The maintenance of a constant extracellular Ca ion concentration depends on the regulation of either the rate of entry into or the rate of exit from the Ca pool. Ca enters the pool through dietary absorption and bone resorption. It exits the pool via faeces and urine and by incorporation into bone and also by transfer to the fetus and mammary gland in the case of pregnant and lactating animals. The endocrine factors controlling Ca homeostasis include parathyroid hormone and the active metabolite of vitamin D, 1,25-dihydroxycholecalciferol (1,25(OH)2D3). Blood Ca concentrations can be increased or maintained by increasing Ca absorption from the diet or by mobilising skeletal stores of Ca. Active transport of Ca across the intestinal mucosa may increase under the influence of 1,25(OH)2D3. The small intestine is considered to be the major site of Ca absorption in many species, although it is unclear whether this is the main site in ruminant animals. There is evidence that the pre-intestinal region (i.e. the rumen) has significant Ca absorption capacity in sheep( Reference Höller, Breves and Kocabatmaz 1 – Reference Wadhwa and Care 3 ). The site of Ca absorption in ruminants seems to vary from the pre-intestinal to the intestinal regions depending on a number of factors. These could include the age of the animal as well as the quantity, nature and solubility of the Ca source, the interaction of Ca ions with other substances in the digestive tract, and of course the ability of the dietary supply of Ca to meet the quantitative requirements of the animal.
The availability of a simple, inexpensive test to measure Ca absorption capacity in vivo would be useful to monitor this component of Ca homeostasis in ruminant animals. Traditional methods for estimating Ca absorption in mammals include balance measurements and measurements of the uptake of orally administered radioactive or stable isotopes of Ca. Balance measurements are expensive and require repeated handling and intensive housing of test animals and therefore are not suitable for use with large numbers of farm animals. The use of radioisotopes is obviously impractical in production animals. Radioactive calcium (45Ca) has a relatively long half-life (165 d), while 47Ca has an extremely short half-life (4·5 d), and both are expensive. However, not all tracers of Ca need to be radioactive because there are some metal elements that are absorbed from the alimentary tract in a similar fashion to Ca and are also incorporated into bone. Stable Sr has some chemical similarity to Ca as both are alkaline earth metals and exist as divalent cations. Ca has an atomic weight of 40·08 and an atomic number of 20, while these values for Sr are 87·6 and 38, respectively. Sr appears to be deposited in bone by the same mechanisms as Ca or by exchange with Ca in hydroxyapatite bone crystals( Reference MacDonald, Nusbaum and Ezmirlian 4 ). Sr salts are inexpensive and readily available, and Sr cation concentrations can be easily measured by atomic absorption spectrometry. Sr is safe when given orally in small amounts and can be administered repeatedly to the same animal. A method has been developed for measuring Sr absorption as an indicator of Ca absorption capacity in humans( Reference Milsom, Ibbertson and Hannan 5 – Reference Zittermann, Sabatschus and Jantzen 7 ).
In the present study, we tested the applicability of the Sr absorption test used in humans for measuring the gastrointestinal Ca absorption capacity in ruminants using Sr as a marker of Ca absorption and validated it by measuring 45Ca isotope absorption. The Sr absorption capacity of the rumen and small intestine was measured separately and the effect of the administration of 1α-hydroxyvitamin D3 (1αOHD3) on the Sr absorption capacity of both these organs was determined. The synthetic analogue 1αOHD3 is 25-hydroxylated in the liver to 1,25(OH)2D3, which is then secreted into blood( Reference Holick, Tavela and Holick 8 ). Although the method used in humans has revealed that the appearance of Sr in plasma follows the absorption profile of Ca very closely, after a few hours the patterns of absorbed Ca and Sr in plasma start diverging. This is caused by differences in the rate of excretion of Sr by the kidneys and in the rate of deposition in bone compared with Ca. Therefore, to use the absorption profile of Sr in blood as an index of Ca absorption capacity, the absorption profile over the first few hours after dosing should be monitored.
Materials and methods
Animals and study design
A total of four mature merino ewes were fed a diet adequate in energy, protein and phosphorus and slightly low in Ca (Table 1). The diet was fed in two equal portions, morning and afternoon, for 10 d before the start of the experiments. Access to drinking-water was provided at all time points. The animals were housed in metabolism crates, which allowed the collection of urine and faeces. All experimental procedures carried out in sheep were approved by the Animal Ethics Committee of the University of Sydney.
Table 1 Nutrient composition of the sheep diet*
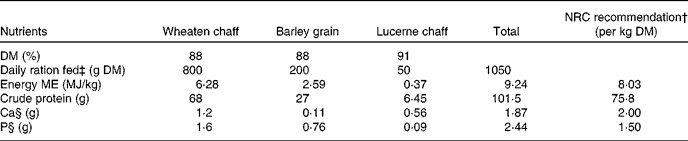
NRC, National Research Council; ME, metabolisable energy.
* Recommended nutrient values obtained from Nutrient Requirements of Small Ruminants, NRC (2007).
† NRC-recommended daily DM intake: 0·91 kg DM.
‡ Daily ration fed to mature merino ewes (45–50 kg body weight): 1·05 kg DM.
§ Ca:P ratio: 0·77.
An aqueous dosing solution (25 ml) containing 1·09 g of Sr as stable strontium chloride and 50 mg of Ca as 45CaCl2 (4·77 MBq 45Ca/50 mg Ca) was administered orally to all the four sheep. An initial blood sample was collected using a jugular catheter before dosing and then blood samples were collected at intervals over the next 48 h. The concentration of Sr in plasma was determined by flame atomic absorption spectrometry (Varian SpectrAA; Varian Inc.) using 0·05 m-KCl as a diluent, and the assay was calibrated with serum samples containing low (500 ng/ml), medium (1000 ng/ml) and high (3000 ng/ml) concentrations of Sr. The concentration of 45Ca in plasma was determined by liquid scintillation spectrometry with Instagel (5 ml) (Perkin Elmer Inc.) as the scintillant solution and quench correction using the channels ratio method.
In these ruminant studies the Sr absorption procedure, developed for human use, was simplified by expressing the results as the percentage of the peak concentration in plasma against time after dosing. The absorption rates of Sr and 45Ca were compared by measuring plasma concentrations at 15 min intervals during the 1st hour after dosing and then at 30 min intervals for a further 3 h, followed by hourly measurement of samples until 8 h after oral dosing. Further blood samples were collected 24 and 48 h after dosing. Blood samples were collected into heparinised syringes and centrifuged immediately. Plasma was stored at 4°C before analysis, which was carried out within 12 h of sample collection.
Measurement of absorption in the rumen
The uptake rate of Sr in the rumen was determined using stable Sr as a tracer analogue of Ca. The four sheep were orally dosed with 5 g of Sr (as strontium chloride) in 25 ml solution, and blood samples were collected at regular intervals from 0 to 8 h after dosing The initial concentration of 5 g Sr ions in the ruminal fluid, if the volume were typical of adult merino sheep of about 6·4 litres( Reference Hecker, Budtz-Olsen and Ostwald 9 ), would have been about 8–9 mm. An oral dose of 1·09 g Sr would have resulted in a Sr concentration of about 2 mm in the rumen.
The same sheep were administered the same dose of Sr by direct injection into the rumen through the abdominal wall to determine whether the Sr uptake pattern observed after oral dosing in fact reflected the absorption rate in the rumen. The injection site (midway from the last rib and the point of the hip on the left-hand side) was clipped, disinfected and anaesthetised by subcutaneous injection of 4 ml lignocaine. The animals were held under the sternum and lifted by an assistant, which caused the rumen to bulge clearly. A sterile 17 gauge needle was plunged into the rumen to a depth of approximately 4 cm. A small amount of gas and ruminal fluid escaping from the needle indicated that it was in position and the solution was administered slowly. Regular blood samples were collected from 0 to 4 h after injection. The concentration of Sr in plasma was determined as described above.
Measurement of absorption in the small intestine
A second oral dose of Sr solution was administered 2 weeks after the first administration, when plasma Sr concentrations had decreased to, or were similar to, the original level, to determine the uptake rate of Sr in the small intestine. The intravenous administration of vasopressin has been used in mature cows in order for orally administered liquid to bypass the rumen and be directed to the abomasum( Reference Scholz and Mikhail 10 ). Previous studies have shown that 15–30 mg of lysine vasopressin given by intranasal spray effectively closes the ruminoreticular groove. The sheep were orally dosed with 5 g of Sr (as strontium chloride) in 25 ml solution, exactly 5 min after intranasal vasopressin administration. Blood samples were then collected as described above. The concentration of Sr in plasma was determined as described above.
Response of the absorption index to elevated plasma concentrations of 1,25-dihydroxycholecalciferol
The uptake rates of Sr in the rumen and small intestine were determined in sheep as described above. The effect of elevated plasma concentrations of 1,25(OH)2D3 on the absorption profile of Sr was then determined. Mini-osmotic pumps (ALZET model 2002; Durect Corporation) delivering 12 μg of 1αOHD3/d (Leo Pharmaceuticals) at a constant rate were implanted subcutaneously, midway over the ribs. The synthetic vitamin D derivative 1αOHD3 is readily converted( Reference Zittermann, Sabatschus and Jantzen 7 ) in vivo to 1,25(OH)2D3 and is less expensive than 1,25(OH)2D3. The implantation site was clipped, disinfected and anaesthetised by the subcutaneous injection of 6 ml lignocaine. The pumps were inserted under the skin through a small incision, which was closed with suture clips. The concentration of 1,25(OH)2D3 was determined using an in-house competitive protein binding assay with the vitamin D receptor prepared from calf thymus( Reference Reinhardt, Horst and Orf 11 ).
Statistical analyses
Data obtained from experiments with two treatment groups were compared using unpaired Student's t test. Data obtained from experiments with more than two treatment groups were assessed using ANOVA. When significant effects were observed, differences between the groups were assessed using a multiple comparison test (least significant difference test). All statistical analyses were carried out using Minitab (version 7.1; Minitab Inc.) or SAS (SAS Institute) statistical packages.
Results
Use of stable strontium to determine calcium absorption capacity
A close correlation was observed between the absorption rates of the two tracers when the percentage of peak plasma concentration was plotted against time (Fig. 1). A correlation coefficient of 0·94 for the absorption rates of the two tracers over the next 48 h revealed that their absorption patterns were similar. Comparison of the values obtained in first 8 h after dosing is more relevant, as this time period most accurately reveals the correlation between the absorption patterns of the two tracers. The correlation coefficient between the absorption rates of the two tracers in the first 8 h after oral dosing was 0·98 (Fig. 2).

Fig. 1 Changes in radioactive calcium (45Ca, ![]() ) and stable strontium (
) and stable strontium (![]() ) concentrations in plasma over 48 h after simultaneous oral administration (at time 0) of 25 ml of aqueous solution of 50 mg of 45Ca (4·77 MBq 45Ca; specific activity: 3·81 MBq/mmol) and 1·09 g of strontium as strontium chloride. Values are means of four sheep, with their standard errors represented by vertical bars.
) concentrations in plasma over 48 h after simultaneous oral administration (at time 0) of 25 ml of aqueous solution of 50 mg of 45Ca (4·77 MBq 45Ca; specific activity: 3·81 MBq/mmol) and 1·09 g of strontium as strontium chloride. Values are means of four sheep, with their standard errors represented by vertical bars.

Fig. 2 Changes in radioactive calcium (45Ca, ![]() ) and stable strontium (
) and stable strontium (![]() ) concentrations in plasma over 8 h after simultaneous oral administration (at time 0) of 25 ml of aqueous solution of 50 mg of 45Ca (4·77 MBq 45Ca; specific activity: 3·81 MBq/mmol) and 1·09 g of strontium as strontium chloride. Values are means of four sheep, with their standard errors represented by vertical bars.
) concentrations in plasma over 8 h after simultaneous oral administration (at time 0) of 25 ml of aqueous solution of 50 mg of 45Ca (4·77 MBq 45Ca; specific activity: 3·81 MBq/mmol) and 1·09 g of strontium as strontium chloride. Values are means of four sheep, with their standard errors represented by vertical bars.
Independent measurements of calcium absorption capacity of the rumen and small intestine
The plasma concentration of Sr increased over 15–30 min after the administration of Sr into the rumen and then remained relatively constant for several hours. The appearance of Sr in blood, 2–3 h after administration into the rumen, is probably due to a combination of continuing absorption in the rumen and absorption in the small intestine of some of the Sr dose that had passed from the rumen and had reached the small intestine. This pattern of absorption was also found upon direct injection of Sr solution through the abdominal wall into the rumen (Fig. 3). In contrast, upon vasopressin treatment before the administration of oral Sr solution, the concentration of Sr in plasma increased gradually over 5 h (Fig. 4). This pattern of absorption was very different from that of ruminal absorption, and it is presumed to follow the absorption pattern of Sr in the small intestine. If the oral dose of Sr were administered 10 min after the administration of intranasal vasopressin spray, the pattern of Sr appearance in plasma would be similar to that observed when Sr is directed into the rumen. Hence, closure of the ruminoreticular groove is short lived and the exact timing of oral dosing is important to obtain repeatable results to determine the rate of absorption in the small intestine.

Fig. 3 Appearance of strontium in plasma after injection of 5 g of strontium as strontium chloride directly into the rumen through the abdominal wall. Values are means of four sheep, with their standard errors represented by vertical bars.

Fig. 4 Absorption profile of strontium in plasma after oral dosing with 5 g strontium as strontium chloride and closure of the ruminoreticular groove by vasopressin treatment to divert the dose to the post-ruminal alimentary tract to determine absorption in the small intestine. Values are means of four sheep, with their standard errors represented by vertical bars.
Response of the absorption index to elevated plasma concentrations of 1,25-dihydroxycholecalciferol
Treatment with 1αOHD3 caused a rapid and highly significant elevation of plasma 1,25(OH)2D3 concentrations (ruminal dosage P= 0·0002, intestinal dosage P= 0·0008; one-tailed paired Student's t test) (Table 2).
Table 2 Concentration of 1,25-dihydroxyvitamin D in plasma
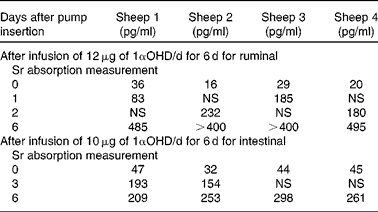
1αOHD, 1α-hydroxyvitamin D; NS, not sampled.
There was a significant increase in Sr absorption after 6 d of 1αOHD3 treatment in both the rumen and small intestine (P= 0·02; ANOVA via a general linear model) (Figs. 5 and 6).

Fig. 5 Absorption profile of strontium in plasma after ruminal administration of 5 g strontium as strontium chloride before (![]() ) and after (
) and after (![]() ) increasing the concentration of 1,25-dihydroxyvitamin D in plasma by subcutaneous infusion of 1α-hydroxyvitamin D. Values are means of four sheep, with their standard errors represented by vertical bars.
) increasing the concentration of 1,25-dihydroxyvitamin D in plasma by subcutaneous infusion of 1α-hydroxyvitamin D. Values are means of four sheep, with their standard errors represented by vertical bars.

Fig. 6 Absorption profile of strontium in plasma after oral dosing with 5 g strontium as strontium chloride and closure of the ruminoreticular groove to divert the dose to the post-ruminal alimentary tract to determine absorption in the small intestine before (![]() ) and after (
) and after (![]() ) increasing the concentration of 1,25-dihydroxyvitamin D in plasma by subcutaneous infusion of 1α-hydroxyvitamin D. Values are means of four sheep, with their standard errors represented by vertical bars.
) increasing the concentration of 1,25-dihydroxyvitamin D in plasma by subcutaneous infusion of 1α-hydroxyvitamin D. Values are means of four sheep, with their standard errors represented by vertical bars.
Discussion
The aim of the present study was to develop a simple, inexpensive method for assessing the gut Ca absorption capacity in ruminants, suitable for use in production animals. The cost and complexity of technique limit the use of the currently available methods for measuring Ca absorption capacity.
Because of its physico-chemical similarity, Sr has been used as a biological analogue of Ca as the transport and distribution of Sr in the body parallel those of Ca. Most of an injected dose of 45Ca or Sr is deposited in bone( Reference Pecher 12 ), and skeletal accumulation of radioactive Sr has been found to be equal to that of Ca in nephrectomised rats( Reference Talmage, Schooley and Comar 13 ) and in rabbits in which kidney function had been destroyed with mercuric oxide( Reference MacDonald, Noyes and Lorick 14 ). In addition, the passage of the two elements from blood to extracellular fluid and spinal fluid is similar( Reference Samachson and Lederer 15 ). However, renal clearance of Sr is much greater than that of Ca( Reference Harrison, Raymond and Tretheway 16 , Reference Spencer, Li and Samaachson 17 ).
Discrimination against Sr in favour of Ca has been shown to occur. Spencer et al. ( Reference Spencer, Li and Samaachson 17 ) found that there is a discrimination against orally administered Sr during absorption in the intestine. Hendrix et al. ( Reference Hendrix, Alcock and Archibald 18 ) showed that Ca and Sr appear to share a common absorption pathway in the gut, and similar findings were reported by Papworth & Patrick( Reference Papworth and Patrick 19 ), who suggested that the common carrier(s) has a greater affinity for Ca than for Sr and mediates the entry of both these divalent cations across the brush border.
A clinical test for measuring Ca absorption in humans has been developed using stable Sr( Reference Milsom, Ibbertson and Hannan 5 , Reference Reid, Pybus and Lim 20 ). This test assesses Ca absorption capacity with sufficient sensitivity and precision to have a wide application in clinical practice. In humans, there is a close correlation between the fractional absorption of stable Sr and the absorption of 45Ca during a 5 h period after the simultaneous oral administration of the two tracers (r 0·98 at 1 and 4 h)( Reference Milsom, Ibbertson and Hannan 5 ). This test has been adapted to assess the gastrointestinal tract Ca absorption capacity in ruminants.
Sequential blood samples obtained from the four sheep after simultaneous administration of an oral dose of stable Sr and 45Ca showed a close correlation between the absorption rates of the two tracers over 48 h after dosing (r 0·94) when the percentage of peak plasma concentration of each tracer was plotted against time. The absorption patterns of the two tracers over the first 8 h after dosing were very similar (r 0·98). The correlation between the absorption patterns of the two tracers was observed during this time period. After 8 h, the percentage of the plasma peak concentration of the two tracers began to diverge as the Ca mixed with the total-body available Ca and the Sr was both cleared by the kidneys and deposited in bone. Studies indicate that there is little bone discrimination between Ca and Sr in vivo, and their skeletal accumulation has been found to be similar in short-term experiments. However, in intact animals bone seems to preferentially releases Sr in vivo and in vitro ( Reference Likins, Posner and Kunde 21 ). Comar & Wasserman( Reference Comar, Wasserman, Comar and Bronner 22 ) suggested that whenever there is a metabolically controlled passage of ions across a membrane, Ca is transported more effectively than Sr. The results of the present study are in agreement with this observation. The percentage of the dose of 45Ca recovered in plasma was higher than that of Sr at all time points, but the curves were of a similar shape when plotted against time. The close correlation between the absorption patterns of the two tracers is in agreement with the findings of previous work in monogastric animals. These studies have shown that stable Sr is a useful qualitative and quantitative tracer of Ca absorption in sheep. Because of a limit in the sensitivity of measurement by atomic absorption spectrometry, the minimum dose of Sr that could be administered to measure uptake rates in plasma was 1 g. For routine study, an oral dose of 5 g of Sr as strontium chloride has been found to yield repeatable values for changes in absorption capacity even though this dose may lead to higher concentrations in the rumen compared with those of Ca derived from food.
Independent measurements of the Ca absorption capacity of the rumen and small intestine were made in sheep, through intranasal administration of lysine vasopressin to stimulate ruminoreticular groove closure and thus direct a dosing solution to the abomasum without passing through the rumen. It is likely that intranasal administration of vasopressin may not divert all of an oral dose of Sr into the abomasums, particularly because closure of the ruminoreticular groove occurs only for a few minutes. Therefore, some of the Sr dose may enter the rumen. Nevertheless, the different patterns of appearance of Sr in blood are in agreement with the interpretation that Sr is being absorbed mainly in the small intestine after treatment with vasopressin and mainly in the rumen when no vasopressin is administered.
There is considerable debate in the literature as to which region of the gastrointestinal tract is the major site of absorption of macrominerals such as Ca and Mg. The rumen has been demonstrated to be the major site of Mg absorption in sheep( Reference Grace, Ulyat and MacRae 23 – Reference Green, Fontenot and Webb 25 ). Pfeffer et al. ( Reference Pfeffer, Thompson and Armstrong 26 ) have concluded that the net movements of Ca and Mg in sheep are low and rather variable, but the net secretion of Ca and Mg apparently occurs in the small intestine and net absorption of Mg in the large intestine. Other factors such as Ca and phosphate ion concentrations in the ruminal fluid also influence Ca absorption in the reticulorumen. Care et al. ( Reference Care, Brown and Farrar 27 ) showed that net absorption of Ca occurred after the administration of an experimental solution containing Ca at a concentration >4 mmol/l. Beardsworth et al. ( Reference Beardsworth, Beardsworth and Care 2 ) found significant net absorption to occur in the reticulorumen of sheep and concluded that the pre-intestinal region is a major site of Ca absorption. Wadhwa & Care( Reference Wadhwa and Care 3 ) suggested that Ca and Sr share a common absorption pathway in the reticulorumen. Thus, it is clear that the reticulorumen is capable of absorbing significant quantities of Ca, although the main site of Ca absorption seems to vary from the pre-intestinal to the intestinal regions in ruminants.
Daily administration of 1αOHD3 by intramuscular injections to lactating ewes has previously been shown to increase, approximately 2-fold, the rate of absorption of dietary Ca from the alimentary tract( Reference Braithwaite 28 ). However, in that in vivo study, determining the region of the alimentary tract where the increase in absorption capacity occurred in response to an increased supply of 1,25(OH)2D3 was not possible. In the present study, the diversion of the oral dose of Sr to the post-ruminal region of the tract led to a significant increase in the absorption capacity of the intestine with 1αOHD treatment (Fig. 6). A similar apparent increase was also observed in the absorption capacity of the rumen upon 1αOHD treatment (Fig. 5). However, a conclusion that ruminal Ca absorption capacity can be stimulated by an increased supply of 1,25(OH)2D3 is not clear cut. Although there was an increase in the appearance rates of Sr in blood, 15 min after the oral dose had been directed to the rumen (Fig. 5), the absorption patterns differed from those observed without an increased supply of 1,25(OH)2D3 (Fig. 3). When there was no increase in 1,25(OH)2D3 supply, the concentration of Sr in plasma reached a peak by 1 h after dosing, with a slight decline over the following 3 h. In contrast, when the plasma concentration of 1,25(OH)2D3 was elevated, that of Sr, derived from that delivered to the rumen, continued to increase steadily over the subsequent 5 h (Fig. 6). Therefore, it is possible that this pattern of absorption is a combination of absorption in the rumen and the stimulated absorption in the small intestine.
In monogastric animals, the vitamin D-mediated increase in transcellular Ca absorption capacity across the small-intestinal mucosa involves an entry ion channel, the transient receptor potential vanilloid channel type 6( Reference Peng, Chen and Berger 29 ), and a cytoplasmic Ca-binding protein, calbindin-D9k( Reference Bronner 30 ). Although these components of Ca transport have been readily demonstrated in the small-intestinal mucosal cells of sheep, they are either undetectable or expressed in only very small amounts in the ruminal mucosa of sheep( Reference Wilkens, Kunert-Keil and Brinkmeier 31 – Reference Schröder, Goebel and Huber 33 ). Furthermore, the concentrations of the vitamin D receptor, which specifically binds to 1,25(OH)2D3 to enable regulation of the expression of its gene, are also very low in the ruminal mucosa of sheep compared with those in the small intestine( Reference Schröder, Goebel and Huber 33 ). In addition, in vitro studies with isolated sheep ruminal mucosa could find no evidence for an effect of vitamin D on the active transport of Ca( Reference Wilkens, Mrochen and Breves 32 , Reference Schröder, Goebel and Huber 33 ). From these mechanistic studies, it was concluded that the absorption of Ca across the ruminal mucosa of sheep and its regulation are probably operating by processes different from those in the small intestine.
Although it cannot be concluded from the present study that Sr absorption patterns in the rumen of sheep are directly modified by 1,25(OH)2D3, the experiments demonstrate that the ruminal Ca absorption process is a major component of dietary Ca absorption in sheep. This suggests that the active transport process of Ca demonstrated in isolated ruminal mucosa (M. R. Wilkens, personal communication) is the same mechanism by which Sr is transported from the rumen in vivo. The mechanism and regulation of this active Ca transport process in the rumen cannot be defined yet and require further investigation.
Conclusion
The present study showed that the typical ruminal absorption profile of Sr exhibits a steady-state plateau, which is reached rapidly after dosing, indicating that the rate of Sr input in blood is equal to that of output. Ruminal injection of Sr confirmed these results. In contrast, intestinal absorption is characterised by a steadily increasing concentration of Sr in plasma. When sheep were treated with 1αOHD3, there was a significant increase in plasma 1,25(OH)2D3 concentrations, which led to a significant increase in the Ca absorption capacity of the intestine. Thus, it can be concluded that both the rumen and small intestine have Ca absorption pathways and that the capacity of each can be determined by monitoring the concentration of Sr in plasma after the administration of a small oral dose of this Ca analogue.
Acknowledgements
The authors thank Ms A. Trube and Ms I. van Ekris for their valuable technical laboratory assistance and Mr K. McKean and Ms K. Eardley for their expert assistance with animal care. The present study was financially supported by the Australian Dairy Research and Development Council.
Both authors contributed to the design and execution of the experimental research and the writing of the manuscript.
Neither author has any conflicts of interest with any aspect of the study.










