CVD, as a result of an atherosclerotic process, are the leading cause of morbidity and mortality in developed countries, in which the diet is one of the most important environmental factors associated with the atherothrombotic process. However, epidemiological studies have shown a lower incidence of CHD in populations of Mediterranean countries(Reference Keys1, Reference Trichopoulos and Lagiou2), a circumstance that could be attributed to the beneficial health effects of the so-called Mediterranean diet(Reference Trichopoulou, Costacou and Bamia3), which is characterised by a high intake of vegetables, legumes, fruits and nuts, together with a widespread use of olive oil(Reference Willett, Sacks and Trichopoulou4). Nowadays, it is recognised that many of the nutrients in these foods have some protective effects against the atherogenic process. In the Mediterranean region, three nuts (walnuts, almonds and hazelnuts) are used to constitute part of the dietary source of energy, but have not been included in many dietary recommendations due to their high fat content(Reference Fraser5–Reference Ros and Mataix7). Nevertheless, recent clinical evidence suggests that consumption of nuts promotes a healthy lipid profile associated with a lower risk of CVD(Reference Abbey, Noakes and Belling8–Reference Sabate, Oda and Ros16). Several studies have attributed this quality to their nutrients, mainly their MUFA and PUFA. Apart from that, nuts are complex foods that are sources of other nutrients: vegetable protein, fibre, vitamins, minerals and many bioactive constituents such as antioxidants, phytosterols and other phytochemicals(Reference Sabate, Ros and Salas-Salvado6, Reference Kris-Etherton, Yu-Poth and Sabate17). To provide an experimental understanding of the results of epidemiological studies in human subjects and to tackle the possible beneficial mechanisms of nut intake in atherosclerotic lesions, apoE-deficient mice were used in the present experimental study. The animals in this model develop spontaneous atherosclerosis, even with low-fat and low-cholesterol diets, and the disease features are similar to those observed in humans and other species(Reference Sarria, Surra and Acin18). Therefore, the aim of the present study was to compare the effect of supplementation with a mixture of three nuts on the development of atherosclerotic lesions with that of an isoenergetic diet containing palm oil in apoE-deficient mice.
Materials and methods
Animals
Female and male apoE-deficient mice (n 23 each group), aged 2·7 (sem 0·03) months from a C57BL/6J × Ola129 genetic background, were fasted for 6 h and anaesthetised with isoflurane (Forane), and their blood was drawn from the retro-orbital plexus. Then, plasma cholesterol was determined. For each sex, two groups with similar plasma cholesterol concentrations were established. The animals had free access to food and water during the 12-week experimental period, and they were housed in a temperature-controlled facility and handled observing the criteria from the European Union for care and use of laboratory animals in research. The protocol was approved by the Ethics Committee for Animal Research of the University of Zaragoza.
Diets
In the present study, we established two study groups, one of which received a chow diet (Teklad Mouse/Rat Diet no. 2014, Harlan Teklad; Harlan Ibérica) supplemented with 3 % (w/w) edible whole nuts (nut group) in a mix composed of 50 % walnuts, 25 % almonds and 25 % hazelnuts (n 11 for both female and male mice). This represents a daily dose of 3 g nuts/kg body weight per mouse which, considering the higher metabolic rate of mice(Reference Demetrius19), would translate into the dose of 30 g/d or 0·4 g nuts/kg used in human interventions and the same proportion of nuts, so as to reproduce in mice the effects observed in human subjects(Reference Estruch, Martínez-González and Corella20–Reference Salas-Salvado, Fernandez-Ballart and Ros22). The other group received the same chow diet but was supplemented with 2 % (w/w) palm oil. Both diets were isoenergetic and provided the same amount of fat and MUFA (Table 1). Diets were prepared by milling the chow diet, adding the corresponding supplement and 3·0 % (w/w) of wheat starch as agglutinant to facilitate pellet formation, and then drying at 60°C for 48 h. All diets were prepared weekly and stored in an N2 atmosphere at − 20°C. Fresh food was provided daily. Preparation of extractable and non-extractable phenolic compounds from the diets was carried out according to Arranz et al. (Reference Arranz, Saura-Calixto and Shaha23) and quantified using the method of Singleton et al. (Reference Singleton, Orthofer and Lamuela-Raventós24). The contents of extractable phenolic compounds and hydrolysable polyphenols were higher (21 and 26 %, respectively) in the nut diet than in the palm oil diet (Table 1). The animals were fed the experimental diets for 12 weeks; both were well tolerated.
Table 1 Characteristics of the experimental diets

P:S, polyunsaturated:saturated ratio.
Experimental procedures
At 1 week before killing and after fasting, blood samples were obtained for integrin αM (ITGAM) and integrin α4 (ITGA4) expressions and paraoxonase activity. At killing, the animals were euthanised by suffocation with CO2 and exsanguinated by cardiac puncture. The liver was split; one piece was stored in neutral buffered formaldehyde for histological analysis and the remainder immediately frozen in liquid N2. The heart and the arterial tree were perfused with 5 ml of cold PBS (pH 7·4) under physiological pressure. After that, the heart was removed, embedded in OCTTM (Tissue-Tek), frozen in dry ice-cooled isopentane (Panreac) and stored at − 80°C until analysis.
Plasma analysis
Total plasma cholesterol and TAG concentrations were measured in a microtitre assay, using Infinity™ commercial kits (Thermo Scientific). Plasma HDL-cholesterol was quantified in the supernatant by a fluorometric enzyme assay (Amplex Red; Molecular Probes) after precipitation of apoB particles with phosphotungstic acid–MnCl2 (Roche). Paraoxonase was assayed as arylesterase activity determined by the rate of phenylacetate hydrolysis, as described previously(Reference Acin, Navarro and Carnicer25). ApoA1 and apoA4 were quantified by ELISA using specific polyclonal antibodies (Biodesign and Santa Cruz Biotechnology), as described previously(Reference Navarro, Carpintero and Acin26). Plasma lipoprotein profile was determined in 100 μl of pooled plasma samples from each group by fast protein liquid chromatography gel filtration(Reference Calleja, Paris and Paul27) using a Superose 6B column (Amersham Pharmacia), and the cholesterol in each fraction was measured with the fluorescent method described above.
Blood cell analysis
Expression of ITGAM and ITGA4 proteins in blood cells from samples taken 1 week before killing was analysed using a fluorescence-activated cell sorter (Becton-Dickinson), as described previously(Reference Acin, Navarro and Perona28). The results are expressed as the proportion (%) of the marker-positive cells recovered in the region corresponding to monocytes.
LDL oxidation susceptibility
LDL oxidisability was assessed using modified procedures of Navab et al. (Reference Navab, Hama and Hough29) to determine the presence of reactive oxygen species by measuring the conversion of 2′,7′-dichlorofluorescein diacetate into fluorescent dichlorofluorescein. Briefly, LDL fractions (5 μg cholesterol) separated by fast protein liquid chromatography were incubated, at 37°C with 2 μg dichlorofluorescein, in 25 μl of 0·1 % sodium azide and 100 μl PBS, up to a total volume of 150 μl. Fluorescence was measured after 3 h of incubation at an excitation wavelength of 485 nm and an emission wavelength of 535 nm(Reference Arbones-Mainar, Navarro and Carnicer30).
Evaluation of atherosclerotic lesions
Sections of the proximal aorta from the frozen heart were sliced on a cryostat (Microm HM505E; Thermo Scientific). From each heart, four cryosections (6 μm) were fixed with neutral buffered formaldehyde, stained for lipids with Sudan IV and counterstained with haematoxylin and eosin (Harris solution; Sigma Chemical Company). Images were captured by a digital camera fitted to a Nikon microscope. Aortic lesion areas were blindly quantified using Scion Image software (Scion Corporation).
Hepatic histological analysis
Liver samples stored in formaldehyde were embedded in paraffin. Then, sections (4 μm) were stained with haematoxylin and eosin and images were captured. Hepatic fat content was evaluated by quantifying the lipid droplets in each liver section with Adobe Photoshop CS2 (Adobe Systems Incorporated) and expressed as a percentage of the entire liver section(Reference Guillen, Acin and Navarro31).
RNA isolation
RNA was isolated using TRI Reagent Solution (Ambion; Applied Biosystems). Contaminant DNA was removed by TURBO DNA-free™ DNase treatment and removal reagents from Ambion. RNA was quantified by absorbance at A 260/280 (the A 260/280 ratio was greater than 1·75). The integrity of 28S and 18S ribosomal RNA was verified by agarose gel electrophoresis followed by ethidium bromide staining, and the 28S:18S ratio was greater than 2.
Quantification of mRNA
Equal amounts of DNA-free RNA from each sample of all animals were used in quantitative RT-PCR analyses. First-strand complementary DNA synthesis and PCR were performed using the First-Strand complementary DNA Synthesis Kit (Fermentas) and Power SYBR® Green PCR Master Mix (Applied Biosystems) according to the manufacturers' instructions and as described previously(Reference Arbones-Mainar, Navarro and Acin32). Primers were designed by Primer Express® (Applied Biosystems) and checked by BLAST analysis (NCBI) to verify specificity and selective amplification of the target gene, as well as to ensure amplification of complementary DNA and not of genomic DNA. The sequences were as follows: for Pla2g7 – sense, 5′-GCG TTT GTA CTA CCC AGC TCA AGA-3′, antisense, 5′-TGC AGG AGT TGT CAG AGA ACCA-3′; for prenylcysteine oxidase 1 (Pcyox1) – sense, 5′-GGT TCA GTC ATT CAC CCC CTA A-3′, antisense, 5′-TAC AAA CCA GCT GCT CTC CTC A-3′; for paraoxonase 2 (Pon2) – sense, 5′-GCA CGC TGG TGG ACA ATT TATC-3′, antisense, 5′-TGT CAC TGAT GGC TTC TCG GAT-3′. Real-time PCR were performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems) following the standard procedure. The specificity of the PCR was confirmed by observing a single dissociation curve. The relative amount of all mRNA was calculated using the comparative 2− ΔΔCq method. Ppib mRNA was used as the reference gene(Reference Guillen, Navarro and Arnal33).
Statistical analyses
Data were analysed by the Kolmogorov–Smirnov test to check for the normal distribution of variables and by Bartlett's test to assess the homogeneity of variance. Student's t test was used when both tests satisfied the corresponding hypothesis, and when either of these tests did not, differences between pairs were tested using the Mann–Whitney U test. Unless otherwise stated, results are presented as means with their standard errors. Correlations between variables were tested by calculating Spearman's correlation coefficient using SPSS software, version 15.0 (SPSS, Inc.). Differences were considered significant when P< 0·05.
Results
Body and liver weight
Somatic variables in the animals are shown in Table 2. At killing, males and females had significant differences in body weight (P< 0·001) and body-weight changes (P= 0·01). However, we found no significant differences between the two diets in terms of body-weight change, liver weight, feed intake or energy intake.
Table 2 Effect of the experimental diets on somatic variables in apoE-knockout mice (Mean values with their standard errors)
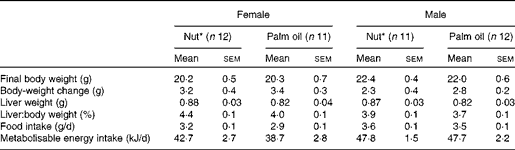
* Nut, diet supplemented with 3 % nuts, composed of 50 % walnuts, 25 % almonds and 25 % hazelnuts. Statistical analysis was carried out using Student's t test.
Plasma lipid parameters
The effect of the different diets on plasma lipid parameters after the 12-week experimental period is shown in Table 3. The same-sex groups had similar initial cholesterol levels, which were significantly higher in males than in females, and this difference remained after the experimental period. In females, no significant differences were observed between the two diets in terms of total cholesterol. However, male mice consuming the nut diet had significantly lower plasma cholesterol than those in the palm oil group. There were no significant differences in plasma HDL-cholesterol in the two dietary groups. VLDL+LDL-cholesterol were significantly lower in mice of both sexes receiving the nut diet. Plasma TAG levels were higher (P< 0·001) in males than in females, but neither sex showed significant diet-related differences. ApoA1, the principal protein in HDL particles, and in agreement with the results for HDL-cholesterol, was not modified by the dietary intervention. ApoA4 was significantly decreased in males on the nut diet. A significant increase was observed in arylesterase activity in female mice receiving the nut diet.
Table 3 Effect of dietary intervention on plasma parameters in apoE-knockout mice according to sex (Mean values with their standard errors)
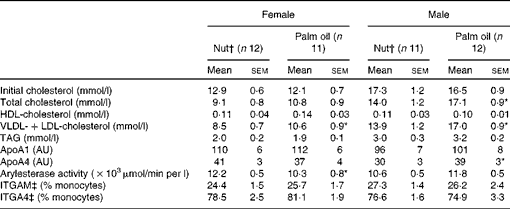
AU, arbitrary units; ITGAM, integrin αM; ITGA4, integrin α4.
* Mean values were significantly different from those of the nut diets (P< 0·05; Mann–Whitney U test).
† Nut, diet supplemented with 3 % nuts, composed of 50 % walnuts, 25 % almonds and 25 % hazelnuts.
‡ ITGAM and ITGA4 protein levels are shown.
Monocyte activation
ITGAM and ITGA4 protein expressions of circulating monocytes are also shown in Table 3. The percentages of monocytes expressing ITGAM and ITGA4 showed no significant differences between the diets in either sex.
Quantification of atherosclerotic lesion area
Fig. 1 shows the atherosclerotic lesion area in apoE-knockout mice consuming the different diets. After consumption of a diet supplemented with nuts or palm oil for 12 weeks, the aortic atherosclerotic lesion areas tended to show higher values in males than in females, although the difference did not reach statistical significance (94·1 (sem 12·0) v. 70·8 (sem 7·3) × 103μm2, P< 0·11). A sex-related difference in the pattern of response to the different diets was noted; while female mice fed the nut diet showed significantly lower values than those on the palm oil diet, this effect was not observed in males receiving the same diets. Moreover, males consuming the nut diet showed two types of response: one group of mice (n 6) presented a marked reduction in lesion area (34·5 (sem 8·4) × 103μm2), while the other (n 5) showed a considerable increase (158·5 (sem 9·6) × 103μm2), suggesting an individual genetic response to nut supplementation.

Fig. 1 Effect of supplementation with nuts or palm oil on aortic atherosclerotic lesions in apoE-knockout mice according to sex. Blinded quantification of atherosclerotic lesion areas was carried out in aortic cross-sections from female mice receiving the nut (○) and palm oil (●) diets and male mice receiving the nut (△) and palm oil (▲) diets, respectively. Individual data and medians are shown. * Median values were significantly different from those of the nut diet (P= 0·05; Mann–Whitney U test).
Oxidative stress variables
As shown in Fig. 2(a), LDL prepared from the nut groups showed significantly lower levels of reactive oxygen species than LDL from palm oil-fed animals in both sexes. The decrease was particularly important in females.

Fig. 2 Effect of supplementation with nuts (□) or palm oil (![]() ) on LDL reactive oxygen species (ROS) content and hepatic paraoxonase 2 (Pon2) and prenylcysteine oxidase 1 (Pcyox1) mRNA expressions according to sex. (a) Presence of ROS in LDL prepared from the different experimental groups and assayed as described in the Materials and methods section. (b) Hepatic Pon2 mRNA expression. (c) Hepatic Pcyox1 mRNA expression. Data are presented as box plots showing medians and interquartile ranges. * Median values were significantly different from those of the nut diet (P< 0·05; Mann–Whitney comparison between pairs).
) on LDL reactive oxygen species (ROS) content and hepatic paraoxonase 2 (Pon2) and prenylcysteine oxidase 1 (Pcyox1) mRNA expressions according to sex. (a) Presence of ROS in LDL prepared from the different experimental groups and assayed as described in the Materials and methods section. (b) Hepatic Pon2 mRNA expression. (c) Hepatic Pcyox1 mRNA expression. Data are presented as box plots showing medians and interquartile ranges. * Median values were significantly different from those of the nut diet (P< 0·05; Mann–Whitney comparison between pairs).
Since PON2 is a ubiquitous antioxidant enzyme(Reference Precourt, Amre and Denis34), we investigated whether the test diets induced any differential hepatic mRNA expression. According to the results depicted in Fig. 2(b), female mice consuming the nut diet showed a significant increase in the hepatic expression of this message. On the other hand, the hepatic mRNA expression of Pcyox1 (a pro-oxidant enzyme of LDL(Reference Banfi, Brioschi and Barcella35)) was also tested (Fig. 2(c)), and no change was observed in females, while it increased in males consuming the nut diet.
Association among parameters
In an association analysis including all animals, atherosclerotic lesion areas showed a positive relationship with plasma cholesterol concentrations (r 0·49, P= 0·001). When the two sexes were analysed separately, this association was stronger in females (r 0·71, P= 0·00; Fig. 3(a)). Likewise, a significant inverse association was observed between atherosclerotic lesion areas and hepatic Pon2 mRNA expression (r − 0·55, P= 0·02; Fig. 3(b)).

Fig. 3 Relationships among parameters in female apoE-deficient mice consuming the different diets. (a) Relationship between the atherosclerotic lesion area and total cholesterol (R s= 0·71; P= 0·00). (b) Association between the atherosclerotic lesion area and hepatic paraoxonase 2 (Pon2) mRNA expression (R s= − 0·55; P= 0·02). Correlations were calculated according to Spearman's test and values corresponding to all experimental groups have been included where ○ and ○ correspond to the nut and palm oil groups, respectively. AU, arbitrary units.
Discussion
Atherosclerotic lesions in female and male apoE-deficient mice showed differential responses to a diet supplemented with 3 % mixed nuts compared with a diet with a similar contribution of fat provided by palm oil (saturated fat). In both sexes, the addition of a small amount of nuts decreased non-HDL-cholesterol and the content of reactive oxygen species in LDL. However, an increase in serum paraoxonase activity and in the hepatic expression of Pon2 was observed only in females consuming the nut diet. The decrease in atherosclerotic lesion areas was significant and correlated positively with plasma cholesterol and negatively with Pon2 expression. These results indicate that female mice benefit from nut intake, as it lowers their LDL and endows them with a higher antioxidant defence through PON2.
The decreased non-HDL-cholesterol in males and females on the nut diet compared with the saturated fat-enriched diet, palm oil in this case, agrees with numerous human clinical studies that have consistently shown a favourable plasma lipid response to diets including almonds, hazelnuts or walnuts(Reference Abbey, Noakes and Belling8, Reference Durak, Koksal and Kacmaz9, Reference Jenkins, Kendall and Marchie11–Reference Sabate, Oda and Ros16). The beneficial effects of nuts on the lipid profile are mainly attributed to their favourable fatty acid composition – low in saturated and high in unsaturated fatty acids. In the present experiment, the nut supplement contributed to the increase in the PUFA content of this diet, leading to a higher and more favourable polyunsaturated:saturated ratio than palm oil (3·9 v. 1·6, respectively; Table 1). The decrease in plasma cholesterol produced by a high PUFA in comparison with a saturated fat diet has also been reported in male apoE-deficient mice(Reference Calleja, Paris and Paul27), in both sexes in LDL receptor- and apoE-deficient mice(Reference Merkel, Velez-Carrasco and Hudgins36), in male rabbits(Reference Kritchevsky, Tepper and Wright37) and in male hamsters(Reference Terpstra, van den Berg and Jansen38). Therefore, using this experimental design, non-HDL-cholesterol in apoE-deficient mice fed nuts follows the human pattern and could be attributed to the fatty acid composition of the diets.
With respect to the finding of a lack of effect on HDL-cholesterol, or on its main apoA1, human studies involving diets enriched in almonds, hazelnuts or walnuts have also shown contradictory results, reporting increases(Reference Abbey, Noakes and Belling8, Reference Jenkins, Kendall and Marchie11, Reference Zambon, Sabate and Munoz14), decreases(Reference Sabate, Fraser and Burke12) or absence of changes(Reference Spiller, Jenkins and Bosello13, Reference Torabian, Haddad and Cordero-MacIntyre39). Furthermore, there is no agreement on the effect of PUFA-containing diets on plasma HDL-cholesterol in animal studies: PUFA tend to reduce HDL-cholesterol in rabbits receiving a diet enriched with maize oil compared with olive or avocado oil(Reference Kritchevsky, Tepper and Wright37), but tend to increase it in comparison with coconut oil. Meanwhile in male hamsters, the substitution of maize oil as a source of polyunsaturated fat for palm oil decreased HDL-cholesterol(Reference Terpstra, van den Berg and Jansen38). Moreover, a different HDL response to diets has been reported in LDL receptor- and apoE-deficient mice, two models of atherosclerosis(Reference Merkel, Velez-Carrasco and Hudgins36). While apoE-deficient mice consuming PUFA diets compared with diets containing SFA showed decreased HDL-cholesterol in females as well as in males, no differences were observed between these diets in terms of plasma HDL in LDL receptor-deficient mice of either sex. Overall, these results point to an interaction among dietary composition, sex and genetic background that makes dietary response complex.
In the present study, there were sex-related differences in the response of atherosclerotic lesions to dietary nut supplementation, a circumstance that does not seem to be the case in human studies dealing with risk factors(Reference Sabate, Oda and Ros16, Reference Ros, Tapsell and Sabate40) or in an intervention analysing carotid atherosclerosis(Reference Murie-Fernandez, Irimia and Toledo41). Despite the fact that we used the same proportion of nuts as the latter study, we should point out the differences between the two reports, including the analysis of different arteries and the particular lipid metabolism in apoE-deficient mice(Reference Sarria, Surra and Acin18), which, moreover, exhibit clear differences between sexes(Reference Calleja, Paris and Paul27, Reference Surra, Guillen and Arbones-Mainar42). In females, the nut-supplemented diet delayed the progression of aortic lesions compared with palm oil, a finding that may be attributed to a synergistic effect on plasma non-HDL-cholesterol levels, on the one hand, and on increased antioxidant defence of LDL, on the other hand. The latter could be executed through an increase in Pon2 expression as an ubiquitous antioxidant enzyme(Reference Precourt, Amre and Denis34) and without any change in Pcyox1, the pro-oxidant agent of these particles(Reference Banfi, Brioschi and Barcella35). In fact, PON2 has been shown to protect against atherosclerosis development(Reference Devarajan, Bourquard and Hama43–Reference She, Zheng and Wei46). In addition, its expression could also be modified by sex as occurs with Pon1, another member of the three-gene paraoxonase family(Reference Bin Ali, Zhang and Lim47).
Favourable effects of nuts may be attributed to their fatty acid profiles or the presence of other minor components. One limitation of our experimental design using palm oil for comparison is the inability to discriminate between the two components, but a positive aspect is that it proposes a practical and reasonable combination to be recommended for populations. Indirectly, the changes observed in hepatic Pon2 gene expression cannot be explained by the different fatty acid profile. In macrophages, this enzyme has been induced by polyphenols from pomegranate(Reference Shiner, Fuhrman and Aviram48), and some of these substances are also present in walnuts, but not in hazelnuts or almonds(Reference McKay, Chen and Yeum49). In fact, our nut diet provides a higher amount of hydrolysable polyphenols. Therefore, the present observation would represent an initial contribution to defining a role for these compounds in the regulation of PON2 via its mRNA and possibly in mediating LDL reactive oxygen species content. Several observations suggest that nut antioxidants have favourable effects on CVD(Reference Blomhoff, Carlsen and Andersen50). Indeed, ellagic acid and polyphenol-rich extracts from walnuts were effective inhibitors of in vitro plasma LDL oxidation(Reference Anderson, Teuber and Gobeille51, Reference Torabian, Haddad and Rajaram52), and flavonoids from the almond peel protected LDL from oxidation in hamsters(Reference Chen, Milbury and Lapsley53). However, other authors have found no differences in plasma antioxidant status following walnut consumption, despite an increase in thiols(Reference McKay, Chen and Yeum49), nor have Bullo et al. (Reference Bullo, Nogues and Lopez-Uriarte54) observed differences between walnuts and walnut-skin extracts. In this respect, despite the slightly high hydrolysable polyphenol content of the nut diet, the different response observed in females may also be attributed to possible differences in nut polyphenol species with respect to palm oil, and to combinations and interactions among them and with the matrix. Phenolic compounds may exert both anti- and pro-oxidant effects(Reference Filipe, Haigle and Silva55, Reference Joubert, Winterton and Britz56). In this respect, Acin et al. (Reference Acin, Navarro and Arbones-Mainar57) reported that the oral administration of hydroxytyrosol to apoE-deficient mice increased the atherosclerotic lesion area, suggesting that phenolic compounds, outside the original matrix, could not only fail to be useful, but could even be harmful. Another source of variation is introduced by sex, which, in this species, modifies the response to exogenous factors and genetic background(Reference Surra, Guillen and Arbones-Mainar42, Reference Surra, Guillen and Barranquero58). The clarification of these aspects will require further studies.
In conclusion, the present results in apoE-deficient mice show that, compared with consumption of a palm oil-enriched diet, consumption of a mixed nut diet is associated with decreased aortic atherosclerosis in females but not in males, an effect that appears to be related to both a reduction in non-HDL-cholesterol and an enhanced expression of Pon2 with protection of LDL from oxidation.
Acknowledgements
We thank Silvia Garcés and Mª Pilar Lierta for their help in maintaining the animals, and Belén Aguado and Asun Callizo for their contribution to diet preparation. We also thank Martha Messman for her assistance in manuscript editing. This study was funded by grants CICYT-FEDER (SAF2010-14958), Gobierno de Aragón (PI025/08) and Redes FSE-DGA (B-69). N. G. and M. A. N. were recipients of a Fundación Cuenca Villoro fellowship and a Miguel Servet contract, respectively. CIBER Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The contribution of each author was as follows: J. C. S. carried out the design of the diets, atherosclerotic analysis and prepared the manuscript; M. P. T. carried out apo analysis and prepared the manuscript; I. O. carried out the flow cytometer experiments; J. C. S. evaluated the flow cytometer experiments and prepared the manuscript; N. G. was involved in animal management and sample preparation; M. A. N. carried out plasma lipid analysis and statistical analysis; C. B. carried out apo analysis and prepared and analysed the mRNA expression; C. A. carried out the histological analyses; J. O. supervised the experimental design, obtained funding and prepared the manuscript. The authors report no conflicts of interest.








