It is generally accepted that non-digestible dietary carbohydrates – resistant to digestion in the small intestine – are the main substrates available for fermentation by bacteria in the human colon( Reference Drakoularakou, Rastall and Gibson 1 ). When this fermentation is carried out by selective bacteria, causing a beneficial effect on the gut microbiota and consequently on the host, they are considered prebiotics( Reference Charalampopoulos and Rastall 2 – Reference Gibson, Scott and Rastall 4 ). Many of the beneficial health effects are related to soluble dietary fibre (SDF) and non-digestible oligosaccharides, such as the regulation of metabolic disorders related to obesity and reduction of cancer risk( Reference Charalampopoulos and Rastall 2 , Reference Corzo, Alonso and Azpiroz 3 , Reference Courtois 5 ). The most important health-promoting bacteria of the gut microbiota are bifidobacteria and lactobacilli. Both are common targets for dietary intervention that improves health( Reference Drakoularakou, Rastall and Gibson 1 , Reference Manning and Gibson 6 – Reference Saliminen, Ramos and Fonden 8 ). Other bacteria such as streptococci, enterococci, eubacteria and Bacteroides can be classified as potentially beneficial to health or as potentially harmful depending on the species( Reference Roberfroid, Gibson and Hoyles 7 ). Healthy bacteria are beneficial to the host through their metabolisms such as SCFA formation (principally acetate, propionate and butyrate), absence of toxin production and synthesis of defensins or vitamins( Reference Rastall and Gibson 9 – Reference Macfarlane and Macfarlane 11 ).
Typical prebiotics include SDF, inulin-derived fructans (fructo-oligosaccharides; FOS) and galacto-oligosaccharides (GOS)( Reference Corzo, Alonso and Azpiroz 3 , Reference Roberfroid, Gibson and Hoyles 7 , Reference Rastall and Gibson 9 , Reference Slavin 12 ), but nowadays there is great interest in finding novel prebiotics from waste biomass or by-products from food industry( Reference Rastall and Gibson 9 , Reference Gullon, Gullon and Moure 13 – Reference Mateos-Aparicio, Redondo-Cuenca and Villanueva 15 ). New candidates for prebiotics include polydextrose, lactosucrose, malto-oligosaccharides, gluco-oligosaccharides, xylo-oligosaccharides and soyabean oligosaccharides( Reference Drakoularakou, Rastall and Gibson 1 , Reference Corzo, Alonso and Azpiroz 3 , Reference Gibson, Scott and Rastall 4 , Reference Moura, Barata and Carvalheiro 16 ). One of these promising potential prebiotics is Okara, an abundant and inexpensive by-product obtained after extraction of the soluble fraction from soyabean seed for tofu or soyamilk production( Reference Mateos-Aparicio, Redondo-Cuenca and Villanueva 17 – Reference Espinosa-Martos and Ruperez 20 ), and its re-valorisation would be economically valuable. Okara is an insoluble by-product and has a more complete nutritional profile than current prebiotics in the market (inulin, FOS, GOS) as it contains not only dietary fibre but also protein, oil and minerals. Okara has a high total dietary fibre (TDF) content of 54–55 % (50–51 % insoluble dietary fibre (IDF) and 4·5 % SDF) and 3·9 (sd 0·2) % of low molecular weight (MW) carbohydrates (LMWC) (0·4 (sd 0·1) % inulin, 1·4 (sd 0·1) % stachyose+raffinose, 0·2 (sd ≤0·1) % glucose)( Reference Mateos-Aparicio, Redondo-Cuenca and Villanueva 17 , Reference Espinosa-Martos and Ruperez 20 – Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 ). Okara has been proven to be a potential weight-loss supplement, with potential prebiotic effect because of its high TDF content and beneficial effects on lipid metabolism( Reference Redondo-Cuenca, Villanueva and Mateos-Aparicio 18 – Reference Espinosa-Martos and Ruperez 20 , Reference Villanueva, Yokoyama and Hong 24 , Reference Jimenez-Escrig, Tenorio and Espinosa-Martos 25 ).
The traditional treatment of plant polysaccharides to obtain prebiotic oligosaccharides is with enzymes, but recently there is an increasing interest in the use of new technologies such as autohydrolysis with elevated temperature and pressure applied to by-products( Reference Rastall and Gibson 9 , Reference Gullon, Gullon and Moure 13 , Reference Berardini, Knodler and Schieber 26 ). Furthermore, new technologies have been applied to soyabean and even to Okara. For example, high-pressure microfluidisation and fermentation by Lactobacillus delbrueckii subsp. bulgaricus of soyabean waste produce an increase in SDF by degradation of insoluble polymers into simple carbohydrates( Reference Tu, Chen and Wang 27 ). Moreover, high hydrostatic pressure (HHP) has been previously used for SDF maximisation in Okara( Reference Mateos-Aparicio, Mateos-Peinado and Ruperez 21 , Reference Li, Qiao and Lu 22 , Reference Perez-Lopez, Mateos-Aparicio and Ruperez 28 ), with the advantage that it does not affect organoleptic attributes and can extend the shelf-life of products( Reference Lambert, Demazeau and Largeteau 29 – Reference Vervoort, Van der Plancken and Grauwet 31 ). In addition, the use of enzymes to increase SDF content in food products has been reported, including soyabean( Reference Nakamura, Furuta and Maeda 32 – Reference Ruperez, Perez-Cozar and Redondo-Cuenca 35 ). A food-grade enzyme (Ultraflo ® L; Novozymes) has been used to digest Okara at atmospheric pressure( Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 , Reference Kasai, Murata and Inui 36 ) and the combined effects of both, HHP and Ultraflo ® L, have been successfully applied to maximise the SDF content of Okara by our group( Reference Perez-Lopez, Mateos-Aparicio and Ruperez 28 ).
Evaluation of potential prebiotics includes different approaches. First, the capacity of certain beneficial bacteria to grow in culture media containing the selected ingredient has to be verified( Reference Corzo, Alonso and Azpiroz 3 , Reference Gibson, Scott and Rastall 4 ). This effect as well as its non-digestible nature have been proven in native and enzymatically treated Okara( Reference Espinosa-Martos and Ruperez 20 , Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 ). Next, the potential prebiotic ingredient could be fermented in vitro, before an in vivo animal experiment followed by human trials( Reference Corzo, Alonso and Azpiroz 3 , Reference Gibson, Scott and Rastall 4 ). Native Okara has demonstrated a beneficial effect on lipid profiles of plasma in Syrian hamsters( Reference Villanueva, Yokoyama and Hong 24 ), as well as a potential weight loss and prebiotic effect in Wistar rats( Reference Prestamo, Ruperez and Espinosa-Martos 19 , Reference Jimenez-Escrig, Tenorio and Espinosa-Martos 25 ). However, as far as we know, a fermentative colonic model has not been used to demonstrate the prebiotic effect of Okara.
Therefore, the present study aimed to evaluate – using in vitro batch culture systems modelling the human gut – the potential prebiotic properties of native Okara and after its treatment for SDF maximisation via HHP assisted by a food-grade enzyme.
Methods
Substrate
Fresh Okara, obtained as an industrial by-product from soyabean (Glycine max (L.) Merr), was provided by Toofu-Ya S.L., a local food processing company (Arganda del Rey). At the laboratory, fresh Okara was freeze-dried (Virtis Bench Top 3L; Hucoa-Erlöss S.A.), then defatted by extraction with ethylic diethyl ether in a Soxtec System (Tecator) and kept in airtight containers at room temperature until use. Before enzymatic or HHP treatment, Okara was re-hydrated in water (15 %, w/v) with constant shaking in a Heidolph Reax 2 rotatory shaker (Heidolph Instruments GmbH & Co. KG) overnight.
All solutions, including dilutions and mobile phases for HPLC, were prepared with ultrapure water.
High hydrostatic pressure treatment assisted by Ultraflo® L applied to Okara
Pre-hydrated Okara solution, 15 % (w/v), was treated simultaneously with Ultraflo ® L (concentration of 0·025 %), a food-grade β-glucanase (endo-β-1,3(4)-), with both cellulase and xylanase activities (Novozymes), under HHP (pressure of 600 MPa) at 40°C for 30 min, not considering the pressure build up and release time. These conditions were previously optimised( Reference Perez-Lopez, Mateos-Aparicio and Ruperez 28 ).
The treatment was performed in vacuum-sealed plastic bags (Doypack, 110×200×35-mm size, 75-μm-thick film, Polyskin XL; Amcor Flexible Hispania) in Stansted SFP 7100:9/2C HHP equipment (Stansted Fluid Power Ltd), using water as the pressure-transmitting medium. After HHP+Ultraflo ® L treatment, samples were stored at −20°C and then freeze-dried.
Dietary fibre analysis of Okara treated with high hydrostatic pressure and assisted by Ultraflo® L
After HHP and Ultraflo ® L treatment, SDF and IDF in untreated control and HHP+Ultraflo ® L-treated samples were determined according to the Association of Official Analytical Chemists( 37 ) enzymatic–gravimetric method with dialysis (12 kDa MW cut-off)( Reference Mateos-Aparicio, Mateos-Peinado and Ruperez 21 , Reference Mañas and Saura-Calixto 38 ). In the SDF fraction, uronic acids (UA) were spectrophotometrically quantified by the method of Scott( Reference Scott 39 ), with galacturonic acid as the standard and neutral sugars (NS) by the anthrone method( Reference Loewus 40 ) with glucose as the standard. Moreover, SDF and IDF were hydrolysed with H2SO4 (1 m) at 105°C for 1·5 h, and reducing sugars were spectrophotometrically measured by the dinitrosalicylic acid method (DNS)( Reference Miller 41 ). Every spectrophotometric method was conveniently adapted for microplate reading, and the absorbance was read on a Biotek PowerWawe XS spectrophotometer (BioTek Instruments, Inc.). Thus, SDF was calculated either as reducing sugars (DNS method) or as UA+NS (from UA and anthrone methods). IDF was calculated as reducing sugars (DNS) and TDF was calculated as SDF plus IDF.
Batch culture fermentations
Batch culture fermentation vessels (100-ml working volume), previously sterilised, were filled with 45 ml of sterile complex colonic model growth medium. The composition of this medium included, among others, peptone water (5 g/l), yeast extract (4·5 g/l), starch (5 g/l), tryptone (5 g/l), NaCl (4·5 g/l), KCl (4·5 g/l), mucin (4 g/l), casein (3 g/l), pectin (2 g/l), xylan (2 g/l), arabinogalactan (2 g/l) and inulin (1 g/l)( Reference Macfarlane, Macfarlane and Gibson 42 , Reference Tejero-Sarinena, Barlow and Costabile 43 ), trying to simulate a common and complex human diet. All media and chemicals were purchased from Oxoid and Sigma. Subsequently, the vessels were connected to a circulating water bath at 37°C and sparged with O2-free N2 gas overnight to create anaerobic conditions before inoculation. The pH was adjusted between 6·7 and 6·9 using pH meter controllers with NaOH or HCl (Electrolab260; Electrolab Ltd), and then 5 ml of faecal slurry, prepared as 10 % w/v in 0·1 m sterile PBS (pH 7), was inoculated into each vessel. Three different experiments, with different healthy human donors, were completed. The volunteers were free of any known metabolic and gastrointestinal diseases, were not taking probiotic or prebiotic supplements and had not taken antibiotics for 6 months before faecal sample donation. Verbal informed consent was obtained from all donors, according to the ethical guidelines of the University of Reading. In total, four vessels were used, in triplicate (four vessels per donor), with either 0·5 g of freeze-dried HHP+Ultraflo ® L Okara or native Okara samples, 0·5 g of FOS (Orafti® P95; BENEO GmbH) as a positive control and another vessel without any sample (negative control). Pre-digestion of Okara was not needed according to our previous studies( Reference Espinosa-Martos and Ruperez 20 ). Batch cultures were run for 48 h, and 5-ml aliquots were taken at times 0, 4, 8, 24 and 48 h for analysing bacterial populations by fluorescent in situ hybridisation (FISH) and for SCFA and lactic acid analyses by HPLC.
Enumeration of bacterial populations by fluorescence in situ hybridisation analysis
The bacterial groups Chis 150 – Clostridium histolyticum ( Reference Franks, Harmsen and Raangs 44 ), Lab 158 – lactobacilli( Reference Harmsen, Elfferich and Schut 45 ), Erec 482 – Clostridium coccoides and Eubacterium rectale ( Reference Franks, Harmsen and Raangs 44 ), Prop 853 – Clostridial cluster IX( Reference Walker, Duncan and McWilliam Leitch 46 ), Rfla 729-Rbro 730 – Ruminococcus albus and Ruminococcus flavefaciens/Clostridium sporosphaeroides, Ruminococcus bromii and Clostridium leptum ( Reference Harmsen, Raangs and He 47 ), Bac 303 – Bacteroides( Reference Manz, Amann and Ludwig 48 ), Bif 164 – Bifidobacterium ssp.( Reference Langendijk, Schut and Jansen 49 ) and Eub 338 I-II-III-domain bacteria( Reference Daims, Bruhl and Amann 50 ) were identified using synthetic oligonucleotide probes targeting specific regions of the 16S ribosomal RNA molecule, labelled with the fluorescent dye Cy3.
An aliquot (375 µl) from each vessel at each time point was fixed during 4 h (4°C) in 1125-µl (4 % w/v) paraformaldehyde. Next, the samples were centrifuged at 13 000 g for 5 min and washed twice in 1-ml, sterilised PBS. The pellets were re-suspended in 150-µl PBS+150-µl ethanol and stored (−20°C).
For hybridisation, samples were diluted in an appropriate amount of PBS/SDS for each probe. Aliquots (20 µl) were applied in each well of a six-well polytetrafluoroethylene and poly-l-lysine-coated six-well slide (Tekdon Inc.). After drying for 15 min in a drying chamber (at 46 or 50°C), the slides were sequentially dehydrated in alcohol (50, 80 and 96 % v/v, ethanol) for 3 min in each solution. Gram+bacterial groups needed a previous treatment with lysozyme (20 µl), followed by ethanol dehydration. A 50-µl aliquot of an appropriate hybridisation buffer and 5 µl of a fluorescent-marked oligonucleotide probe were added to the slide, and incubated for 4 h in a microarray hybridisation incubator (Grant Boekel). Next, hybridisation slides were washed in 50-ml washing buffer, containing 20 µl of 4',6-diamidino-2-phenylindole dihydrochloride (50 ng/µl; Sigma), for 15 min and dried with compressed air. The composition of the hybridisation and wash buffers depended on the rRNA probe as reported in probe Base( Reference Loy, Horn and Wagner 51 ). A 5-µl aliquot of anti-fade reagent (polyvinyl alcohol mounting medium with DABCO® antifading; Sigma) was added to each well and a coverslip was placed. Finally, the slides were counted (fifteen different fields for each sample) with an epifluorescence microscope (Eclipse 400; Nikon) using the Fluor 100 lens. The means of the three donors were expressed as log10 cells/ml( Reference Martin-Pelaez, Gibson and Martin-Orue 52 , Reference Costabile, Walton and Tzortzis 53 ).
Analysis of SCFA and lactic acid
Samples (1 ml) from each fermentation time point were centrifuged (13 000 g , 10 min), and supernatants were filtered through 0·2-µm Acrodisc® Syringe Filters with hydrophilic polyvinylidene fluoride membrane, 13 mm (Pall Corporation). Aliquots (20 µl) were injected into an HPLC system (Merck), equipped with a refraction index detector. The column used was an ion-exclusion REZEX-ROA organic acid column (Phenomenex Inc.), maintained at a constant temperature of 85°C. The eluent was sulphuric acid in ultrapure water (0·0025 mmol/l), with a flow rate of 0·5 ml/min. Calibration curves for lactate, acetate, propionate and butyrate (12·5–100 mm) were accomplished for SCFA quantification. The mean metabolite concentrations were expressed as mm ( Reference Tejero-Sarinena, Barlow and Costabile 43 ).
Statistical analysis
Results were expressed as means and standard deviations. At least, three different measurements were accomplished for each mean. Comparison of dietary fibre means was performed by one-way ANOVA with a significance level of P<0·05. Statgraphic version 5.1 was used. Bacterial counts by FISH and SCFA and lactic acid data were analysed by 2-way ANOVA with Bonferroni post-tests with P<0·05. In addition, a paired t test was applied in order to assess the significance of the results of single pairs of data using GraphPad Prism 5.0 (GraphPad Software).
Results
Dietary fibre analysis
In order to obtain a SDF-enriched product, HHP treatment assisted by Ultraflo ® L was applied to Okara, and the dietary fibre content was determined according to the AOAC( 37 ) enzymatic–gravimetric method with dialysis (12 kDa MW cut-off)( Reference Mateos-Aparicio, Mateos-Peinado and Ruperez 21 , Reference Mañas and Saura-Calixto 38 ).
Dietary fibre contents in native Okara and after treatment with HHP assisted by enzymes are shown in Table 1. An overall increase in SDF was reported when Okara was treated with HHP and Ultraflo ® L. When SDF was expressed as the sum of UA and NS, a SDF value that was 1·58-fold higher was reported. However, low SDF content was reported in native Okara when reducing sugars were measured by the DNS method (6·32-fold higher with the treatment). IDF and TDF showed a 0·60- and 0·66-times reduction, respectively, when the treatment was applied.
Table 1 Dietary fibre in native Okara and after treatment with high hydrostatic pressure assisted by Ultraflo ® L (Mean values and standard deviations, n 3)
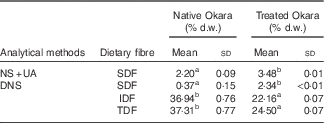
d.w., dry weight; NS, neutral sugars; UA, uronic acid; DNS, 3,5-dinitrosalicylic acid; SDF, soluble dietary fibre; IDF, insoluble dietary fibre; TDF, total dietary fibre.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Enumeration of bacterial populations by fluorescence in situ hybridisation analysis
The potential prebiotic effect of native Okara and HHP assisted by Ultraflo ® L-treated Okara on the main bacterial groups constituting the human intestinal microbiota were assessed by FISH analysis. Specific microbiota groups such as lactic acid bacteria and butyrate producers were chosen as they are the most important bacteria, whose growth has been related to the prebiotic effect. Other bacterial groups, mainly related to dietary fibre fermentation, were also included. Potentially harmful bacterial groups were selected to monitorise a possible decrease. The analyses were performed at 0, 4, 8, 24 and 48 h of fermentation as reported in Fig. 1.
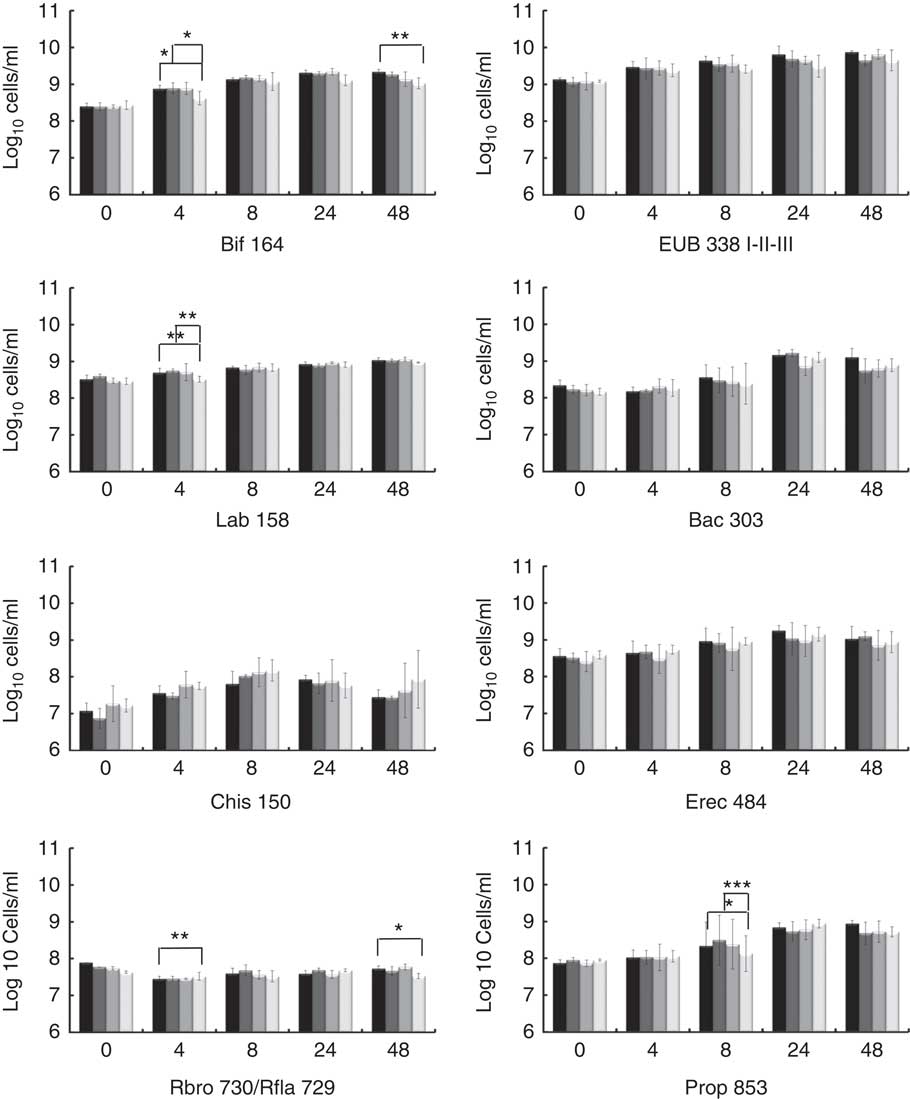
Fig. 1 Fluorescence in situ hybridisation analysis (FISH) of bacterial population in pH-controlled faecal batch cultures on Okara treated with high hydrostatic pressure (HHP) and assisted by Ultraflo
® L (![]() ), native Okara (
), native Okara (![]() ), FOS (
), FOS (![]() ) and negative control (
) and negative control (![]() ) as substrates. FOS (Orafti® P95): fructo-oligosaccharides. Results are mean values of triplicate analyses and are expressed as log10 cells/ml, and standard deviations. * P<0·05, ** P<0·01, *** P<0·001 are significantly different.
) as substrates. FOS (Orafti® P95): fructo-oligosaccharides. Results are mean values of triplicate analyses and are expressed as log10 cells/ml, and standard deviations. * P<0·05, ** P<0·01, *** P<0·001 are significantly different.
For total bacteria (Eub 338 I- II- III), no differences between treatments were found by two-way ANOVA with Bonferroni post-tests, but t test (P<0·05) showed a prolonged growing stage when HHP+Ultraflo ® L-treated Okara was added as the substrate. HHP+Ultraflo ® L and native Okara showed an in vitro bifidogenic activity (Bif 164) at 4 (both) and 48 h (only HHP+Ultraflo ® L Okara) of fermentation (4 h: HHP+Ultraflo ® L Okara, log10/ml 8·88 (sd 0·09) and native Okara, log10/ml 8·89 (sd 0·15), 48 h: HHP+Ultraflo ® L Okara, log10/ml 9·34 (sd 0·06)) (Fig. 1 and 2) compared with negative control (4 h: log10/ml 8·62 (sd 0·18) and 48 h: log10/ml 9·02 (sd 0·15)). Both treated and native Okara exhibited a significant increase in bifidobacteria up to 8 h (t test, P<0·05).
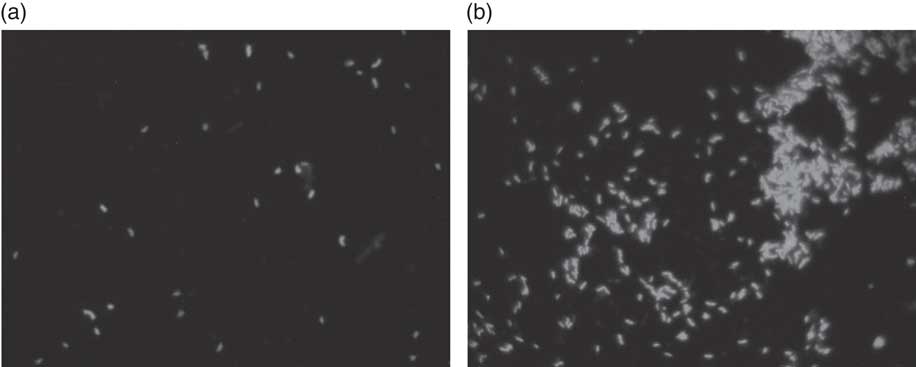
Fig. 2 Fluorescence in situ hybridisation (FISH) analysis of Bifidobacterium in batch culture at 48 h growing on (a) negative control, and (b) Okara treated with HHP and assisted by Ultraflo ® L. ** P<0·001, significantly different.
For lactobacillus/enterococcus spp., an increase at 4 h was noticed for treated and native Okara with two-way ANOVA (log10/ml 8·73 (sd 0·08) and log10/ml 8·72 (sd 0·03) respectively), compared with negative control (log10/ml 8·51 (sd 0·08)). With HHP+Ultraflo ® L-treated Okara, lactobacilli grew constantly, whereas with native Okara the growth was mainly found between 4 and 24 h.
No statistical differences between treatments were found for Bacteroides spp. (Bac 303), C. coccoides and E. rectale group (Erec 482) and the C. histolyticum group (Chis 150). However, Bacteroides spp. significantly increased after 24 h of incubation, whereas treated Okara promoted a lower growth rate at 24 h (HHP+Ultraflo ® L Okara, log10/ml 9·16 (sd 0·13) and native Okara, log10/ml 9·22 (sd 0·10)). An increase in Erec 482 was noticed after 8 h of incubation when treated Okara or when native Okara was added in both cases (t test, P<0·05). Numbers of clostridia only increased in the first 4 h of incubation and decreased after 24 h. Moreover, treated Okara had a smaller Chis 150 population than native Okara, and both were lesser than FOS and negative control. Rfla 729-Rbro 730 (R. albus and R. flavefaciens – C. sporosphaeroides, R. bromii and C. leptum) revealed a low growth rate, with statistical differences between treated Okara and negative control at 4 h (the negative control was higher) and 48 h (HHP+Ultraflo ® L: log10/ml 7·73 (sd 0·08), and negative control: log10/ml 7·53 (sd 0·07)) (Fig. 1). Differences in Clostridial cluster IX (Prop 853) between Okara treated with HHP and assisted by Ultraflo ® L, native Okara and negative control were appreciated at 8 h (HHP+Ultraflo ® L: log10/ml 8·69 (sd 0·21), Okara: log10/ml 8·88 (sd 0·14) and negative control: log10/ml 8·12 (sd 0·49)). Remarkable differences in growth kinetics among all treatments and negative control could be appreciated for Prop 853, as the increase in bacteria was first appreciated at 8 h for every treatment except for negative control, which started at 24 h.
Analysis of SCFA and lactic acid
Differences between both native and HHP+Ultraflo ® L-treated Okara and negative control (P<0·05) and FOS (P<0·01) were appreciated in the production of acetic acid after 24 h of fermentation, whereas changes in propionic acid production were revealed at 8 and 48 h (P<0·001) (Table 2). When comparing total increase in organic acids, HHP+Ultraflo ® L-treated Okara produced 1·12- and 1·36-fold higher acetic acid and propionic acid, respectively, compared with native Okara. No differences in butyric acid production between treatments were appreciated. An increase was only noticed (t test P<0·05) in HHP assisted by Ultraflo ® L-treated Okara after 24 h of fermentation. Nevertheless, butyric acid production was 2·68-fold higher after 48 h of fermentation, and 1·55-fold higher when HHP+Ultraflo ® L-treated Okara was added instead of native Okara. Lactic acid presented differences among treatments at 4 h (native Okara was 2·45- and 2·60-fold higher than FOS and negative control, respectively). After 8 h, lactic acid was not detected. Considerable differences between donors were found for all organic acids. No significant levels of branched-chain fatty acids from the fermentation of resistant protein were found( Reference Henningsson, Björck and Nyman 54 ).
Table 2 SCFA and lactic acid contents of batch cultures with Okara treated with high hydrostatic pressure and assisted by Ultraflo ® L, native Okara, fructo-oligosaccharides (FOS) and a negative control (Mean values and standard deviations, n 3)
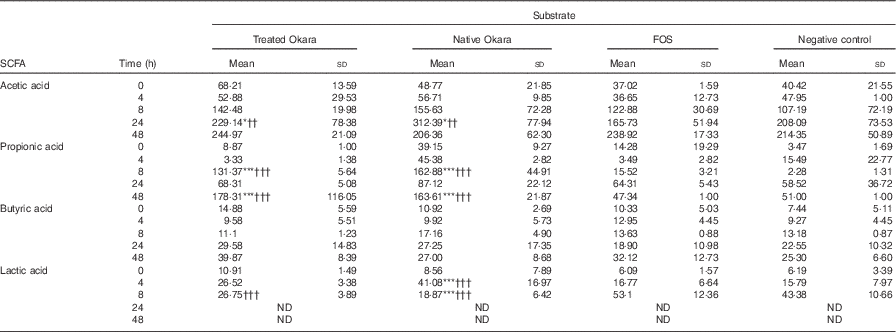
* P<0·05, *** P<0·001, significantly different from negative control. †† P<0·01, ††† P<0·001, Significantly different from FOS.
Discussion
According to our present results, a potential prebiotic effect of native Okara and HHP+Ultraflo ® L-treated Okara has been found, with capacity to promote the growth of beneficial bacteria, including bifidobacteria after 4 and 48 h (Fig. 2) and of lactobacilli after 4 h of in vitro faecal batch culture fermentation simulating the human gut. Previous digestion of Okara was not necessary as it is indigestible( Reference Espinosa-Martos and Ruperez 20 ).
Results obtained from the in vitro batch culture systems suggest that potential prebiotic effect is shown by Okara of soyabean, particularly after HHP treatment (600 MPa, 40°C, 30 min) assisted by Ultraflo ® L (0·025 %), which needs further research to assess the effect in vivo. In fact, differences between samples were noticed, as a bifidogenic effect of treated Okara after 4 and 48 h of batch culture (Fig. 2), whereas native Okara did not bring about such effects at 48 h (Fig. 1). Moreover, even if there were no statistical differences at 8 h in lactobacilli, HHP+Ultraflo ® L-treated Okara performed better, whereas at 4 h differences with the negative control were observed in both Okara samples. Other potentially beneficial bacteria such as the Ruminococcus group showed an increase in HHP+Ultraflo ® L Okara at 48 h. SCFA values also suggested a better potential prebiotic response when treated Okara was fermented, especially in acetic acid (48 h) and butyrate (24–48 h) contents (Table 2).
The prebiotic effect of soyabean oligosaccharides has been previously suggested. For example, raffinose and stachyose have been found to be growth promoters of Bifidobacterium infantis ( Reference Roberfroid, Gibson and Hoyles 7 ). Moreover, Okara can be fermented by Streptococcus thermophilus and L. delbrueckii subsp. bulgaricus ( Reference Tu, Chen and Wang 27 ) and in vitro fermentation by Bifidobacterium bifidum and Lactobacillus acidophilus of native Okara or Okara treated by Ultraflo ® L showed positive results after 48, 72 and 96 h of incubation, with a significant production of acetic, followed by propionic and butyric acids (93:5:2 at 96 h)( Reference Espinosa-Martos and Ruperez 20 , Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 ). In our batch culture experiments (Table 2), acetic acid was also predominant, followed narrowly by propionic acid, with a ratio of 13:12:1 after 8 h of incubation with HHP+Ultraflo ® L Okara and 18:19:2 with native Okara, respectively. These SCFA are a source of energy for the colonic mucosa, stimulate cell proliferation, reduce cholesterol levels and have anti-proliferative effects in colorectal cancer as well as beneficial effect within the muscles, kidneys, brain and heart( Reference Bergman 10 – Reference Slavin 12 ). In our study, however, acetic and propionic acid levels showed differences between treatments (Table 2), only butyrate increased with time. E. rectale is one of the main producers of butyrate in the colon( Reference Flint, Duncan and Scott 55 ), and no statistically significant differences have been detected in FISH (Erec 484) (Fig. 1). On the other hand, the Ruminococcus group (Rfla 729-Rbro 730) also produces butyrate( Reference Shahrul, Kolida and Gibson 56 ), and HHP+Ultraflo ® L-treated Okara was significantly higher than native Okara at 48 h (Fig. 1), despite the low growth rate, which was also found previously by Walker et al.( Reference Walker, Duncan and McWilliam Leitch 46 ). This could explain the reason why HHP+Ultraflo ® L-treated Okara fermentation showed a tendency to increase butyrate production (Table 2), which is the preferred energy source for colonic epithelial cells and promotes normal cell differentiation and proliferation( Reference Slavin 12 ). Bifidobacteria are acetate producers, and acetate increase according to their behaviour has been noticed. Furthermore, the main producer of propionic acid has been reported to be Clostridial cluster IX (Prop 853)( Reference Walker, Duncan and McWilliam Leitch 46 ). At 8 h of incubation, both propionic acid levels and Clostridial cluster IX population were higher in native Okara than HHP+Ultraflo ® L-treated Okara groups. Moreover, differences in SCFA production between FOS and Okara substrates could be observed, especially in propionic acid at 48 h (Table 2). This could be explained by the great complexity of Okara’s cell wall( Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 , Reference Kasai, Murata and Inui 36 , Reference Mateos-Aparicio, Mateos-Peinado and Jimenez-Escrig 57 ), which needs longer time to be fermented, allowing a longer growth rate, than other easily digested molecules such as FOS. Lactic acid, produced by lactic acid bacteria including lactobacilli, bifidobacteria, enterococci and streptococci, increased during the first few hours of fermentation, and then it was no longer detected, probably because of its utilisation by other bacteria. In fact, the production of butyric acid from lactic acid has been previously suggested( Reference Duncan, Louis and Flint 58 ), and agree with our results (Table 2). The results also show that some potential pathogenic bacteria could be inhibited when Okara is fermented. In fact, the C. histolyticum group (Chis 150) exhibited a decrease after 24 h of incubation. Other potentially harmful bacteria such as the Bacteroides–Prevotella group (Bac 303) also showed a decrease after 24 h of incubation and a lower rate at 24 h when HHP+Ultraflo ® L-treated Okara was added instead native Okara. Total bacterial levels remained unchanged among treatments, but with an increase in time, and thus variations appear to be inter-population only, as it has been previously appreciated in artichokes( Reference Costabile, Kolida and Klinder 59 ).
The potential prebiotic effect was enhanced by previous treatment of Okara to maximise its SDF content. The effectivity of HHP and enzymatic hydrolysis to increase the amount of SDF (1·58-fold higher) has been previously reported on Okara( Reference Perez-Lopez, Mateos-Aparicio and Ruperez 28 ). HHP has already been used for the hydrolysis of IDF residue from Okara without enzymatic assistance( Reference Mateos-Aparicio, Mateos-Peinado and Ruperez 21 , Reference Li, Qiao and Lu 22 ). Similarly, the food-grade enzymes Ultraflo ® L and cellulase were used at atmospheric pressure on Okara as a substrate( Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 , Reference Ruperez, Perez-Cozar and Redondo-Cuenca 35 , Reference Kasai, Murata and Inui 36 ), with similar results. In addition, LMWC have been identified after Ultraflo ® L hydrolysis of polysaccharides (arabinans, galactans, arabinogalactans, xylogalactans or glucans) present in Okara, and their potential fermentability by B. bifidus and L. acidophilus has been assessed( Reference Espinosa-Martos and Ruperez 20 , Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 ), which agree with the results of our present study. Kasai et al.( Reference Kasai, Murata and Inui 36 ) found an increase in NS after cellulase treatment of Okara. They reported the difficulty to achieve extensive digestion of Okara, as it is composed of indigestible and complex fibres, which could explain the low amount of SDF found in native Okara (Table 1) by DNS method. With this HHP assisted by Ultraflo ® L treatment, a partial hydrolysis of the indigestible fibre has been achieved( Reference Perez-Lopez, Mateos-Aparicio and Ruperez 28 ), as IDF value decreased with the treatment, increasing the amount of terminal reducing sugars, measured by DNS (Table 1). Besides, according to our previous analysis, Okara contains approximately 32, 15 and 3 g/100 g DM of protein, fat and ashes, respectively, before fat extraction( Reference Espinosa-Martos and Ruperez 20 ). Soluble soyabean carbohydrates released by this treatment have other potential health benefits, such as reduction of cholesterol levels( Reference Lukaczer, Liska and Lerman 60 , Reference Jenkins, Kendall and Marchie 61 ), improvement of glucose tolerance in diabetes, and anti-inflammatory and anti-carcinogenic effects on the digestive tract( Reference Courtois 5 , Reference Roberfroid, Gibson and Hoyles 7 , Reference Slavin 12 ).
All these in vitro fermentability data support the idea that Okara from soyabean has potential prebiotic effects. According to previous studies( Reference Drakoularakou, Rastall and Gibson 1 , Reference Corzo, Alonso and Azpiroz 3 , Reference Gibson, Probert and Van Loo 62 , Reference Tenorio, Espinosa-Martos and Prestamo 63 ), soyabean-derived oligosaccharides have not presented enough evidence to be considered as prebiotics yet, but they are promising candidates. However, although in vivo studies are needed to demonstrate that HHP+Ultraflo ® L-treated Okara selectively stimulates the growth of bacterial groups in the gut that confer health benefits to the host, all these promising results from the in vitro study, in combination with previous results, support the idea that Okara from soyabean has, in fact, potential prebiotic effects, attributable to its SDF content( Reference Prestamo, Ruperez and Espinosa-Martos 19 , Reference Espinosa-Martos and Ruperez 20 , Reference Villanueva, Perez-Cozar and Redondo-Cuenca 23 – Reference Jimenez-Escrig, Tenorio and Espinosa-Martos 25 ). The batch culture fermentation methodology was appropriate for studying the selectivity of fermentation, changes in the main groups of the microbiota and SCFA production( Reference Gibson, Scott and Rastall 4 , Reference Roberfroid, Gibson and Hoyles 7 ). Treatment with HHP (600 MPa, 40°C, 30 min) assisted by Ultraflo ® L (0·025 %) could have enhanced the potential prebiotic effects of Okara according to our results. In addition to its prebiotic effect, Okara is interesting from a nutritional point of view as a complete and healthy by-product from soyabean. Its re-valorisation would have an economic impact and could be used for food applications in bakery and pastry industries as a substitute of cereal flours or as a gluten-free flour for snacks( Reference Waliszewski, Pardio and Carreon 64 ). These are preliminary results, but further in vivo studies are needed to determine whether these potential prebiotic effects possess beneficial health-promoting effects in humans.
Acknowledgements
The authors thank Takazumi from Toofu-Ya S.L. for providing the Okara by-product and Martínez-Gutiérrez from Novozymes Spain, S.A., for providing Ultraflo ® L.
E. P.-L. acknowledges the predoctoral training programme of the Education, Language Policy and Culture of the Basque Government (Spain) (grant no. PRE_2013_1_682) for her work experience contract at the Department of Metabolism and Nutrition of ICTAN-CSIC in Madrid and for providing financial support during her short stay abroad at the Food & Nutritional Sciences Unit, School of Chemistry, Food and Pharmacy, University of Reading, UK (where the batch culture experiments were performed), under the supervision of A. C.
The author contributions are as follows: E. P.-L. was the principal investigator and contributed to the study design, analysis and interpretation of the results and wrote the manuscript; D. C. contributed to data analyses; A. C. contributed to the study design, analyses and supervision; I. M.-A. and P. R. contributed to the study design and supervision.
There are no conflicts of interest to declare.







