Fe-deficiency anaemia remains a major public health problem worldwide. Based on national surveys, the WHO reported that a total of 800 million preschool children and women were anaemic in 2011, representing a global prevalence of 42·6 % in preschool children and 38·2 % in pregnant women and 29·4 % in all women of reproductive age. It is estimated that 42–50 % of global anaemia could be eliminated by improving Fe intakes( 1 ). With a Human Development Index of 0·471, Haiti ranks amongst the twenty countries with the lowest development worldwide( 2 ). The prevalence of anaemia is high in Haiti, with 62 % of children and 37 % of women of reproductive age affected.
Fe fortification of staple foods is considered the most practical, sustainable and cost-effective solution to control Fe deficiency( Reference Zimmermann and Hurrell 3 ). Wheat flour is a staple food in Haiti, with an estimated consumption of about 120 g/d based on the production volume and the total population numbers. Only one flour mill exists in Haiti (Les Moulins d’Haiti), which produces about 60 % of all flour consumed in the country. The remaining 40 % are imported, mainly from the USA. To improve the nutritional situation in Haiti, the government has drafted a new fortification law foreseeing the addition of different micronutrients to flour, palm oil and salt (J Marhone, unpublished results). The currently discussed recommendation for Fe fortification is 30 mg Fe/kg in the form of NaFeEDTA to all wheat flour sold in Haiti.
Fe is probably the most challenging nutrient when it comes to food fortification. Water-soluble Fe compounds generally show the highest bioavailability as they are highly soluble in gastric juices. But they are also most likely to result in organoleptic changes of the fortified products, especially in hot and humid climates. Ferrous sulphate is the most commonly used water-soluble Fe fortificant for flour fortification, but it can cause rancidity and off-colours in stored flours. Ferrous fumarate (FeFum) is an Fe compound insoluble in water but soluble in dilute acid. It has been shown to be as bioavailable as ferrous sulphate in adults but causes less sensory changes in the fortified foods. NaFeEDTA is a water-soluble form of Fe that is better absorbed than ferrous sulphate from flours with a high phytate content. NaFeEDTA does not promote lipid oxidation in stored flours and may produce less sensory changes in these products compared with ferrous sulphate. However, NaFeEDTA is considerably more expensive (approximately 16 times the costs of ferrous sulphate per mg Fe); the costs for FeFum are about twice that of ferrous sulphate( Reference Allen, de Benoist and Dary 4 ).
Helicobacter pylori infection is common worldwide; it is estimated that approximately 50 % of the global population is infected, with especially high rates in low-income countries. Depending on the chronicity of H. pylori infection as well as environmental and host factors, gastric acid production can be either increased, decreased or unchanged in infected persons( Reference Atherton 5 ). Previous studies are equivocal on whether H. pylori infection affects Fe absorption( Reference Ciacci, Sabbatini and Cavallaro 6 , Reference Sarker, Davidsson and Mahmud 7 ).
As costs are an important issue in the flour fortification programme in Haiti, the aim of this study was to investigate Fe absorption from wheat flour fortified with FeFum, NaFeEDTA or a combination of the two in young women in Haiti and their preschool age children. We hypothesised that: (1) Fe absorption would be higher from NaFeEDTA alone or combined with FeFum, compared with FeFum alone, and (2) Fe absorption from both compounds would be lower in individuals infected with H. pylori.
Methods
Participants
Study participants were twenty-two mother–child pairs living in Port au Prince, Haiti. As two of the most vulnerable groups for Fe deficiency, we intended to include both young women and young children in the study. For convenience reasons in terms of logistics during recruitment, we decided to recruit mother–child pairs. The mothers were between 18 and 45 years of age, generally healthy, weighed <65 kg and were neither pregnant nor breast-feeding. The children were between 2·5 and 5 years of age, generally healthy and neither wasted (<−2 z scores for weight for height) nor stunted (<−2 z scores for height for age). Women and children with known metabolic, chronic and gastrointestinal disease, as well as women and children taking long-term medication (except for oral contraceptives for the women) were not included in the study. Further exclusion criteria were as follows: consumption of vitamin and/or mineral supplements 2 weeks before the study and during the study, blood donation or substantial blood loss within 4 months of the beginning of the study. Women and children were only included as pairs. All women provided written informed consent for themselves and their children. The study was conducted according to the Declaration of Helsinki and ethical approval for the study was provided by the ethical review committees at ETH Zurich as well as the Ministry of Health in Haiti. The study was registered at clinicaltrials.gov (NCT02096250).
Study design
The flow of study participants is shown in Fig. 1. A randomised cross-over design with single meals was chosen for test meals A (FeFum) and B (NaFeEDTA), where each women and child served as their own control. This design was chosen to eliminate a potential effect of the first test meal on Fe absorption of the second meal. Test meal C (FeFum+NaFeEDTA) was given to all the women at the same time point for technical reasons. In total, each woman consumed three test meals and each child consumed two meals.

Fig. 1 Study design and participant flow chart. FeFum, ferrous fumarate; FeFum+NaFeEDTA, ferrous fumarate and sodium iron EDTA at a 1:1 ratio for iron.
In the week before test meal consumption, interested study participants were invited to a community health centre in Port au Prince for an information session. They had to present at the health centre in the morning after an overnight fast (no food and drinks after midnight). The study was explained to them in detail by the staff of the Ministry of Health of Haiti. After the information, each woman was individually talking to one of the investigators and informed consent was obtained from those women still willing to participate. Consenting women and their children were directly screened: weight and height were measured and the general health status was assessed using a questionnaire. Following this procedure, women had to provide a small urine sample for a pregnancy test. In addition, a venous blood sample was collected (4 ml into a heparin-coated tube) for baseline measurements of Fe status, inflammation and presence of H. pylori.
On all study days, study participants presented at the health centre in the morning after an overnight fast (no food or drinks after midnight). In the week after screening, study participants presented at the health centre on 2 consecutive days for the consumption of test meals A and B, one on each day. The order of the two meals was randomised for each participant by using a random number list generated in EXCEL. A period of 14 d after consumption of the second test meal, a venous blood sample (4 ml into an EDTA-coated tube and 4 ml into a heparin-coated tube) was taken from each participant (women and children) for the determination of Hb, Fe-stable isotopes incorporated into erythrocytes as well as Fe and inflammatory status. Directly following blood sampling, test meal C was consumed by the women only. A last blood sample was taken from the women another 14 d later in the same way as described above. All test meals were labelled with the stable Fe isotopes 57Fe and 58Fe. Per test meal, 4 mg of Fe was administered to the women and 2 mg of Fe was administered to the children. 57Fe was used to label the FeFum in test meal A and 58Fe to label the NaFeEDTA in test meal B. In test meal C, both Fe compounds were labelled with 57Fe. The amount of Fe used in the test meal was chosen to simulate the fortification level currently discussed by the Haitian government.
Test meals
All test meals consisted of white bread rolls with processed cream cheese (La Vache qui Rit; Fromageries Bel) and a carbonated beverage (Sékola Citron; Séjourné). For each test meal, the women received two bread rolls (approximately 94 g) with 30 g of cream cheese and 410 g of Sékola Citron. The children received one bread roll (approximately 47 g) with 15 g of cream cheese and 160 g of the same beverage. The bread rolls were specifically prepared for the study by a local baker using non-fortified wheat flour (77 % extraction) from the Haitian mill. The bread rolls were prepared from one single batch of flour collected directly at the mill but at two time points (for test meals A and B together and again for test meal C). Bread rolls were re-heated in an oven before consumption and cut into halves. Isotopes, pre-weighed in individual doses, were added to one side of the bread before spreading the cheese on top using a disposable plastic knife for each participant. The FeFum (57Fe) was added in a powder form from glass vials directly onto the bread. After emptying the vial, it was rinsed twice with 0·25 ml water, which was also poured on the bread. The NaFeEDTA was added in a liquid form from Teflon vials directly onto the bread. The vials were rinsed once with 0·5 ml of water, which was also poured on the bread.
Preparation of isotopically labelled iron
Isotopically labelled 57Fe fumarate was prepared from isotopically enriched elemental Fe (Dr Paul Lohmann) and isotopically labelled Na58FeEDTA and Na57FeEDTA was prepared using the method described by Loots et al.( Reference Loots, Lieshout and Lachmann 8 ). Isotopic enrichment was 96·3 % for 57Fe and 99·86 % for 58Fe.
Blood analysis
Blood samples were stored in a cool box at the health centre until transport to the laboratory following completion of sampling each day. All heparinised samples were centrifuged and plasma aliquoted and stored at −20°C until analysis. EDTA blood was used for the analysis of Hb on the day of sampling using an automated haematology analyser (Sysmex KX-21N; Sysmex). The remaining EDTA blood was aliquoted and stored at −20°C until analysis of isotopic composition. All samples were shipped to ETH Zurich on dry ice for analysis. Serum ferritin (SF), soluble transferrin receptor (TfR), C-reactive protein (CRP), α1-acid glycoprotein (AGP) and retinol binding protein (RBP) were determined using the multiplex ELISA method described by Erhardt et al.( Reference Erhardt, Estes and Pfeiffer 9 ). H. pylori was determined using a qualitative rapid test kit (D-HPD20, Rapid Anti-H. Pylori Test; Rapid Labs). This test has a sensitivity of 94·88 % and a specificity of 95·38 % compared with the standard ELISA method.
Body Fe stores (BIS) were calculated from SF and TfR using the following equation: BIS (mg/kg)=−(log(TfR (mg/l)/(SF (μg/l)/1000))−2·8229)/0·1207. Acute inflammation was defined as CRP concentrations >5 mg/l for both women and children, and chronic inflammation was defined as AGP>1 g/l. Fe deficiency was defined using either SF or TfR with the following cut-offs: mothers: SF<15 μg/l in subjects without inflammation and SF<30 μg/l in subjects with elevated CRP or AGP, TfR>8·3 mg/l; children: SF<12 μg/l and SF<30 μg/l in subjects with elevated CRP or AGP, TfR>8·3 mg/l. Vitamin A deficiency was defined as RBP concentrations<0·7 μmol/l and moderate vitamin A deficiency was defined as RBP<1·05 μmol/l.
Each isotopically enriched blood sample was analysed in duplicate for its isotopic composition under chemical blank monitoring. Whole blood was mineralised by microwave digestion and Fe was separated by anion-exchange chromatography, followed by a precipitation step with ammonium hydroxide( Reference Walczyk, Davidsson and Zavaleta 10 ). All isotopic analyses were performed by negative thermal-ionisation MS (MAT 262; Finnigan MAT).
Calculation of iron absorption
Total circulating Fe was calculated based on blood volume, which was estimated based on weight and height using the equation by Brown et al.( Reference Brown, Hopper and Hodges 11 ). For the calculation of fractional absorption, 80 % incorporation of the absorbed Fe into the erythrocytes was assumed( Reference Walczyk, Davidsson and Zavaleta 10 ). For the calculation of absorbed Fe from test meal C, the isotopic composition in the blood sample taken on day 16 was used as the baseline.
Statistical analysis
Based on data from previous Fe-absorption studies at our laboratory, we calculated a sample size of twenty subjects to be sufficient to detect a difference of 30 % in fractional Fe absorption between meals with a β of 0·8 and an α of 0·05. To account for potential dropouts during the study, we increased the sample size by 10 % and aimed to include twenty-two women with their children. Statistical analysis was done using SPSS 22 (IBM SPSS Statistics). All data were checked for normal distribution before analysis. Normally distributed data are presented as means and standard deviations (age, height, weight, Hb, BIS) and non-normally distributed data are presented as geometric means and 95 % CI (SF, TfR, CRP, AGP, RBP, fractional Fe absorption). Comparisons of fractional Fe absorption between test meals were done using repeated-measures ANOVA with post hoc Bonferroni correction for the mothers and a paired-samples t test for the children. Associations between Fe absorption and parameters of Fe status as well as inflammatory status were investigated using Pearson’s correlations. The level of significance was defined as P<0·05.
Results
The study population consisted of twenty-two women (mean age 28·3 (sd 7·3) years) and their twenty-two children aged between 2·5 and 5 years (mean age 3·6 (sd 0·7) years). All women consumed all three test meals, but absorption results from two women had to be excluded because no enrichment could be detected in the final blood samples. Absorption data of three children are missing for test meal A and of two for test meal B. Three of those values are missing because the children did not consume the entire test meal with the isotopes and two because no blood sampling was possible from the child at endpoint. Baseline blood samples were available from all women and children.
Anthropometric characteristics as well as Fe and inflammatory status of all study participants are shown in Table 1. Seven children and one woman had CRP concentrations >5 mg/l, indicating acute inflammation. Furthermore, five mothers and fourteen children had elevated AGP concentrations (>1 g/l). Seven of the children had Hb concentrations <110 g/l and were thus diagnosed as anaemic, and five women had Hb concentrations <120 g/l, the cut-off for anaemia in women. Based on a low SF, 27 % of women and none of the children were Fe deficient. Based on an elevated TfR, 23 % of women and 64 % of children were Fe deficient.
Table 1 General characteristics of the study population (Mean values and standard deviations; geometric means and 95 % confidence intervals)

CRP, C-reactive protein, AGP, α1-acid glycoprotein; RBP, retinol binding protein.
Geometric mean fractional Fe absorption from the different test meals and the total amount of Fe absorbed from the labels are shown in Table 2. Fractional absorption was not significantly different comparing mothers with children for either test meal. For both groups, fractional absorption was significantly higher from meal B (NaFeEDTA) compared with meal A (FeFum) (P=0·029). Absorption from test meal C (FeFum+NaFeEDTA) was not significantly different from test meals A or B (P>0·05), and showed an intermediate value. Fe absorption of the women from all three test meals is shown in Fig. 2. The total amount of Fe absorbed from the labels was not significantly different comparing the three meals in women (P>0·05), while it was significantly higher in the meal with NaFeEDTA compared to FeFum in children (P=0·002).

Fig. 2 Fractional iron absorption in mothers from three different iron compounds in an iron absorption study in Haiti (n 22). A, ferrous fumarate; B, NaFeEDTA; C, ferrous fumarate+NaFeEDTA.
Table 2 Fractional iron absorption and amount of iron absorbed from different test meals in mothers and their children in Haiti (Geometric means and 95 % confidence intervals)
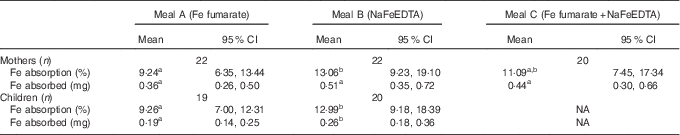
NA, not applicable.
a,b Mean values with unlike superscript letters were significantly different (repeated-measures ANOVA with post hoc Bonferroni correction for the mothers, paired-samples t test for the children, P<0·05).
In all, nineteen of the women were H. pylori positive and only two were negative. Of the children, thirteen were H. pylori positive and eight were negative (for the remaining children, sufficient serum was not available to determine an infection). The Fe-absorption values in women and children grouped by infection status are shown in Table 3. There was no significant difference in Fe absorption comparing H. pylori positive with negative study participants among both women and children. However, the small numbers of women who were H. pylori negative limit comparisons in this group. When comparing Fe absorption between H. pylori-positive and -negative study participants, the combined data from both women and children for test meals A and B showed no significant differences (P=0·226 for meal A and P=0·397 for meal B).
Table 3 Fractional iron absorption comparing Heliobacter pylori-negative and -positive subjectsFootnote * (Geometric means and 95 % confidence intervals)
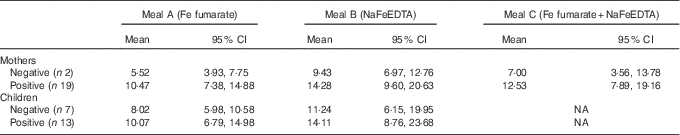
NA, not applicable.
* For mothers and children and for each test meal, values were compared between H. pylori-positive and -negative subjects using an independent-samples t test. No significant differences were detected for any of the groups (P>0·05).
Fe absorption was significantly higher from the NaFeEDTA meal compared with the FeFum meal in the H. pylori-positive children (P=0·010). The difference was not significant in the negative children (P=0·113). The differences between the three meals were not significant in either group of women. In mothers, log Fe absorption from both test meals was significantly negatively correlated with SF, BIS, CRP and AGP (all log data, P<0·05). Furthermore, log Fe absorption from test meal B was significantly positively correlated with log TfR (r 0·567, P=0·006), whereas the correlation was not significant for test meal A (r 0·376, P=0·085). In children, the only significant correlation was between log Fe absorption from test meal A and log CRP (r −0·483, P=0·036). In a linear stepwise regression model with Fe absorption as the dependent variable and CRP, AGP, BIS and H. pylori as independent variables, only CRP remained a significant predictor of Fe absorption from test meal A (mothers (CRP): R 2 0·337, P=0·006, β=−0·581; children (CRP): R 2=0·233, P=0·036, β=−0·483). The overall predictive value of the model increased with all independent variables included (mothers: r 2 0·447, children: r 2 0·295), but none of them showed a significant prediction independently (all P>0·05). Using SF instead of BIS in the regression model did not change the results.
Discussion
Wheat flour is the vehicle most frequently chosen for large-scale Fe-fortification programmes and programmes were in place or in planning in seventy-eight countries worldwide in 2008( Reference Hurrell, Ranum and de Pee 12 ). In the draft of the national flour fortification programme of Haiti, it is suggested to use 30 mg Fe in the form of NaFeEDTA per kg of flour (J Marhone, unpublished results). However, NaFeEDTA is the most expensive of the common Fe fortificants and the additional costs may jeopardise the success of the programme. Furthermore, as the flour produced and used in Haiti is low-extraction flour (77 % extraction) with a relatively low phytic acid content, the benefit of using NaFeEDTA over other fortificants such as FeFum is unclear. In this study, we have therefore investigated the bioavailability of NaFeEDTA, FeFum, as well as a combination of the two, from a test meal based on wheat flour in young women and their children in Haiti.
Our results show that despite the low extraction rate of the flour, NaFeEDTA was significantly better absorbed (+40 %) by both women and children compared with FeFum. Considering an estimated daily consumption of wheat flour in adults of 120 g, a fortification level of 30 mg Fe/kg and the measured Fe bioavailability in this study in women, the anticipated amount of absorbed Fe would be 0·47 mg/d for NaFeEDTA and 0·33 mg/d for FeFum. Assuming daily Fe needs of women of childbearing age of 1·4 mg, this absorbed amount would only cover 33 % in the case of NaFeEDTA and 24 % in the case of FeFum and may therefore be insufficient to achieve adequate Fe nutrition in the population. Increasing the amount of absorbed Fe could either be achieved by improving the bioavailability or by increasing the fortification level. The WHO recommendation for wheat flour fortification in low-extraction flour and with a consumption of 75–149 g flour/d is 40 mg Fe in the form of NaFeEDTA/kg flour or 60 mg Fe as FeFum/kg flour( 13 ). Using these fortification levels, based on our absorption data, the anticipated amount of absorbed Fe from 120 g wheat flour would be 0·63 mg when using NaFeEDTA and 0·67 mg for FeFum. Thus, using a 50 % higher fortification level with FeFum might result in an equal amount of absorbed Fe and therefore could be more cost-effective.
An advantage of a fortification mixture of FeFum and NaFeEDTA is that the EDTA moiety may enhance Fe absorption from both Fe compounds as well as the native Fe in the meal. In infants and children consuming inhibitory meals fortified with FeSO4, the addition of NaFeEDTA or Na2EDTA enhances Fe absorption by about 50 %( Reference Davidsson, Walczyk and Zavaleta 14 – Reference Chavasit, Porasuphatana and Suthutvoravut 16 ). Whether EDTA can enhance Fe absorption from FeFum is uncertain( Reference Walter, Pizarro and Olivares 17 , Reference Fidler, Davidsson and Zeder 18 ). In our study, the absorption from a combination of NaFeEDTA and FeFum did not differ significantly from FeFum alone, suggesting that, in low-extraction wheat flour, there is no strong enhancing effect of the EDTA moiety of Fe absorption from co-fortified FeFum.
In acute and chronic inflammation, the concentrations of cytokines, such as IL-6, are increased, stimulating hepcidin production( Reference Dunn, Rahmanto and Richardson 19 , Reference Viatte and Vaulont 20 ). Increased circulating hepcidin concentrations can then reduce dietary Fe absorption( Reference Weiss 21 ). In this study, a high number of subjects, especially in the group of children, showed either acute or chronic inflammation as determined by CRP and AGP, respectively, which may have influenced the results of the Fe-absorption studies. This is suggested by the significant negative associations shown between Fe absorption and inflammatory markers in both groups. Another important predictor of hepcidin and consequently Fe absorption is Fe status( Reference Dunn, Rahmanto and Richardson 19 , Reference Viatte and Vaulont 20 ). In the women, where only five subjects were affected by inflammation, the expected inverse relationship between Fe absorption and Fe status was apparent: there were significant associations between Fe absorption and Fe status (SF and TfR). In contrast, in the children, these associations were not visible, possibly because of the much higher rate of inflammation, and the confounding effect of inflammation on SF, an acute-phase protein( 22 ).
H. pylori infection has repeatedly been associated with Fe-deficiency anaemia( Reference Barabino, Dufour and Marino 23 – Reference Marignani, Angeletti and Bordi 25 ). Although these conditions may simply cluster together in low-resource settings, one possible explanation for a causal effect is reduced Fe absorption from the diet as a result of impaired gastric acid secretion( Reference Sarker, Davidsson and Mahmud 7 , Reference Berg, Bode and Blettner 24 ). Ciacci et al.( Reference Ciacci, Sabbatini and Cavallaro 6 ) investigated Fe absorption using the serum Fe appearance method in H. pylori-positive and -negative adults. Fe appearance was significantly higher in H. pylori-negative compared with -positive subjects and it improved significantly in the latter group after antibiotic treatment. However, in this study, the administered Fe was already in the ferrous form (Fe2+), whereas most dietary non-haeme Fe is in the ferric form (Fe3+) and needs to be reduced before absorption into enterocytes( Reference Zimmermann and Hurrell 3 ). Thus, it is unclear whether the effect of H. pylori infection on absorption from the Fe administered in the study by Ciacci et al.( Reference Ciacci, Sabbatini and Cavallaro 6 ) would be comparable with its effect on dietary Fe.
Sarker et al.( Reference Sarker, Davidsson and Mahmud 7 ) investigated gastric acid secretion and Fe absorption in children in Bangladesh. Even though they found lower gastric acid output in H. pylori-infected children, Fe absorption from test meals with FeFum and ferrous sulphate did not differ significantly from the non-infected children. In our present study there was a tendency towards higher, rather than lower Fe absorption in H. pylori-positive subjects, both in the group of mothers and in children. However, the sample sizes in the subgroups were small; there were only two H. pylori-negative mothers and seven H. pylori-negative children, which made a statistical comparison difficult. Nevertheless, the trends observed were similar to those observed by Sarker et al., where absorption values for H. pylori-negative subjects were also lower for ferrous sulphate, although not significantly( Reference Sarker, Davidsson and Mahmud 7 ). The pathogenesis of H. pylori infection shows large variations depending on the virulence of the infecting H. pylori strain, the extent and type of the host immune response as well as other modulating factors such as diet and smoking. Depending on the location and type of gastric inflammation, H. pylori infection can result in increased or decreased secretion of gastric acid. Which kind of inflammation develops is not well understood to date, but host genetics as well as environmental factors seem to play a role besides strain type( Reference Atherton 5 ). We did not determine gastric acid secretion in our study, but one possible explanation for our findings may be that our subjects may have higher rather than lower gastric acid secretion with H. pylori infection.
Our study has several strengths. We used Fe-stable isotopes to accurately quantify Fe bioavailability from Haitian wheat flour in both young women and children, two key target groups for Fe-fortification programmes, and directly compared three different Fe fortificants. Our study also has limitations: not all of the subjects were Fe deficient and this may have contributed towards lower fractional absorption of the fortificant Fe; the low numbers of H. pylori-negative subjects limited comparisons based on H. pylori status and the power of the study would allow us to only detect a difference of at least 30 % in fractional Fe absorption between meals. In conclusion, in Haitian women and children consuming low-extraction wheat flour, Fe absorption from NaFeEDTA was 40 % higher than from FeFum, and the combination of FeFum and NaFeEDTA did not lead to a significant increase in Fe absorption compared with FeFum alone. In the context of Haiti, where the high costs of NaFeEDTA may not be affordable, the use of FeFum 60 mg Fe/kg flour may be a preferable, cost-effective fortification strategy.
Acknowledgements
The authors would like to thank all women and children who participated in the study. Further thanks are due to the study team of the Ministère de la Santé Publique et de la Population in Port au Prince for their support during the study and to Christophe Zeder, Adam Krzystek and Jürgen Erhardt for their support with sample analysis.
This study was funded by the International Atomic Energy Agency (IAEA).
I. H.-A., C. U. L. and M. B. Z. formulated the research question and designed the study; I. H.-A., K. E. and J. M. P. carried out the field work and collected the samples; Y. R. was responsible for laboratory analyses; and I. H.-A. carried out the statistical analysis and wrote the first draft of the manuscript. All authors have reviewed the manuscript.
None of the authors has any conflicts of interest to declare.







