Obesity has been reported as a risk factor for low serum 25-hydroxyvitamin D (25(OH)D) concentrations( Reference Wortsman, Matsuoka and Chen 1 ). Both obesity and vitamin D deficiency are major public health issues( Reference Shakur, Tarasuk and Corey 2 , 3 ) that have been associated with insulin resistance and a higher risk of developing type 2 diabetes( Reference Song, Wang and Pittas 4 ).
Weight loss of at least 5–7 % resulting from lifestyle changes has been associated with a reduced risk of developing type 2 diabetes in overweight or obese individuals with prediabetes( Reference Knowler, Barrett-Connor and Fowler 5 , Reference Tuomilehto, Lindstrom and Eriksson 6 ). Moreover, serum 25(OH)D concentrations have been shown to increase following weight loss( Reference Mason, Xiao and Imayama 7 , Reference Rock, Emond and Flatt 8 ). It has been established that weight loss increases insulin sensitivity and secretion, and improves β-cell function( Reference Kitabchi, Temprosa and Knowler 9 ). Similarly, some studies have reported that vitamin D supplementation also leads to improved glucose metabolism( Reference Mitri, Dawson-Hughes and Hu 10 , Reference von Hurst, Stonehouse and Coad 11 ). To our knowledge, only one study has demonstrated that increased serum 25(OH)D concentrations after a clinically significant weight loss correlated with decreased insulin resistance( Reference Tzotzas, Papadopoulou and Tziomalos 12 ). However, this study had some limitations including the small sample size (n 44) and the lack of adjustment for seasonality, which is an important confounding factor to take into account. Moreover, insulin secretion and β-cell function were not assessed.
The main objective of the present study was to determine whether a rise in serum 25(OH)D concentrations observed after a clinically significant weight loss ( ≥ 5 %) contributes to the improvement in insulin sensitivity. The secondary objectives were to determine whether this increase in serum 25(OH)D concentrations improves insulin secretion and β-cell function. We hypothesised that an increase in serum 25(OH)D concentrations after a clinically significant weight loss is associated with improved insulin sensitivity, insulin secretion and β-cell function, irrespective of the amount of weight loss.
Experimental methods
Subjects
Data were combined from two prospective studies, IG( Reference Gagnon, Brown and Couture 13 ) and Lawson (J.-P. Baillargeon, unpublished results), in which men and women at high risk for type 2 diabetes followed lifestyle-modifying weight loss interventions for 1 year. The interventions took place at the Centre hospitalier universitaire de Sherbrooke located in Québec, Canada. The IG study was completed in 2006, while the Lawson study ended in 2011. The research was conducted according to the Declaration of Helsinki; the Centre hospitalier universitaire de Sherbrooke and the University of Sherbrooke ethics review board of research on humans approved the study protocols, and written informed consent was obtained from each participant. Both studies were registered on the clinicaltrials.gov database: NCT00991549 (IG) and NCT00969007 (Lawson).
Both studies included overweight or obese men and women with a BMI ≥ 27 kg/m2 and a diagnosis of prediabetes defined as either impaired fasting glucose (fasting glucose 6·1–6·9 mmol/l) or impaired glucose tolerance (fasting glucose < 7·0 mmol/l; 2 h glucose post-oral glucose tolerance test (OGTT) 7·8–11·0 mmol/l; IG study, n 49; Lawson study, n 73). Potential participants from the original studies were excluded if they were unable to follow the intervention, if they consumed medications for obesity or known to alter glucose tolerance 3 months before study entry, if they had bariatric surgery or were on the waiting list for surgery, if they planned pregnancy or if they had a pacemaker (for bioelectrical impedance analysis). Participants were also excluded for the present study if they started medications known to alter glucose homeostasis (n 4) or vitamin D supplements (n 12) during the study, if frozen plasma was not available at baseline or at follow-up for the measurement of serum 25(OH)D (n 2) or if insulin values were missing for homeostasis model assessment of insulin sensitivity (HOMA%S) calculation (n 5). Finally, eighty-four subjects were included in the analysis (IG, n 31; Lawson, n 53). Baseline characteristics of the participants who were excluded were similar to those who were included in the analysis.
Intervention
In the IG study, participants were randomised to one of two weight loss interventions involving lifestyle changes for 1 year, as described previously( Reference Gagnon, Brown and Couture 13 ). Briefly, participants randomised to the interdisciplinary approach attended individual meetings with a nurse, a dietitian and an endocrinologist every 6 weeks. Upon request by the team, individual consultations with a psychologist or a kinesiologist were organised. In contrast, both participants randomised to the group, and individual approaches were invited to attend twenty-five group seminars of about 45 min covering topics such as obesity, diet, exercise and behaviour modification. In the Lawson study (designed to identify predictors of success to lifestyle intervention), all participants followed the same interdisciplinary approach described for the IG study.
Anthropometry, body composition and physical capacity
Anthropometric measurements and body composition were evaluated at baseline and after 1 year of intervention. Body weight was measured with a mechanical scale to the nearest 0·1 kg. Height was measured with a stadiometer to the nearest 0·1 cm. Waist circumference was measured twice at the midpoint between the lowermost rib and the iliac crest. Multiple measurements were averaged. Body composition was assessed by bioelectrical impedance analysis (Tanita model TBF 300A). A 6 min walk test, validated in this population( Reference Beriault, Carpentier and Gagnon 14 ), was used to evaluate the physical capacity before and after the intervention( 15 ).
Vitamin D supplementation and seasonality
Information about consumption of vitamin D supplement was collected before and after the intervention. Participants were divided in two groups according to the month of study entry: spring/summer (from April to September) and fall/winter (from October to March).
Biochemical measurements
Blood samples were collected after a 12 h fast at baseline and after 1 year. Glucose level was measured using Vitros 950 (Johnson & Johnson Diagnostics) before 2009 and Roche Diagnostics Modular thereafter. These two methods were highly correlated, and the resulting linear equation was y= 0·988+0·197. Insulin and C-peptide levels were measured using a human RIA (EMD Millipore). Glycosylated Hb (HbA1c) was measured using the Tosoh G7 automated HPLC analyser. Serum 25(OH)D concentrations were measured using an electrochemiluminescent assay (Elecsys Vitamin D Total; Roche Diagnostics).
Insulin sensitivity, insulin secretion and β-cell function
Fasting blood samples were collected and 2 h-75 g-OGTT were performed at baseline and after 1 year to evaluate insulin sensitivity, insulin secretion and β-cell function. Blood samples were collected for all participants at times 0, 10, 20, 30, 60, 90 and 120 min during the OGTT to measure glucose, insulin and C-peptide levels. For some participants, blood samples were also available at times − 30, − 20 and − 10 min, and in this case, results were averaged to derive fasting values. Insulin sensitivity was estimated using HOMA%S ((22·5/fasting insulin × fasting glucose) × 100) and Matsuda indices: 10 000/(fasting glucose × fasting insulin × (mean glucose × mean insulin))1/2. Insulin secretion was estimated using the AUC for C-peptide. β-Cell function was calculated using the disposition index (Matsuda × AUC C-peptide).
Statistical analyses
Participants were divided into two groups based on their percentage of weight loss at 1 year (non-responders, < 5 %; responders, ≥ 5 %). Normality of the data was assessed using visual inspection (histogram) and both a skewness value between − 1 and 1 and a kurtosis value between − 4 and 4. Data that did not follow normality were log- or Box cox-transformed. Baseline characteristics of the participants as well as changes in serum 25(OH)D concentrations and metabolic variables were compared between weight loss groups using an unpaired t test (or a Wilcoxon test for variables that failed to follow normality after transformation) for continuous variables and a χ2 test for categorical variables. Changes in serum 25(OH)D concentrations and metabolic variables were compared within each group using a paired t test. Finally, a multivariate linear regression analysis was used to evaluate whether changes in serum 25(OH)D concentrations were independently associated with changes in insulin sensitivity, insulin secretion and β-cell function after adjusting for changes in body weight ( ≥ 5 % v. < 5 %), age, sex, season of study entry, physical capacity, HbA1C and serum 25(OH)D concentrations at baseline.
Results
Baseline characteristics of the participants
Baseline characteristics of the participants based on the percentage of weight loss at 1 year ( < 5 %, non-responders v. ≥ 5 %, responders) are shown in Table 1. Participants were overweight or obese French-Canadians with a mean age of 57 years. At baseline, age, sex, body weight, BMI, waist circumference and body fat mass were similar between groups. Fasting glucose and HbA1c levels were significantly higher in the responders that in the non-responders. Moreover, responders had a significantly higher baseline serum 25(OH)D concentration than the non-responders (62·7 v. 52·5 nmol/l). The prevalence of vitamin D deficiency (serum 25(OH)D ≤ 50 nmol/l) was, however, similar between groups (41·1 and 32·1 % for the non-responders and responders, respectively). No significant difference was observed between groups for the season of study entry, prevalence of vitamin D supplement consumption, physical capacity and other metabolic variables.
Table 1 Baseline characteristics of the participants (Mean values, standard deviations and percentages)

OGTT, oral glucose tolerance test; HbA1c, glycosylated Hb; HOMA%S, homeostasis model assessment of insulin sensitivity; 25(OH)D, 25-hydroxyvitamin D.
* For the difference between groups using an unpaired t test or Wilcoxon test, as appropriate.
† Body fat mass was assessed by bioelectrical impedance analysis.
Changes in anthropometry, metabolic variables and serum 25-hydroxyvitamin D concentrations following weight loss
Changes in selected variables are shown in Table 2. By design, a higher decrease in body weight was observed in the responders than in the non-responders, corresponding to a mean weight loss of 9·5 (sd 4·2) v. 0·8 (sd 2·8)%. Moreover, a higher reduction in waist circumference (7·0 (sd 4·0) v. 0·8 (sd 3·2)%) and body fat mass (11·0 (sd 1·0) v. 1·4 (sd 5·5)%) was observed in the responders than in the non-responders (P< 0·0001 for all). Concordant with the improvements in anthropometry, significantly greater reductions in fasting glucose, fasting insulin, 2 h glucose post-OGTT and HbA1c levels were found in the responders than in the non-responders. No significant difference in physical capacity was shown after the intervention between the groups.
Table 2 Changes in serum 25-hydroxyvitamin D (25(OH)D) concentrations and selected variables after 1 year according to the percentage of weight loss (Mean values and standard deviations)
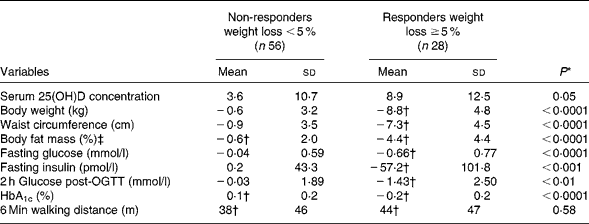
OGTT, oral glucose tolerance test; HbA1c, glycosylated Hb.
* For the difference between groups using an unpaired t test or Wilcoxon test, as appropriate.
† The P value for the difference within the group is ≤ 0·05 using a paired t test.
‡ Body fat mass was assessed by bioelectrical impedance analysis.
Fig. 1 shows the changes in serum 25(OH)D concentrations, insulin sensitivity, insulin secretion and β-cell function according to the weight loss group. The responders had a greater rise in serum 25(OH)D concentrations compared with the non-responders (8·9 (sd 12·5) v. 3·6 (sd 10·7) nmol/l, P= 0·05), corresponding to an increase of 19·6 (sd 29·5) v. 8·4 (sd 23·1)%. Compared with the non-responders, responders had a significantly greater improvement in HOMA%S (11·8 (sd 26·7) v. − 0·5 (sd 9·2), P< 0·0001), Matsuda index (18·5 (sd 26·7) v. 1·3 (sd 12·2), P< 0·0001) and in disposition index (3562 (sd 6208) v. − 678 (sd 4343), P= 0·001). Finally, no significant change in insulin secretion, as assessed by the AUC C-peptide, was observed after the intervention between the groups ( − 52 (sd 163) v. − 35 (sd 109) nmol/l 120 min, P= 0·58).
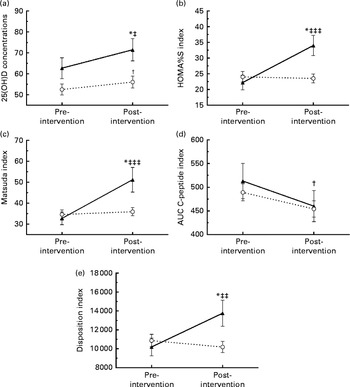
Fig. 1 Changes in serum 25-hydroxyvitamin D (25(OH)D) concentration, insulin sensitivity, insulin secretion and β-cell function after 1 year according to the percentage of weight loss (weight loss < 5 % (![]() ); weight loss ≥ 5 % (
); weight loss ≥ 5 % (![]() )). (a) Changes in serum 25(OH)D concentrations; (b) change in homeostasis model assessment of insulin sensitivity (HOMA%S) index; (c) change in Matsuda index; (d) change in AUC of C-peptide; (e) change in disposition index. Values are means, with standard deviations represented by vertical bars. * Significant change from baseline for the group that lost ≥ 5 % of their weight (P≤ 0·05; paired t test). † Significant change from baseline for the group that lost < 5 % of their weight (P≤ 0·05; paired t test). Mean value was significantly different from that of the group that lost < 5 % weight: ‡ P= 0·05, ‡‡ P= 0·001, ‡‡‡ P< 0·0001 (unpaired t test).
)). (a) Changes in serum 25(OH)D concentrations; (b) change in homeostasis model assessment of insulin sensitivity (HOMA%S) index; (c) change in Matsuda index; (d) change in AUC of C-peptide; (e) change in disposition index. Values are means, with standard deviations represented by vertical bars. * Significant change from baseline for the group that lost ≥ 5 % of their weight (P≤ 0·05; paired t test). † Significant change from baseline for the group that lost < 5 % of their weight (P≤ 0·05; paired t test). Mean value was significantly different from that of the group that lost < 5 % weight: ‡ P= 0·05, ‡‡ P= 0·001, ‡‡‡ P< 0·0001 (unpaired t test).
Associations between change in serum 25-hydroxyvitamin D concentration, change in body weight and change in insulin sensitivity, insulin secretion and β-cell function following weight loss
To evaluate whether the change in serum 25(OH)D concentration was independently associated with the changes in insulin sensitivity, insulin secretion and β-cell function, we performed a multivariate linear regression analysis for each outcome variable (Table 3). While a ≥ 5 % change in weight was significantly associated with changes in HOMA%S, Matsuda index and disposition index, change in serum 25(OH)D concentration was not found to be associated with any of these metabolic markers of type 2 diabetes. The change in insulin secretion was not associated with either the percentage of weight loss or the change in serum 25(OH)D concentration. Adjustment for age, sex, season of study entry, physical capacity, baseline HbA1c levels and serum 25(OH)D concentrations did not change the results. Moreover, similar results were obtained when participants with BMI ≥ 40 kg/m2 were excluded.
Table 3 Associations between changes in serum 25-hydroxyvitamin D (25(OH)D) concentration, percentages of weight loss and changes in metabolic variables in multivariate regression analyses (β-Coefficients and P values)
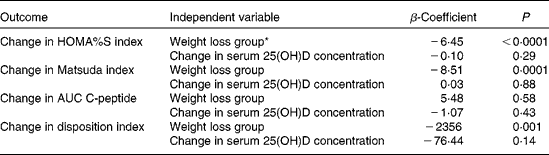
HOMA%S, homeostasis model assessment of insulin sensitivity.
* Weight loss ≥ 5 % v. < 5 %.
Discussion
The present study aimed to determine whether an increase in serum 25(OH)D concentrations observed after a clinically significant weight loss ( ≥ 5 %) contributes to the improvement in insulin sensitivity, insulin secretion and β-cell function. We hypothesised that an increase in serum 25(OH)D concentration after a clinically significant weight loss would be associated with improved insulin sensitivity, insulin secretion and β-cell function, independently of the amount of weight loss. We demonstrated that a weight loss of at least 5 % was associated with a significant increase in serum 25(OH)D concentration as well as an improvement in insulin sensitivity and β-cell function, as expected, but not in insulin secretion. Moreover, participants who lost at least 5 % of their weight had a significantly greater reduction in fasting glucose, fasting insulin, 2 h glucose post-OGTT and HbA1c levels. However, contrary to our hypothesis, the increase in serum 25(OH)D concentration following weight loss did not contribute to the improvement in insulin sensitivity and β-cell function.
To our knowledge, only two studies have assessed whether change in serum 25(OH)D concentration following weight loss has been associated with change in insulin sensitivity( Reference Tzotzas, Papadopoulou and Tziomalos 12 , Reference Reinehr, de Sousa and Alexy 16 ). No previous study has examined the relationship between change in 25(OH)D concentration and change in insulin secretion or β-cell function. Contrary to our findings, Tzotzas et al. ( Reference Tzotzas, Papadopoulou and Tziomalos 12 ) demonstrated that an increase in serum 25(OH)D concentration after a weight loss similar to that observed in the present study (10 %) is significantly correlated with a reduction in insulin resistance evaluated by HOMA-insulin resistance (r − 0·43, P< 0·05) in twenty-six women who followed a low-energy diet for 5 months. However, in this study, seasonality, which is an important factor influencing serum 25(OH)D concentrations, was not taken into account. Indeed, 5 months of intervention corresponds to a change in season and the concomitant improvement in serum 25(OH)D and insulin sensitivity could potentially be explained by this factor. It is, however, also possible that the correlation between serum 25(OH)D and insulin sensitivity is observed in the early stages of weight loss and disappears at 1 year. In contrast, similar to what we found, Reinehr et al. ( Reference Reinehr, de Sousa and Alexy 16 ) showed that changes in serum 25(OH)D concentrations following a 1-year lifestyle-oriented weight loss programme did not correlate with changes in insulin sensitivity (HOMA%S) in sixty-seven obese children, after adjusting for the change in weight. This study and the present study highlight the importance of taking into account seasonality in the design of the study or adjusting for this factor in the analyses.
In agreement with several studies, the present results demonstrated that weight loss improves insulin sensitivity as well as β-cell function( Reference Kitabchi, Temprosa and Knowler 9 , Reference Gagnon, Brown and Couture 13 ). Weight loss of at least 5–7 % due to the change in lifestyle had been associated with a 58 % decreased risk of developing type 2 diabetes in overweight or obese individuals with prediabetes( Reference Knowler, Barrett-Connor and Fowler 5 , Reference Tuomilehto, Lindstrom and Eriksson 6 ). The factors responsible for the improvement in glucose homeostasis after weight loss remain incompletely understood. Chronic inflammation associated with obesity may be one of the factors contributing to insulin resistance and impaired insulin secretion( Reference Festa, D'Agostino and Williams 17 ). Indeed, there is a strong positive correlation between body fat and circulating C-reactive protein, IL-6 and TNF-α concentrations( Reference Festa, D'Agostino and Williams 17 – Reference Ho, Zhao and Courville 19 ), and these inflammatory markers have been associated with impaired insulin sensitivity and secretion( Reference Shoelson, Lee and Goldfine 20 ).
After weight loss, the decrease in inflammatory markers has been significantly associated with improved insulin sensitivity( Reference Ryan and Nicklas 21 ). Another factor that could potentially explain the improvement in insulin resistance following weight loss is the change in adiponectin concentrations. Adiponectin is an adipose tissue-specific protein that increases insulin sensitivity. Patients with the metabolic syndrome, obesity and type 2 diabetes have lower adiponectin concentrations that have been associated with insulin resistance( Reference von Frankenberg, do Nascimento and Gatelli 22 – Reference Hotta, Funahashi and Arita 24 ). The change in adiponectin following weight loss could also be involved in the amelioration of insulin sensitivity( Reference Mather, Funahashi and Matsuzawa 25 ).
In the present study, we observed, in accordance with other studies( Reference Mason, Xiao and Imayama 7 , Reference Rock, Emond and Flatt 8 ), that a weight loss of at least 5 % results in a significant increase in serum 25(OH)D concentrations, after adjustment for vitamin D supplementation and seasonality. Although several prospective observational studies have shown that higher serum 25(OH)D concentrations have been associated with a reduced risk of developing type 2 diabetes( Reference Song, Wang and Pittas 4 , Reference Gagnon, Lu and Magliano 26 ) as well as with improved insulin sensitivity, insulin secretion and β-cell function( Reference Kayaniyil, Retnakaran and Harris 27 ), we were unable to demonstrate that the rise in serum 25(OH)D concentrations following weight loss is a significant contributor to the improvement in insulin sensitivity and β-cell function. One of the reasons may be that the increase in serum 25(OH)D concentrations after a clinically significant weight loss was small in the present study, averaging 9 nmol/l. The mean serum 25(OH)D concentration increased from 63 to 72 nmol/l. Thus, 61 % of the participants who lost at least 5 % of their weight did not reach the target serum 25(OH)D concentration of 75 nmol/l that has been identified as the optimal value for glucose homeostasis in epidemiological studies( Reference Forouhi, Luan and Cooper 28 ). Moreover, it is likely that there is a great inter-individual variability in the metabolic response to a change in vitamin D status. Indeed, recent studies have suggested that the presence of certain polymorphisms in the vitamin D receptor gene or in the insulin receptor substrate 1 (IRS-1) gene may affect the change in insulin sensitivity following an increase in serum 25(OH)D concentrations( Reference Zheng, Parnell and Smith 29 , Reference Jain, von Hurst and Stonehouse 30 ). Alternatively, it is also possible that the change in serum 25(OH)D concentrations after weight loss does not contribute to the metabolic improvement observed after a change in lifestyle. Indeed, serum 25(OH)D may just be a marker of better health (physical activity, sunlight exposure and nutrition) rather than a causal factor leading to obesity and type 2 diabetes. A Mendelian randomisation study would be useful to better assess the relationship between the change in serum 25(OH)D concentrations and insulin sensitivity, insulin secretion and β-cell function following weight loss.
The present study has limitations that need to be acknowledged. Most importantly, given the relative small sample size, it may not have the power to detect a weak association between serum 25(OH)D concentrations and markers of type 2 diabetes risk following weight loss. There was also a significant difference between groups at baseline in factors that have been shown to predict future diabetes risk namely fasting plasma glucose, HbA1c and serum 25(OH)D concentrations. These factors were included in the analyses, and were not found to affect the results. Moreover, it was not possible to adjust for some of the potential confounders such as dietary vitamin D intake and sunlight exposure, determinants of vitamin D status that can be improved after a lifestyle intervention programme. Finally, the number of participants with serum 25(OH)D < 50 nmol/l at baseline was small (n 32), precluding us from performing analyses in this subgroup. It thus remains possible that an association between serum 25(OH)D and insulin sensitivity, insulin secretion and β-cell function could be found in individuals with vitamin D deficiency. The present study also has strengths that need to be highlighted. First, the lifestyle intervention lasted for 1 year, which has the advantage of comparing markers of type 2 diabetes risk during the same month of the year, thus reducing the confounding by seasonality. Second, we obtained a detailed profile of glucose homeostasis including fasting glucose, 2 h glucose post-OGTT, HbA1c as well as OGTT-derived measures of insulin sensitivity, insulin secretion and β-cell function.
In conclusion, the present study revealed that although serum 25(OH)D concentrations increase significantly following a weight loss of at least 5 %, this increase is not a major contributor of the improvement in glucose homeostasis in overweight or obese individuals with prediabetes undergoing a lifestyle modification programme.
Acknowledgements
The authors would like to thank Rafael Nascimento Soares who helped with the literature review. V. T. received a scholarship from Diabète Québec to work on this project. C. G., M.-F. L. and J.-P. B. are scholars from the Fonds de la recherche du Québec-Santé (FRQ-S). C. G. received start-up funds from Laval University and CHU de Québec Research Centre to measure serum 25(OH)D concentrations in the study sample. A. C. C. is the recipient of the Canadian Institutes of Health Research-GlaxoSmithKline (CIHR-GSK) Chair in Diabetes. The Centre de recherche du Centre hospitalier universitaire de Sherbrooke and the CHU de Québec Research Centre are funded by the FRQ-S. The Lawson Foundation and Novo Nordisk Canada provided funding for the original cohorts.
V. T. and A.-S. M. reviewed the literature, analysed and interpreted the data and wrote the first draft of the manuscript. C. B., A. C. C., J.-P. B. and M.-F. L. conducted the original studies (IG and Lawson), helped in the interpretation of the data and reviewed the manuscript. C. G. designed the study, interpreted the data and reviewed the manuscript. All authors accepted the final version of the manuscript.
None of the authors has any conflict of interest to declare.







