Numerous studies have demonstrated that n-3 PUFA, especially EPA (20 : 5n-3) and DHA (22 : 6n-3), are associated with a reduced risk of developing several pathologies such as cardiovascular(Reference Riediger, Othman and Suh1), neurodegenerative(Reference Dyall and Michael-Titus2), inflammatory(Reference Calder3) diseases and cancer(Reference Saini and Keum4). Indeed, EPA and DHA are directly implicated in lipid metabolism by lowering triacylglycerolaemia (Reference Simopoulos5) but they are also precursors of eicosanoids with strong anti-inflammatory properties(Reference Calder3, Reference Zarate, el Jaber-Vazdekis and Tejera6). Results from the latest French epidemiological study(Reference Dubuisson, Carrillo and Dufour7) have shown that, even if the consumption of total lipids was in line with the recommendations (34 % of the total energy intake for 35–40 % recommended), EPA and DHA consumption was at least 32 % lower than the French guidelines (117 and 169 mg/d, respectively, for recommendations of 250 mg/d of each n-3 long-chain (LC) PUFA)(Reference Astorg, Bougnoux and Calvarin8, Reference Mortureux9). In order to cover the n-3 LC PUFA physiological needs, increasing EPA and DHA intakes represents a major nutritional challenge. One of the strategies consists in improving their bioavailability, without changing the total lipid intake. Several studies have demonstrated that lipid bioavailability depends on physicochemical factors of the dietary intake(Reference Cansell10–Reference Michalski, Genot and Gayet14). Indeed, nowadays, EPA and DHA are essentially consumed as oily fish for food intake or as fish oil (mainly TAG or ethyl esters) in food supplements. In the case of food supplements, the interest for marine phospholipids (PL) has recently increased. However, the limited data concerning the impact of the molecular lipid species (TAG v. PL) of lipid intake on the n-3 LC PUFA fate leads to conflicting results. On the one hand, n-3 LC PUFA levels were increased in the liver and brain of rats fed EPA and DHA in the form of PL compared with TAG(Reference Graf, Duchateau and Patterson15, Reference Rossmeisl, Jilkova and Kuda16). On the other hand, accretion of DHA in rat liver, plasma and kidney was significantly lower when it was provided as PL(Reference Song, Fujimoto and Miyazawa17, Reference Song and Miyazawa18). Human studies were even less conclusive. In a double-blind, randomised, placebo-controlled, crossover study, a 4-week consumption of 600 mg of n-3 LC PUFA in PL increased the n-3 LC PUFA levels in plasma and erythrocytes compared with the TAG form(Reference Ramprasath, Eyal and Zchut19). In contrast, no effect of the molecular species of the dietary lipids (TAG v. PL) was observed on n-3 LC PUFA levels in plasma and erythrocytes after 28 consecutive days of supplementation with 816 mg EPA per d and 522 mg DHA per d(Reference Yurko-Mauro, Kralovec and Bailey-Hall20). These contradictory results may account for different experimental designs, especially different EPA:DHA ratios, and prevent accurate comparison. Moreover, the impact of the molecular lipid species form was often studied by using fish oil for TAG and krill oil for PL. However, it is worth noting that krill oil only provides 40–60 % of n-3 PUFA in the form of PL, while the remaining fraction originates from TAG and NEFA(Reference Castro-Gómez, Holgado and Rodríguez-Alcalá21, 22). As a result, the role of PL as a lipid carrier on n-3 LC PUFA metabolic fate is not clearly established. To our knowledge, only one study provided n-3 PUFA only as TAG or as PL and showed a better lymphatic absorption of n-3 LC PUFA for rats fed PL v. TAG(Reference Cansell, Nacka and Combe23). However, PL were structured in liposomes while TAG were provided as fish oil in bulk phase, so that the physical state of the two formulations was not comparable.
Thereby, the objective of the present study is to provide data on the digestibility and the intestinal absorption of n-3 LC PUFA according to their molecular lipid species, only in the bulk oily phase. Special attention was paid to ensure that the EPA:DHA ratio in the dietary lipid sources remained the same in order to ensure comparable results. n-3 LC PUFA esterified in TAG or PL were first subjected to an in vitro lipolysis in a titrimetric model. Intestinal absorption of n-3 LC PUFA was studied in vivo in a rat lymph duct fistula model. The lipid and fatty acid (FA) compositions of the lymph were analysed. It is worth noting that in vivo rodent models are considered as appropriate study models to assess oral compound bioavailability. They are often used in single-dose protocols or supplementation studies(Reference Couëdelo, Amara and Lecomte11, Reference Couëdelo, Boué-Vaysse and Fonseca12, Reference Michalski, Genot and Gayet14, Reference Cansell, Nacka and Combe23). Indeed, similar physiological and physicochemical events, in particular those implying lipolytic enzymes, are described in both rodents and humans. Moreover, lymph analysis gives direct information on intestinal bioavailability, in contrast to blood, where the lipid composition may be contaminated by lipoproteins from liver metabolism. Thus, rat studies provide useful data to better understand the early stages of lipid digestion and investigate the intestinal bioavailability of FA of interest.
Methods
Animals
Male Wistar rats (8 weeks old, body weight: 300–350 g) were obtained from Elevage Janvier (Saint Genest sur l’Isle). All experiments were carried out in compliance with the ethic committee in Bordeaux and accepted by The French Ministry under no. 2017031014448864. Rats were treated in accordance with the European Communities Council Guidelines for the Care and Use of Laboratory Animals (2010/63/EU). All experiments conformed to the Guidelines for the Handling and Training of Laboratory Animals. Rats were housed four per cage (480 mm × 375 mm × 210 mm) for at least 7 d before the experiment in a controlled environment, at constant temperature (22 ± 1°C) and humidity (60 %) with free water and food access and a 12 h light–12 h dark cycle.
Experimental design
At 24 h before surgery, rats were fed a fat-free diet (SAFE) with free access to water. Each rat was placed under anaesthesia by an intra-peritoneal injection of a ketamine–xylazine mixture (100/10 mg/kg, respectively; Axience). A polyethylene catheter (0·95 mm × 15 cm; Biotrol) was inserted into the main mesenteric lymph duct as described by Bollman et al. (Reference Bollman, Cain and Grindlay24) and Couëdelo et al. (Reference Couëdelo, Boué-Vaysse and Fonseca12). Immediately after surgery, rats were randomly assigned to one of the three experimental groups (six rats/group), corresponding to one of the three n-3 LC PUFA-enriched formulations (TAGn-3, TAGn-3+PLveg, and TAGveg+PLn-3). A volume, equivalent to 1 g of formulation, was orally administrated by gavage, providing, respectively, 8·6 and 22·3 mg of EPA and DHA per rat. Each rat was then placed in an individual restraining cage (rat compartment 228 mm × 89 mm), in a warm environment with freely available water. After gavage, lymph was collected during 6 h in a collection tube stored in ice. To prevent pain, rats received an intra-peritoneal injection of buprenorphine (0·02 mg/kg; Axience) 1 h before and 2 h after surgery. At 6 h post-intubation, rats were euthanised by an intra-peritoneal injection of sodium pentobarbital (Axience) and lidocaine (Ceva).
Lipid formulations
The lipid formulations were prepared at room temperature under nitrogen in order to prevent PUFA oxidation. Three marine formulations were designed to provide a similar amount of FA, but differed by the chemical structure of lipids. More precisely, EPA and DHA were exclusively esterified either on TAG or on PL molecules (Table 1).
Table 1. Fatty acid and phospholipid (PL) compositions of the three experimental lipid formulations

LC, long-chain; TAGn-3, oil phase with n-3 LC PUFA esterified in TAG; TAGn-3+PLveg, oil and soya lecithin mix with n-3 LC PUFA esterified in TAG; TAGveg+PLn-3, oil and marine PL mix with n-3 LC PUFA esterified in PL.
Marine PL were extracted from Lecimarin F50 (Novastell) by solvent fractionation using cold acetone. Briefly, 1 ml of chloroform–methanol (2:1, v/v) was added to 1 g of marine lecithin and maintained at room temperature until complete solubilisation. After addition of 20 ml of cold acetone (–20°C), the sample was homogenised and then centrifuged (1050 g, 5 min, 4°C; Sorvall ST-40R, Thermo Fisher Scientific). The supernatant, containing the neutral lipids, was removed and the pellet, containing the polar lipids, was extracted twice with cold acetone and stored at –20°C.
The formulation providing EPA and DHA only esterified in PL (TAGveg+PLn-3) was prepared by solubilising marine PL (13 wt.%) to a mixture of vegetable oils (flaxseed–copra–grapeseed–palm–oleic sunflower oils; 1:6:6:30:57, by weight). The formulation including EPA and DHA only on TAG (TAGn-3) was obtained by blending two fish oils (Omegavie 1812 TG and Omegavie Tuna oil 25 % DHA; 1:10, w/w; Polaris) to 11 wt.% of blended vegetable oils (flaxseed–copra–grapeseed–palm–oleic sunflower oils; 1:6:6:31:56, by weight). In order to distinguish the role of PL, acting either as emulsifier or as FA carrier, a third formulation (TAGn-3+PLveg) was prepared including vegetable PL (without n-3 LC PUFA contribution). More precisely, 11 wt.% of the marine oil and 10 wt.% of soya lecithin (Nat&Form) were added to a mixture of vegetable oils (copra, palm and oleic sunflower oils; 6:30:63, by weight).
Phospholipid classes of marine polar lipid extract and of soya lecithin were characterised by proton NMR (1H NMR) according to Cansell et al. (Reference Cansell, Bardeau and Morvan25) (Table 1). The glyceride structure of the marine oil mixture and of the marine PL was enzymatically determined according to the methods described below. In the marine TAG (oil mixture), EPA was mainly esterified in the sn-1/3 positions of marine TAG (26 % in the sn-2 position), whereas DHA was mainly located in the sn-2 position (50 %). In marine PL, both EPA and DHA were essentially distributed in the sn-2 position (>74 %) (see online Supplementary material S1).
Lipid analysis
The total lipids were extracted from the lymph according to the procedure described by Folch et al. ( Reference Folch, Lees and Sloane Stanley26 ). PL and TAG fractions from the lipid extract were separated by TLC (glass plates 20 × 20 cm pre-coated with silica gel 60H) using a solvent mixture composed of hexane–diethyl ether–formic acid (75:35:1, by vol.). After vaporisation of 2,7-dichlorofluorescein (Sigma-Aldrich) and visualisation under UV light, the spots corresponding to PL and TAG were extracted from the silica gel by the addition of 2·5 ml of chloroform–methanol (2:1, v/v). After homogenisation and centrifugation of the scraped fractions (1050 g, 5 min, 20°C), the organic phase containing lipids was collected. The extraction step was repeated by the addition of distilled water (100 µl) and chloroform–methanol 2 ml (2:1, v/v) to the silica gel phase. Lipid extraction from the silica gel was ended by the addition of 2 ml of methanol to the silica gel phase, homogenisation and centrifugation. The overall organic phases were pooled and dried under nitrogen. Finally, 2,7-dichlorofluorescein was removed using 0·4 ml of a KCl solution (0·8 % in distilled water, w/v) and 2 ml of chloroform–methanol (2:1, v/v). The organic phase was washed twice by the addition of 0·8 ml of a mixture of chloroform–methanol–KCl 0·8 % in distilled water (15:240:235, by vol.). Samples were stored at –20°C until analysis.
The FA composition of the lipid formulations, the samples from the in vitro lipolysis experiments and those collected from the lymph, that is, total lipids, TAG and PL fractions, were submitted to the trans-methylation procedure adapted from Castro-Gomez(Reference Castro-Gómez, Fontecha and Rodríguez-Alcalá27, Reference Sehl, Couëdelo and Fonseca28). The resulting fatty acid methyl esters were analysed by GC analysis (TRACE GC, Thermo Fisher Scientific) equipped with a flame ionisation detector and a split injector. A fused-silica capillary column (BPX 70, 60 m × 0·25 mm internal diameter, 0·25 μm film; SGE) was used with hydrogen as a carrier gas (inlet pressure: 120 kPa). The split ratio was 1:33. The column temperature program was as follows: from 160°C, the temperature increased to 180°C at 1·3°C/min and was maintained for 65 min before increasing at 25°C/min to 230°C, temperature that was maintained for 15 min. The injector and detector were maintained at 250°C and 280°C, respectively. Gas chromatographic peaks were integrated using Chromquest software (Thermofinnigan). Fatty acids were quantified using an internal standard (1 mg/ml in chloroform–methanol (2:1, v/v)) added at 10 % of the lipid weight before the trans-methylation procedure. NEFA 17 : 0 (heptadecanoic acid; Nu-Chek Prep) was used as an internal standard for the in vitro lipolysis study. TAG 17 : 0 (1,2,3-triheptadecanoyl-sn-glycerol; Nu-Chek Prep) and PC 17 : 0 (1,2-diheptadecanoyl-sn-glycero-3-phosphatidylcholine; Avanti Polar Lipids) were used for TAG and PL quantification of the lymph samples, respectively.
The proportion of FA esterified in the sn-2 position of lymph TAG was determined using the regiospecificity of lipase. Lymph TAG (3 mg) were mixed with 1 ml of lipase B Candida antartica (>2000 U/g; 1 U = 1 µEq FA/h; Sigma-Aldrich) in ethanol solution (4 mg/ml, w/v) and placed under stirring, at 30°C for 3 h. Lipids were extracted six times by 10 ml of diethyl ether. Hydrolysis products were separated by TLC using a solvent mixture composed of hexane–diethyl ether–acetic acid (70:30:1, by vol.). After vaporisation of 2,7-dichlorofluorescein and visualisation under UV light, the spots corresponding to the 2-monoacylglycerols (2-MG) were scraped off and transferred into a glass tube for their trans-methylation. The proportion of FA esterified in the sn-2 position of TAG was calculated by considering the proportions of each FA from 2-MG compared with those from the three positions of the TAG, with the following equation:
The glyceride structure of lymph PL was achieved by phospholipase A2 (PLA2) hydrolysis according to the Wolff et al. (Reference Wolff, Combe and Entressangles29) method, using PLA2 from bee venom (600–2400 U/g; 1U = 1 µEq FA/h; Sigma-Aldrich). Briefly, 150 µl of a PLA2 solution (1 mg/ml; 3 mM CaCl2) was added to 2 mg of extracted lymph PL and 1 ml of diethyl ether. Samples were incubated under stirring, at 30°C for 1 h. The reaction was stopped by solvent evaporation under nitrogen. Hydrolysis products were separated by TLC using a solvent mixture of chloroform–methanol–distillated water (65:25:4, by vol.). NEFA were scraped off and transferred into a glass tube for methylation. The proportion of FA esterified in the sn-2 position of PL was calculated by considering the proportions of each FA released as NEFA compared with those from the two positions of the PL, with the following equation:
In vitro intestinal lipolysis of lipid formulations
The enzymatic lipolysis of the lipid formulations was assessed using an in vitro digestion model that mimics the physiological conditions of the intestinal lumen according to Carrière et al. (Reference Carrière, Renou and Lopez30) and Minekus et al. (Reference Minekus, Alminger and Alvito31) with slight modifications. The lipolysis was initiated by 5 ml of a 25 % lipid emulsion stabilised by 1·4 % (w/v) of methyl cellulose (Sigma-Aldrich) and dispersed in 15 ml of an assay solution containing 0·6 % (w/v) of methyl cellulose, 150 mM NaCl (Thermo Fisher Scientific), 1·4 mM CaCl2 (Sigma-Aldrich) and 20 mM sodium deoxycholate (Sigma-Aldrich). This mixture was stirred in a temperature-controlled reaction vessel at 37°C and the pH was adjusted to 7·5. The lipolysis was initiated by adding 1 ml of porcine pancreas extract solution (6·7 mg/ml in distilled water, pH 7·5; Sigma-Aldrich) to the reaction vessel. The pH was kept constant at 7·5 for 60 min with 0·1 M NaOH (Chem-Lab) automatically controlled by a pH-stat device (pHstat Titrando, Metler Toledo). Samples (0·5 ml) were collected at times ranging from 0 to 60 min for lipid extraction and analysis. Lipid extraction was immediately performed by mixing each sample vigorously (0·5 ml) with 200 μl HCl (0·1 M; Thermo Fisher Scientific) and 5 ml chloroform–methanol (2:1, v/v) in a glass tube. After phase separation, the upper aqueous phase was removed and the lower phase containing the total lipids was dried under nitrogen, dissolved in 3 ml chloroform–methanol (2:1, v/v), and stored at –20°C until analysis. NEFA, from TAG and PL lipolysis, were isolated from other lipid fractions by TLC (glass plates 20 × 20 cm pre-coated with silica gel 60H (Merck KGaA)) using a solvent mixture composed of hexane–diethyl ether–acetic acid (80:20:1, by vol.). Spots were visualised under UV light after vaporisation of 2,7-dichlorofluorescein in ethanol solution (0·2 %, w/v). NEFA were scraped off and transferred into a glass tube for methylation and GC analysis. At each time point of the kinetics (0, 5, 10, 15, 30 and 60 min), the hydrolysis level (% NEFA/total FA) was calculated. A deprived n-3 LC PUFA formulation composed of 10 wt.% of soya lecithin dissolved in a vegetable oil mixture (copra–palm–oleic sunflower oil; 6:30:63, by weight; TAGveg+PLveg) was also assessed as a control (see online Supplementary material S2). Lipolysis experiments were performed in triplicate, for each formulation, that is, TAGn-3, TAGn-3+PLveg and TAGveg+PLn-3.
Characterisation of lymph chylomicrons
The size of the lymph chylomicrons (CM) was measured for each rat group by dynamic light scattering using VascoTM (Cordouan Technologies). Lymph samples were firstly diluted in NaCl solution in water (0·9 %, w/v). Experiments were performed considering a water refractive index of 1·331 and a viscosity of 0·888 cP. The real part and the imaginary part of particles were fixed at 1·47 and 0·01, respectively.
Statistical analysis
Data were expressed as means and standard deviations. Intergroup comparisons were made on the basis of their respective mean. For the in vitro lipolysis experiment (n 3), multiple comparisons were analysed using a two-way ANOVA with two independent factors (lipid formulation and time of lipolysis) followed by a Bonferroni post hoc test, using GraphPad Prism version 7. Data issued from the in vivo intestinal absorption experiment (n 6) were compared by a one-way ANOVA test followed by a Tukey post hoc test, using R version 3.5.1 (Rcommander package). P values lower than 0·05 were considered to be statistically significant.
Results
In vitro digestion of n-3 long-chain PUFA is modulated by their molecular lipid species of intake
Marine formulations (TAGn-3, TAGn-3+PLveg, TAGveg+PLn-3) were subjected to in vitro lipolysis in a one-step static digestion model, with a focus on the duodenal lipolysis (Fig. 1). Results revealed that all lipid formulations were well hydrolysed during the time course of the lipolysis but to a different extent according to the formulation used (P time<0·001; P lipids=0·05 and P interaction<0·001; two-way ANOVA test). At the end of the lipolysis kinetics (60 min), the presence of soya lecithin in the marine formulation (TAGn-3+PLveg) tended to inhibit the total lipolysis compared with TAGn-3 (lipolysis level of 21·3 (SD 4·7) % and 14·5 (SD 1·5) %, for TAGn-3 and TAGn-3+PLveg, respectively). The lipolysis of TAGn-3+PLveg formulation was decreased by 47 % when compared with the formulation free of n-3 LC PUFA (lipolysis level of 14·5 (SD 1·5) % and 27·3 (SD 1·9) % for TAGn-3+PLveg and TAGveg+PLveg, respectively; P<0·05). When n-3 LC PUFA were esterified in marine PL (TAGveg+PLn-3), the lipolysis seemed to be slower at the beginning of the kinetics compared with the control curve (TAGveg+PLveg); however at 60 min, the lipolysis levels were not statistically different (21·5 (SD 4·3) % and 27·3 (SD 1·9) % for TAGveg+PLn-3 and TAGveg+PLveg, respectively).
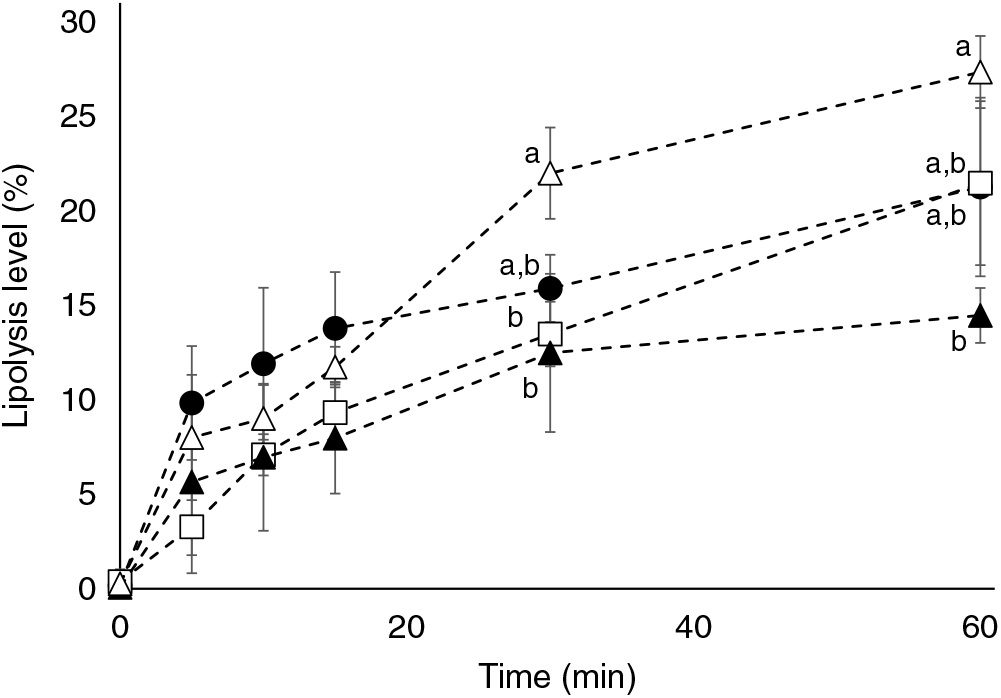
Fig. 1. Effect of lipid formulation on lipolysis levels using an in vitro digestion model. TAG n-3 (•), TAG n-3+PLveg (▴), TAGVeg+PL n-3 (□) and TAGveg+PLveg (Δ). Lipolysis levels are expressed as % NEFA v. total acyl chains in the residual TAG, diacylglycerols, monoacylglycerols and NEFA. Values are means with standard deviations (n 3). a,b Values with unlike letters for the same time are significantly different (P<0·05; two-way ANOVA followed by Bonferroni post hoc test).
Influence of the molecular lipid species of the dietary lipids on the intestinal absorption of n-3 long-chain PUFA
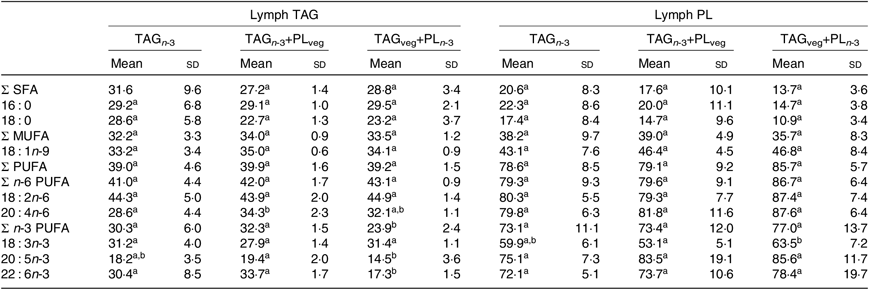
At 6 h after lipid ingestion, no adverse effect was observed consecutive to lipid formulation intubation. The total FA concentration in the rat lymph was similar for the three marine formulations (Table 2). The proportions of EPA and DHA in lymph total FA did not show statistical differences between all groups. Lymph TAG and PL were separated and their FA compositions were analysed separately (Table 3). The relative proportions of lymph TAG and PL were similar regardless of the molecular n-3 LC PUFA carrier (P>0·05). Thereby, TAG was the major lipid fraction (71 % of total FA) while PL only represented 7 % of total FA. Lymph TAG presented a similar FA profile between the three groups (P>0·05). SFA and MUFA were the two major FA classes, and PUFA were mainly represented by n-6 PUFA. Considering n-3 PUFA, EPA and DHA levels in lymph TAG were similar in the three groups. In lymph PL, the incorporation of n-3 LC PUFA and specifically EPA was quite low. DHA levels tended to be higher in the TAGveg+PLn-3 than in TAGn-3 and TAGn-3+PLveg (P=0·10). The proportions of the main FA esterified in the sn-2 position of lymph lipids were defined (Table 4). We noted that SFA, MUFA and PUFA were equally distributed in the three positions of lymph TAG for all groups. In lymph PL, SFA were mostly esterified in the sn-1 position and PUFA in the sn-2, irrespective of the groups. Considering n-3 LC PUFA, EPA and DHA were mainly located in the sn-2 position of lymph PL regardless the molecular FA carrier (TAG v. PL). DHA was equally esterified in the three positions of lymph TAG when provided as marine TAG (TAGn-3 and TAGn-3+PLveg), whereas it was mainly located in the sn-1/3 positions when ingested as marine PL (TAGveg+PLn-3; P<0·001). More precisely, the intake of marine PL decreased by 46 % the proportion of DHA esterified in the sn-2 position of lymph TAG. However, EPA was mainly inserted in the sn-1/3 positions of lymph TAG in all groups.
Table 2. Effect of the molecular carrier form of n-3 long-chain (LC) PUFA on fatty acid composition (g/100 g of total fatty acids) of total lipids of lymph of rats*, 6 h after intubation
(Mean values and standard deviations)
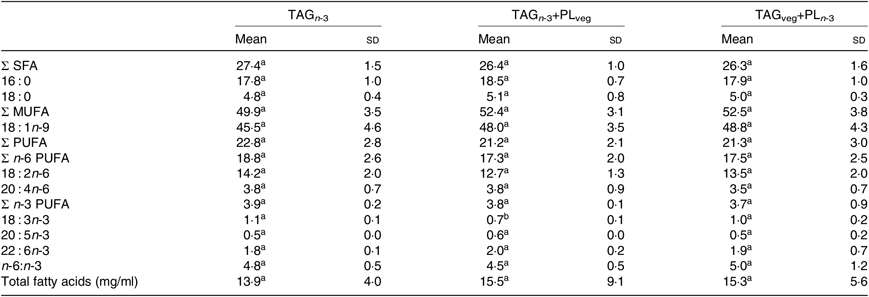
PL, phospholipids; TAGn-3, oil phase with n-3 LC PUFA esterified in TAG; TAGn-3+PLveg, oil and soya lecithin mix with n-3 LC PUFA esterified in TAG; TAGveg+PLn-3, oil and marine PL mix with n-3 LC PUFA esterified in PL.
a,b Mean values within a row with unlike superscript letters are significantly different (P<0·05; ANOVA followed by Tukey’s post hoc test).
* n 6.
Table 3. Effect of the molecular carrier form of n-3 long-chain (LC) PUFA on fatty acid composition (g/100 g of total fatty acids) of TAG and phospholipids (PL) of lymph of rats*, 6 h after intubation
(Mean values and standard deviations)

TAG n-3, oil phase with n-3 LC PUFA esterified in TAG; TAG n-3+PLveg, oil and soya lecithin mix with n-3 LC PUFA esterified in TAG; TAGveg+PL n-3, oil and marine PL mix with n-3 LC PUFA esterified in PL.
a,b Mean values within a row with unlike superscript letters are significantly different (P<0·05; ANOVA followed by Tukey’s post hoc test).
* n 6.
Table 4. Effect of the molecular carrier form of n-3 long-chain (LC) PUFA on fatty acid distribution (% esterified in the internal sn-2 position) in TAG and phospholipids (PL) of lymph of rats*, 6 h after intubation
(Mean values and standard deviations)
TAGn-3, oil phase with n-3 LC PUFA esterified in TAG; TAGn-3+PLveg, oil and soya lecithin mix with n-3 LC PUFA esterified in TAG; TAGveg+PLn-3, oil and marine PL mix with n-3 LC PUFA esterified in PL.
a,b Mean values within a row with unlike superscript letters are significantly different (P<0·05; ANOVA followed by Tukey’s post hoc test).
* n 6.
Characterisation of lymph chylomicrons
Lymph CM were analysed in terms of particle size. Data revealed that the mean diameter of CM were comparable between the three groups (223 (SD 28), 248 (SD 46) and 227 (SD 47) nm for TAGn-3, TAGn-3+PLveg and TAGveg+PLn-3, respectively).
Discussion
The present study aims at evaluating the impact of the molecular lipid species of dietary n-3 LC PUFA on (i) their in vitro digestibility and (ii) their intestinal bioavailability, in rats fed marine TAG or PL. Only few studies tried to couple in vitro lipid hydrolysis with in vivo absorption and they dealt with plant lipids(Reference Couëdelo, Amara and Lecomte11, Reference Couëdelo, Boué-Vaysse and Fonseca12). A recent study used marine lipids(Reference Murota, Takagi and Watanabe32). However, the formulations did not have a similar EPA:DHA ratio and did not allow comparison of PL as n-3 carrier or as emulsifier. In our work, lipid formulations were designed to provide a similar amount of EPA and DHA, esterified exclusively either in TAG or in PL molecules. Because PL display surfactant properties that may influence lipid digestibility and absorption, a formulation containing soya lecithin instead of marine PL (TAGn-3+PLveg) was also assessed. Results showed that even if the in vitro lipolysis level was dependent on the type of the formulation used, EPA and DHA were similarly absorbed, in vivo, regardless of the lipid carrier. However, they were differently distributed in lymph lipid fractions. Specifically, when provided as marine PL, n-3 LC PUFA tended to be better incorporated in lymph PL and DHA was more externalised in the glycerol backbone of lymph TAG. To our knowledge, this is the first study analysing the molecular structure of lymph lipid fractions using marine TAG and PL as dietary n-3 LC PUFA carriers.
Gastro-intestinal digestion can be considered as a limiting step of bioavailability. This is because dietary lipids first have to be hydrolysed in the gastro-intestinal tract, before being absorbed by the intestinal brush border. In our study, we did not perform a gastric phase before the duodenal phase, which could be a limitation. However (1) duodenal lipolysis represents nearly 70 % of total lipolysis(Reference Carriere, Barrowman and Verger33) and (2) the efficiency of the intestinal lipolysis is not called into question by the gastric phase. Our data confirmed that lipolysis extent depended on the affinity of pancreatic lipase towards the lipid species. The presence of n-3 LC PUFA esterified in marine TAG (TAGn-3+PLveg v. TAGveg+PLveg) dramatically impaired the lipolysis rate, due to the molecular structure of EPA and DHA which led to a steric hindrance effect in in vitro studies(Reference Akanbi, Sinclair and Barrow34, Reference Bottino, Vandenburg and Reiser35). In agreement with other studies(Reference Couëdelo, Boué-Vaysse and Fonseca12, Reference Nishimukai, Hara and Aoyama36), we observed an inhibitory effect of soya PL (TAGn-3 v. TAGn-3+PLveg). This could be interpreted as an impediment of the binding of pancreatic lipase/co-lipase at the oil–water interface, decreasing TAG availability for hydrolysis(Reference Borgström37, Reference Larsson and Erlanson-Albertsson38). Moreover, the PL nature influenced the lipid hydrolysis level (TAGveg+PLveg v. TAGveg+PLn−3). In our experiment, the porcine pancreas extract contained PLA2. It is well known that PLA2 activity is modulated by the nature of the polar head group of PL and to a lesser extent by the FA nature, that is, chain length or unsaturation degree(Reference de Haas, Postema and Nieuwenhuizen39, Reference Jain and Rogers40). Several studies using liposomes(Reference Ghomashchi, Yu and Berg41, Reference Singer, Ghomashchi and Le Calvez42) and emulsion droplets(Reference Bahnson43) showed that PLA2 would preferentially bond to anionic PL rather than zwitterionic PL. Thus, in our study, a part of the decreased lipolysis observed with TAGveg+PLn−3 formulation could be attributed to the PL composition (5 % of anionic PL in PLn-3 v. 39 % in PLveg).
The products generated by the lipid hydrolysis (NEFA, 2-MG, and lysophospholipids) are micellised by biliary salts before being absorbed through the intestinal brush border. In the enterocytes, the lipolysis products are re-synthesised in TAG and PL, compacted into CM to be released into the lymph compartment before reaching the bloodstream. In our experiment, the CM diameter was similar, whatever the molecular carriers used, suggesting that the structural properties of CM were impacted neither by the presence of PL in the formulation nor by the molecular n-3 LC PUFA carrier used, nor by the variations of lipolysis rates observed in vitro. This finding is in accordance with a previous study demonstrating that the lipid composition of CM did not differ in rats fed fish oil or marine PL(Reference Cansell, Nacka and Combe23).
In all experimental groups, the FA quantification and FA profile of total lymph lipids were similar, suggesting that, under our test conditions, the digestion and the intestinal absorption of n-3 LC PUFA were not modulated by the molecular n-3 LC PUFA carrier (TAGn-3 v. PLn-3). Moreover, lymph lipids were mainly composed of PL and TAG and the incorporation of EPA and DHA in lymph lipid fractions occurred independently of the molecular lipid species used (i.e. TAG, PL, lysophospholipids, NEFA)(Reference Cansell, Nacka and Combe23, Reference Singer, Ghomashchi and Le Calvez42–Reference Subbaiah, Dammanahalli and Yang44). Indeed, the FA profile of lymph TAG revealed similar n-3 PUFA levels between the three groups, suggesting that EPA and DHA were incorporated in lymph TAG regardless of the molecular FA carrier. However, in lymph PL, DHA levels tended to increase when provided as marine PL (TAGveg+PLn-3) compared with the TAG form of intake (TAGn-3 and TAGn-3+PLveg). These data agree with a previous study where the proportions of n-3 LC PUFA in CM-PL were increased with marine PL compared with fish oil(Reference Cansell, Nacka and Combe23, Reference Murota, Takagi and Watanabe32). It has been suggested that FA absorption could be lower in anaesthetised rats than in conscious animals. Nonetheless, the relative distribution of DHA in lymph lipid fractions is expected to be similar regardless of the consciousness state of the rats(Reference Subbaiah, Dammanahalli and Yang44).
The analysis of the glyceride structure of TAG clearly showed that the molecular n-3 LC PUFA carrier influenced the mechanisms involved in the re-synthesis of lymph lipids. In the enterocyte, lymph TAG are mainly synthesised by the 2-MG pathway. We found that marine TAG intake (TAGn-3 and TAGn-3+PLveg) led to an equimolar distribution of DHA in the three positions (33 % of each) of lymph TAG, whereas the DHA provided was 56 % esterified in the sn-2 position in marine TAG formulation. This showed that the sn-2 position of the dietary TAG was not fully conserved in lymph TAG. A similar conclusion was drawn when structured TAG rich in α-linolenic acid (18 : 3n-3) were used, that is, only 50 % of α-linolenic acid located in the sn-2 position of dietary TAG were recovered at this position in rat lymph TAG(Reference Couëdelo, Vaysse and Vaique45). This was interpreted as either (i) an isomerisation of 2-MG into 1-MG prior to the lipolytic activity of pancreatic lipase(Reference Mattson and Volpenhein46) or (ii) a hydrolysis of the absorbed 2-MG by an intestinal lipase in the enterocyte(Reference Chon, Zhou and Dixon47). This suggested that new metabolic pathways could take place as soon as the concentration of a lipid species varied greatly, thus ensuring a ‘homeostatic’ state. On the contrary, in marine PL, DHA was exclusively (>74 %) esterified in the sn-2 position of the glycerol backbone. After PL digestion, DHA was mainly released as NEFA, due to a predominant activity of PLA2, specific of the sn-2 position of PL(Reference Cohn, Wat and Kamili48). Thus, the presence of DHA in the sn-2 position of lymph TAG suggested that the glycerol-3-phosphate pathway was involved for the de novo TAG synthesis using un-esterified DHA. Indeed, the glycerol-3-phosphate pathway could account for at least 15 % of lymph TAG synthesis. Nevertheless, the major pathway for re-synthesis of lymph TAG seemed to be the 2-MG pathway, leading to the incorporation of a large part of free DHA on the 2-MG backbone. In this way, TAGveg+PLn-3 ensured a DHA enrichment in the sn-1/3 positions of lymph TAG compared with the marine TAG groups (TAGn-3 and TAGn-3+PLveg). The analysis of the glyceride structure of PL showed the same n-3 LC PUFA repartition in the three dietary groups. Lymph PL mainly originate from the re-acylation of 1-lysophospholipids; this suggested that, irrespective of the amount of free DHA in the enterocyte, the composition of lymph PL is highly regulated with an incorporation mainly in the sn-2 position.
These mechanisms are confirmed with the metabolic fate of EPA. Indeed, EPA was less esterified in the sn-2 position of lymph TAG when provided as marine PL than as marine TAG. Nevertheless, since EPA was mainly distributed in the sn-1/3 positions of marine TAG and in sn-2 position of marine PL, it was essentially absorbed as NEFA after gastro-intestinal hydrolysis. Free EPA was then incorporated either in the sn-1/3 positions of lymph TAG or in the sn-2 position of lymph PL.
In conclusion, the present study was undertaken to analyse the impact of different molecular carriers of n-3 LC PUFA that could increase their bioavailability, based on in vivo data. Our results showed that dietary PL from vegetable or animal sources added to the oily formulations, at the ratio of 10 %, did not increase lipid absorption. Although lipolysis differed according to the composition of the formulation, it seemed that the molecular carrier did not greatly modify the intestinal absorption of n-3 LC PUFA. In contrast, the nature of the dietary lipids might modulate FA incorporation and distribution in lymph lipid fractions. However, it would be interesting to complete the present study with the analysis of gene and protein expressions as well as with the activities of some enzymes implied in the intracellular lipid metabolism pathways to consolidate our data. The fact that, when provided as marine PL, DHA was better incorporated in lymph PL and was externalised in lymph TAG may further affect its metabolic fate and tissue accretion. Indeed, previous reports indicated that using PL as n-3 LC PUFA carriers can be more efficient than TAG regarding the metabolisation and hypoadipogenic effect of EPA and DHA(Reference Cansell10, Reference Graf, Duchateau and Patterson15, Reference Rossmeisl, Jilkova and Kuda16, Reference Awada, Meynier and Soulage49–Reference Destaillats, Oliveira and Bastic Schmid51).
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001491
Acknowledgements
Authors are grateful to Novastell and in particular Thierry Coste (Novastel) for providing Lecimarin F50, and also to Polaris for supplying fish oils. The authors also wish to thank Axelle Grélard from Bordeaux University, CNRS, CBMN-UMR 5248, for RMN analysis.
The study was partly financially supported by the French National Association for Research and Technology (ANRT, no. 2015/1182).
None of the authors has any conflicts of interest to declare.








