provided in Table 1 and a sample menu is provided in Appendix 1.
Table 1 Dietary characteristics of the normal protein (NP) v. higher protein breakfast (HP-B), lunch (HP-L), dinner (HP-D) and equally distributed (HP-E) energy balance (EB) and energy restriction (ER) diets in nine volunteers*
(Mean values with their standard errors)
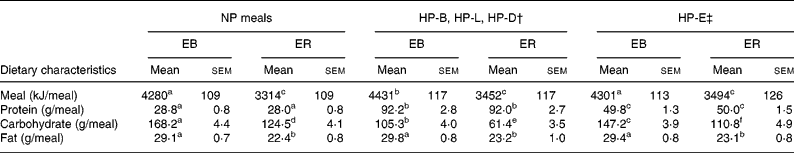
a–f Mean values within a row with unlike superscript letters were significantly different (repeated measures ANOVA within and between treatments; P < 0·05).
* For details of subjects and procedures, see Materials and methods.
† Dietary characteristics only correspond to the HP meal. During each of these trials, the non-HP meals were the same as the NP meals.
‡ Dietary characteristics were consumed at each of the three meals of a given day of the trial.
Participants reported to the metabolic kitchen for all meals on 6 d consecutively for 5 weeks. Breakfast, lunch and dinner were served between 07.00 and 09.00, 11.00 and 13.00, and 16.00 and 18.00 hours, respectively. Participants ate under supervision of research staff and consumed all foods provided to them. During each testing day, the subjects were instructed to eat only the foods provided during the supervised breakfast, lunch and dinner meals at the metabolic kitchen. While the subjects were allowed to leave the kitchen after each meal was consumed, they were not permitted to eat or drink anything besides water during the time between each meal. If a subject consumed anything during this time, the quantity and type of food or drink was recorded. None of the volunteers reported eating or drinking anything extra.
Appetite
Fullness, hunger and desire to eat were assessed over 15 h on days 3 and 6 by responses to questions such as ‘How strong is your feeling of fullness?’ during the following times: pre-meal ( − 30 min), +30, +60, +90, +120, +180 min post-meal for breakfast, lunch and dinner. Responses were recorded on a thirteen-point, numbered, linear category scale. The end anchors were ‘not at all’ to ‘extremely’. Participants were instructed to circle the vertical dash along the horizontal line corresponding to their feelings at that moment, and the results are reported using arbitrary units (au).
Data and statistical analyses
To assess the acute appetite responses, the meal-related 3 h postprandial fullness, hunger and desire to eat area under the curves were calculated for the HP meal in each HP treatment, each NP meal in the NP treatment, and each HP meal in the HP-E treatment. To assess the sustained appetite response, the 15 h (overall) area under the curves were determined for each treatment. All area under the curve measurements were calculated using the trapezoidal rule(Reference Wolever, Jenkins, Jenkins and Josse10). The NP treatment was incorporated into the study design to provide the reference (i.e. ‘normal’) responses for the meal-related and overall feelings of fullness, hunger and desire to eat. All data throughout each of the HP treatments are therefore expressed as the difference (Δ) from NP.
To examine the main effect of protein timing, a repeated measures ANOVA was performed on the meal-related and overall appetite responses during EB and ER. When main effects were detected, post hoc analyses were performed using Least Significant Difference procedures. This statistical approach led to greater than 80 % power (0·87) to detect differences in meal-related and overall appetite among treatments. All measurements are expressed as means and their standard errors. An α level of P < 0·05, two-tailed, was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Meal-related (3 h postprandial) fullness
While no difference in meal-related fullness was observed among the HP treatments during EB (data not shown), the HP-B during negative EB led to greater meal-related fullness (+137 (sem 44) au × 180 min) v. HP-D ( − 1 (sem 37) au × 180 min; P = 0·003). The response to the HP-B did not differ significantly from the HP-L (+62 (sem 53) au × 180 min; P = 0·188) or HP-E-B (+92 (sem 85) au × 180 min; P = 0·587).
Overall (15 h composite) fullness
No difference in overall fullness was observed among the HP treatments during EB (data not shown). During ER, the HP-B elicited greater fullness throughout the 15 h (+404 (sem 162) au × 900 min) compared to HP-L (+33 (sem 162) au × 900 min; P = 0·009) and HP-D ( − 60 (sem 132) au × 900 min; P = 0·05) (Fig. 1). The response to the HP-B did not differ significantly from the HP-E (+274 (sem 165) au × 900 min; P = 0·188) (Fig. 1).

Fig. 1 Fullness responses across the day during the higher protein (HP)-breakfast (B), lunch (L), dinner (D) or equally divided (E) treatments during energy restriction. (A), Fullness responses at each time-point (○, HP-B; ●, HP-L; △, HP-D, ▲, HP-E; ■, normal protein (NP)). Values are means. au, arbitrary units. (B), Fullness area under the curve over the entire 15 h period. Values are means with their standard errors depicted by vertical bars. a,b Mean values with unlike superscript letters were significantly different (repeated measures ANOVA between treatments; P < 0·05).
Meal-related and overall hunger and desire to eat
No differences in the meal-related and overall hunger and desire to eat responses were observed during EB. During ER, the meal-related and overall hunger and desire to eat responses tended to be comparable to the fullness responses but were not as statistically strong (data not shown).
Discussion
While the satiating property of increased dietary protein has been independently observed during breakfast(Reference Halton and Hu7, Reference Leidy, Mattes and Campbell8) and lunch(Reference Halton and Hu7, Reference Latner and Schwartz11), the relative efficacy of consuming protein in different patterns is not known. We found that during ER, increased dietary protein at breakfast led to greater initial and/or sustained increases in fullness compared to lunch and dinner.
Hunger and desire to eat were measured along with the feelings of fullness. We chose to specifically report the fullness (satiety) response due to the well-established satiating properties of protein(Reference Halton and Hu7) and the strong association with food intake which is not consistently observed with the hunger and/or desire to eat responses(Reference Parker, Sturm, MacIntosh, Feinle, Horowitz and Chapman12, Reference Raben, Tagliabue and Astrup13).
The differences in fullness observed between breakfast and the other meals may be a reflection of the greater change in macronutrients from the subject's habitual breakfast consumption. Since most Americans typically consume a relatively small amount of dietary protein at breakfast (15 % of total daily protein) in comparison to other meal times(Reference Moshfegh, Goldman and Cleveland14), the exposure to a disproportionately higher protein test-meal breakfast could potentially lead to a greater feeling of fullness than at other meal times. This is supported by Long et al. (Reference Long, Jeffcoat and Millward15) who examined whether the appetite responses to a higher protein test meal were influenced by habitual protein intake. The subjects habitually consuming a lower protein diet experienced greater postprandial satiety following the higher protein test meal compared to those who habitually consumed a higher protein diet. This would suggest that our differential satiety response at breakfast may have been simply due to the change in diet and not the timing of protein consumption. We previously evaluated whether protein habitualization during weight loss influenced the acute, meal-related appetitive responses(Reference Leidy, Mattes and Campbell8). Unlike that of Long et al. (Reference Long, Jeffcoat and Millward15), the appetite responses following the higher protein meal v. the normal protein meal in the present study were unaffected by chronic protein intake(Reference Leidy, Mattes and Campbell8).
Along these lines, our experimental design incorporated three meals of equivalent energy intake. Since breakfast is typically much smaller than the lunch or dinner meals(Reference de Castro6), the larger breakfast in the current study may have influenced the raw fullness responses. We chose to compare each treatment according to the difference from the NP treatment, which also incorporated a larger breakfast, to counter any differences. We believe the satiating effects of protein at breakfast in the current study are not a result of the change in habitual diet.
The effects of increased protein when equally divided among all meals were also examined. We anticipated greater overall fullness during this treatment as a small amount of protein at every eating occasion should theoretically sustain feelings of fullness throughout the day. No difference in fullness was observed between this treatment and any of the other higher protein treatments. Further, this treatment led to similar fullness compared to the normal protein treatment. This may be explained by the fact that most increased protein studies incorporate protein amounts on the order of approximately 75 g protein/meal or greater(Reference Halton and Hu7). Our HP-E treatment contained approximately 50 g/meal, suggesting a threshold or graded effect may exist for the amount of protein consumed at one time to yield differences in appetite. Additional studies are needed to characterize the dose–response relationship.
The present study also indicates that the satiating properties of protein are affected by energy status as no difference in fullness was observed between the normal protein and increased dietary protein during EB. In a comprehensive review by Halton & Hu(Reference Halton and Hu7), eleven of fourteen studies found greater satiety with increased dietary protein. This finding is also supported by two recent studies(Reference Moran, Luscombe-Marsh, Noakes, Wittert, Keogh and Clifton16, Reference Lejeune, Westerterp, Adam, Luscombe-Marsh and Westerterp-Plantenga17). Several main differences exist when comparing these studies to our current study: (1) the dietary protein in these studies ranged from 30 to 100 % of total energy intake(Reference Halton and Hu7, Reference Moran, Luscombe-Marsh, Noakes, Wittert, Keogh and Clifton16, Reference Lejeune, Westerterp, Adam, Luscombe-Marsh and Westerterp-Plantenga17), whereas the current study was only 18 % in the EB segment; (2) many of these studies incorporated healthy men and/or women, while the current study targeted overweight and/or obese men; (3) lastly, none of these studies compared the increased dietary protein among all three meals(Reference Halton and Hu7, Reference Moran, Luscombe-Marsh, Noakes, Wittert, Keogh and Clifton16, Reference Lejeune, Westerterp, Adam, Luscombe-Marsh and Westerterp-Plantenga17). The present data imply that the satiating effect of increased dietary protein is blunted when consuming 18 % of total energy intake as protein in an EB state while other data support that consuming larger amounts of dietary protein will lead to greater satiety during EB.
We have suggested an increased sensitivity to dietary protein during breakfast as compared to other meal times. Due to the fact that the satiating properties of dietary protein consumed at dinner were completely abolished (as evidenced by the similar postprandial fullness response to that of the normal protein treatment), it may, in actuality, be the reduced sensitivity to dietary protein during lunch and dinner. This would support the concept that individuals appear to be less satiated towards evening leading to increased meal size and shorter inter-meal intervals throughout the day(Reference de Castro2).
The present data lend further support for the need to incorporate increased dietary protein at breakfast when designing effective energy-restricted diets. Several study limitations exist. First, the findings are derived from acute subjective measures of fullness (satiety) from questionnaires. While the data indicate differential fullness responses among the treatments, the present study design does not allow for the examination of whether these fullness responses would lead to differences in food intake. According to Parker et al. (Reference Parker, Sturm, MacIntosh, Feinle, Horowitz and Chapman12) and Flint et al. (Reference Flint, Raben, Blundell and Astrup18), subjective sensations assessing fullness from questionnaires are highly predictive and/or correlated with food intake. Thus, we are confident that the present data provide important and unique findings concerning the timing of protein consumption in relation to appetite and food intake.
The hormones involved with the regulation of food intake and body weight were not examined. Further research is necessary to confirm the present findings, document changes in food intake, and to explore the underlying mechanisms and long-term implications for EB and body weight control.
Lastly, the present study only examined the effect of increased animal protein (provided from the pork and eggs). While research suggests comparable fullness (satiety) between meals containing animal-based (egg albumin) protein v. plant-based protein (i.e. soya)(Reference Lang, Bellisle, Oppert, Craplet, Bornet, Slama and Guy-Grand19), we did not test this in the present study. Thus, caution should be made concerning the generalizability of the present data.
Conclusions
The differential appetitive responses following increased protein intake at breakfast compared to other meal times suggest that the satiating property of dietary protein is influenced by the timing of protein consumption.
Acknowledgements
The authors would like to thank Nadine S. Carnell, MS, who provided nutritional support concerning the test meals. This study was funded through the National Pork Board and the American Egg Board – Egg Nutrition Center. Support for H. L. was also provided, in part, by an Ingestive Behavior Research Center post-doctoral fellowship from Purdue University. None of the authors had any personal or financial conflicts of interest.
Appendix 1 Sample subject energy restriction menus

FF, fat-free.





