Type 2 diabetes and its long-term complications are a major cause of morbidity and mortality worldwide and the prevalence of the disease continues to riseReference Mokdad, Ford and Bowman1, Reference King, Aubert and Herman2. The increasing incidence of type 2 diabetes is associated with increasing rates of obesity in most countriesReference Seidell3. Obesity, in turn, is often associated with insulin resistance which is considered as one of the underlying causes of diabetesReference Reaven4. Thus, a dietary therapy capable of achieving both weight reduction and improvement of insulin resistance may potentially reduce the incidence of type 2 diabetes in obese subjects prone to develop the disease.
Special attention has been focused on carbohydrates in nutrition. Carbohydrates have the greatest influence on postprandial glucose excursions and are the primary stimulus for postprandial insulin release, thus being directly associated with insulin resistanceReference Sheard, Clark and Brand-Miller5. In Western countries the consumption of cereals constitutes the main source of dietary carbohydratesReference Hu6. Cereals are mostly consumed as highly processed products which have lost a substantial amount of dietary fibre and contain a relatively high concentration of starch because most of the bran and germ is removed in the refining process. In contrast, whole-grain products include the endosperm, germ and bran and are rich in dietary fibreReference Slavin7. Such products typically entail higher satiety than do their refined counterparts thus reducing total energy intakeReference Burton-Freeman8.
The beneficial effect of a diet rich in whole-grain products was demonstrated in several prospective cohort studies showing a strong inverse correlation between whole-grain consumption and the risk of developing type 2 diabetesReference Meyer, Kushi, Jacobs, Slavin, Sellers and Folsom9–Reference Montonen, Knekt, Jarvinen, Aromaa and Reunanen11. Additional studies have dealt with the influence of whole-grain intake on insulin resistance. For example, the consumption of whole grain was inversely correlated with insulin resistance in the Insulin Resistance Atherosclerosis StudyReference Liese, Roach, Sparks, Marquart, D'Agostino and Mayer-Davis12 and in cross-sectional examinations of the Framingham Offspring CohortReference McKeown, Meigs, Liu, Saltzman, Wilson and Jacques13. Above all, two randomized studies showed that dietary intake of whole grain significantly improved insulin resistanceReference Jang, Lee, Kim, Park and Lee14, Reference Pereira, Jacobs, Pins, Raatz, Gross and Slavin15.
Although most obese people prone to develop type 2 diabetes are aware that weight loss and healthy nutrition are of primary importance they obviously fail to integrate a healthy diet in their everyday life. This difficulty may be overcome by dietary products which are fast to prepare and provide a structured eating pattern that is easy to comply with.
In this randomized controlled study we investigated the effects of a hypo-energetic diet with a whole-grain based dietary product (WG) with reduced starch content derived from double-fermented wheat and a nutrient-dense meal replacement product (MR) on insulin resistance in obese subjects prone to develop type 2 diabetes. Special emphasis was placed on the question as to whether insulin resistance was influenced by the products beyond their effects on the amount of body weight lost.
Methods
Subjects and study design
The study employed an open-label, randomized, two-way cross-over design and was conducted at a single study centre (Profil Institut für Stoffwechselforschung GmbH) in Neuss, Germany. We enrolled obese (BMI >29 and < 40 kg/m2) male and female subjects of Caucasian race (age ≥ 18 and ≤ 70 years) with elevated fasting blood glucose (>6·1 and ≤ 7·1 mmol/l). Exclusion criteria were use of oral anti-diabetic agents, insulin treatment and any condition potentially influencing insulin resistance or insulin secretion. The study protocol was approved by the local ethics committee and was conducted according to the guidelines of Good Clinical Practice and the Declaration of Helsinki. Written informed consent of subjects participating in the study was received after detailed oral and written explanation of the study objectives.
Dietary intervention
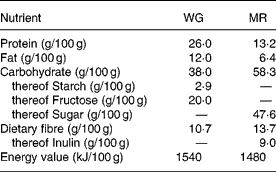
Subjects were randomly assigned to a 4-week treatment period either consuming a starch-reduced WG derived from double-fermented wheat (Balantose™, Cargill GmbH, Freising, Germany) or a nutrient-dense MR product (Slim Fast™, Unilever Toronto, Ontario, Canada). After a wash-out period of 2 weeks on habitual nutrition, subjects switched to the respective other product, again for a 4-week treatment period. Subjects were instructed to replace at least two daily meals (preferentially breakfast and dinner) as well as snacks with WG or MR, respectively, targeting for a daily consumption of 200 g of either product. The nutritional characteristics of WG and MR are detailed in Table 1. WG contained fibre naturally occurring in whole-grain wheat whereas MR represented a mixture containing added dietary fibre components with the main part being inulin.
Table 1 Nutritional values of the whole-grain based dietary product (WG) and the nutrient-dense meal replacement product (MR)
WG and MR were provided as dry powder and prepared in portions equivalent in energy content. WG was prepared by dissolving the powder in 100 ml water, 100 ml milk (0·3 % fat) or 200 ml yoghurt (1·5 % fat) whereas MR was always dissolved in 250 ml milk (0·3 % fat) which is according to manufacturers' recommendations.
All subjects were instructed by a dietitian on how to prepare the dietary meals. Additionally, dietary information was provided on how to prepare the non-substituted meal, preferentially lunch, including lists of suitable food, food to be avoided, cooking and preparation advice plus recipes. General advice was given to limit saturated fat intake, to favour food rich in dietary fibres, to avoid sugared beverages and food, to enhance the intake of fresh fruit and vegetables, and to drink at least 2 litres water/d. WG and MR were supplied free of charge.
During the treatment total daily energy intake was limited to 7120 kJ, of which 2930 kJ were allowed to be delivered by WG or MR. Subjects received a standardized dietary questionnaire in which they daily recorded amount and food matrix chosen for blending WG or MR as well as type and amount of all other ingested food. Based on these records, total daily consumed energy of each subject was calculated by a dietitian. In addition, consumption of WG and MR was determined by balancing supplies, which were weighed before dispense and after return. To check for and ensure subjects' compliance with the diet, they were asked to visit the study centre 1 week after dietary treatment had started. Fasting blood samples were collected (data not shown), body weight, vital signs and potential adverse events were recorded and subjects were again requested to control their body weight weekly. In addition, subjects were contacted by telephone during the second and the third week of the treatment. They were interviewed to elucidate compliance to diet, body weight changes and potential adverse events.
Clinical and metabolic measurements
Body weight was recorded with an accuracy of ± 0·1 kg with a calibrated electronic scale and height was recorded with an accuracy of ± 0·5 cm with a vertical ruler. Anthropometric measures were taken while subjects were wearing lightweight clothing and not wearing shoes. Waist:hip ratio was determined by dividing waist circumference (maximum circumference between the lower costal margin and the iliac crest in standing position) by hip circumference (maximal circumference at the level of the femoral trochanter). Blood pressure was read after subjects sat for 5 min using an electric sphygmomanometer (boso-medicus, Bosch, Jungingen, Germany). Blood samples were collected from an antecubital vein after subjects fasted 12 h to determine fasting blood glucose, fasting serum insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol, fructosamine, and safety laboratory parameters (haematology, biochemistry and coagulation).
Bioanalytics
Blood glucose was measured by a glucose oxidase method (Super GL, Hitado, Delecke-Möhnesee, Germany). Serum immunoreactive insulin was measured using a microparticle enzyme immunoassay (Imx Insulin Assay, Abbott, Wiesbaden, Germany). TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and fructosamine as well as safety parameters were measured by standard laboratory methods. Insulin resistance of subjects was assessed by the homeostasis model assessment insulin resistance score (HOMA-IR, fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l) / 22·5Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner16.
Glycaemic, insulinaemic and satiety index of the whole-grain based dietary product
To determine glycaemic, insulinaemic and satiety index of WG ten healthy subjects (4 male, 6 female; BMI < 27 kg/m2) were enrolled in an additional study. All subjects underwent a clinical examination and interview and gave their written informed consent to participate. After a 12-h overnight fast subjects twice underwent an oral glucose tolerance test (50 g glucose) and consumed a 50 g available-carbohydrate portion of WG at the third occasion. WG was provided as dry powder and prepared by dissolving the powder in water according to the manufacturer's recommendation. Venous blood samples were taken before ingestion of the respective meal and 15, 30, 45, 60, 90 and 120 min after starting to eat. To assess satiety, subjects recorded their feeling of satiety at the fixed time and additionally at 240 min after ingestion using an analogue scale ranging from − 3 (extremely hungry) up to +3 (extremely full) according to Holt et al. Reference Holt, Miller, Petocz and Farmakalidis17. Individual glycaemic, insulinaemic and satiety indices were assessed by dividing the incremental area under curve (AUC) for blood glucose, insulin and satiety after consuming WG by the respective AUC following the oral glucose tolerance test and expressed as percentages.
Statistics
The primary outcome variable was a change in HOMA-IR after 4 weeks of treatment, i.e. hypo-energetic diet with either WG or MR, compared to pre-treatment baseline values. Body weight, fasting blood glucose, fasting serum insulin, TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol, fructosamine and waist:hip ratio were regarded as secondary outcome variables. TAG was logarithmically transformed before analysis as results were not normally distributed. Variables were analysed for carry-over and period effects. With no carry-over or period effect demonstrable, results from both sequences were pooled by treatment and analysed by ANOVA. With such an effect demonstrable, results from each treatment period were analysed separately. In order to correct the effect of potential confounders, a correlation between outcome variable and possible confounder was calculated. If such a correlation proved statistically significant, a covariance analysis (ANCOVA, SAS procedure ‘GLM’) was performed. That is, by means of a regression between outcome variable and confounding variable the outcome variable was re-calculated. Kilogram of body weight (pre- and post-treatment) was used as a covariate in the ANCOVA which was conducted for WG and MR separately resulting in two different coefficients of weight loss v. HOMA-IR score (one for WG and one for MR). The difference between the observed and re-calculated outcome variable, which is independent from confounding effects, was subsequently analysed by ANOVA. These within-group comparisons were completed post hoc by an ANOVA comparing weight-loss adjusted changes of outcome variables between treatment groups. Results were considered to be statistically significant with P values < 0·05. Statistical analyses were performed using SAS (8.2; SAS Institute, Cary, NC, USA). Results were expressed as means and standard deviations throughout the text and as means and standard errors in the figures unless otherwise stated.
Results
Thirty-six subjects were enrolled in the study; two subjects withdrew consent and two subjects dropped out due to intolerance to fructose and oesophageal reflux, respectively. One subject was excluded from analysis due to a clinically significant metabolic deterioration during the course of the study, i.e. the participant was newly diagnosed with diabetes during the first treatment period. Thus, thirty-one subjects (thirteen male, eighteen female, age 51 (sd 13) years, BMI 33·9 (sd 2·7) kg/m2, fasting blood glucose 6·3 (sd 0·8) mmol/l) completed the study and were further analysed. Baseline characteristics (Table 2) of subjects assigned to the treatment sequence WG / MR were not significantly different from those assigned to the treatment sequence MR / WG.
Table 2 Baseline characteristics and changes (Δ) from baseline after 4 weeks of diet with a whole-grain based dietary product (WG) and a meal replacement product (MR) in thirty-one obese subjects with elevated fasting blood glucose

HOMA-IR, homeostasis model assessment insulin resistance.
* P values for the comparison of changes after WG v. MR.
† P < 0·05 for difference from baseline.
‡ Data for the first and second treatment period separately due to period effects.
§ Data for the first and second treatment period separately due to carry-over effects.
Judged from the entries given in the questionnaires, subjects compliantly adhered to the prescribed diet. Based on the questionnaire data, total daily energy consumption comprising all ingested food including the supplement with WG was lower (5270 (sd 1010) kJ/d) than with MR (6360 (sd 1070) kJ/d; P < 0·001) thus remaining below the study requirements in both treatment groups. On the basis of weight differences between supplied and returned dietary products daily consumption of either product was 188 (sd 44) g. Subjects consumed 20 g wheat whole-grain fibre/d from WG while 25·8 g dietary fibre/d of which 17·0 g/d was inulin were provided by MR.
For WG the glycaemic index (GI) assessed was 15·5 (sd 15·1), the insulinaemic index was 43·1 (sd 35·5), and the satiety index was 739 (sd 577). For one portion of WG a glycaemic load (GL) of 2·7, 3·4 or 4·2 was calculated depending on whether the dry powder was dissolved in water, milk or yoghurt. Based on a GI of 35 for the MRReference Denyer, Dickinson and Brand-Miller18 the calculated GL of one portion of MR was 13·1.
Body weight decreased both during diet with WG and MR (P < 0·001 for both treatments; Fig. 1), whereas during the wash-out phase a slight increase of body weight was observed. Fasting blood glucose (Fig. 2(A)) and fasting serum insulin concentrations were lower both after diet with WG and with MR. Consequently, the diet with either product resulted in a decrease of HOMA-IR score after 4 weeks of treatment (Fig. 2(B)). Changes in body weight, fasting blood glucose, fasting serum insulin and HOMA-IR score did not differ between diet with WG and MR (Table 2). However, after statistical adjustment for the amount of body weight lost, HOMA-IR showed a better improvement after diet with WG than with MR (P = 0·049; Table 3). Furthermore, fasting serum insulin decreased after diet with WG, but not with MR (P = 0·031).

Fig. 1 Course of body weight during the study. ○, Subjects randomized to treatment sequence whole-grain based dietary product (WG) / meal replacement product (MR); ●, subjects randomized to treatment sequence MR / WG.

Fig. 2 (A) Fasting blood glucose, (B) homeostasis model assessment insulin resistance (HOMA-IR), and (C) total cholesterol before (![]() ) and after (□) diet with a whole-grain based dietary product (WG) and a meal replacement product (MR). Mean value was significant different from baseline value after adjustment for the amount of body weight lost *P < 0·05.
) and after (□) diet with a whole-grain based dietary product (WG) and a meal replacement product (MR). Mean value was significant different from baseline value after adjustment for the amount of body weight lost *P < 0·05.
Table 3 Mean baseline characteristics and changes (Δ) from baseline after 4 weeks of diet with a whole-grain based dietary product (WG) or a meal replacement product (MR) after statistical adjustment for the amount of body weight lost in thirty-one obese subjects with elevated fasting blood glucose

HOMA-IR, homeostasis model assessment insulin resistance.
* P values for the comparison of changes after WG v. MR.
† P < 0·05 with respect to baseline.
‡ Statistically significant for ΔWG v. ΔMR.
§ Data for the first and second treatment period separately due to period effects.
‖ Data for the first and second treatment period separately due to carry-over effects.
As period or carry-over effects, respectively, were observed for HDL-cholesterol and LDL-cholesterol, results from each treatment period were considered separately. Total cholesterol, LDL-cholesterol and TAG decreased after diet both with WG and with MR (Table 2). HDL-cholesterol decreased only in the first treatment period after diet with MR. Changes in lipids were not statistically different between the two treatments. After statistical adjustment for the amount of body weight lost the comparison between treatments revealed that total cholesterol decreased only after diet with MR (P < 0·001) but not with WG (Table 3).
Waist:hip ratio (P < 0·001) and BMI (P < 0·001) decreased after diet with WG as well as with MR. Fructosamine as well as systolic and diastolic blood pressure did not change during the study (Table 2). No clinically significant changes of safety laboratory parameters were observed, neither after diet with WG nor with MR.
During the study thirty adverse events with respect to the gastrointestinal tract were registered, fifteen during dietary treatment with WG, thirteen during dietary treatment with MR and two during the wash-out phase (Table 4). There was one conspicuous difference between the dietary treatments, i.e. during diet with WG three subjects complained about constipation (one of them in two cases) while during diet with MR constipation did not occur. Constipation was transient and occurred early in the treatment period.
Table 4 Gastrointestinal adverse events
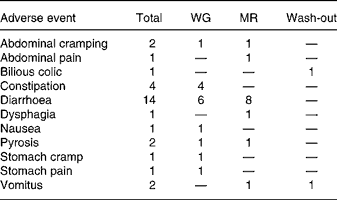
WG, diet with a whole-grain based dietary product; MR, diet with a meal replacement product.
Discussion
In the present study we showed that a hypo-energetic diet with both a WG and a nutrient-dense commercially available MR resulted not only in a loss of body weight, but at the same time caused a decrease of fasting blood glucose, fasting serum insulin and insulin resistance in obese subjects with elevated fasting blood glucose. Although initially no statistically significant difference between treatments was demonstrable, a comparison between both groups, after adjustment for the amount of body weight lost, revealed that insulin resistance improved better after diet with WG than with MR.
Whereas the total dietary fibre content of WG and MR was similar, the fibre composition of both products showed a substantial difference. The basic element of WG is wheat whole-grain which is modified during the manufacturing process. The whole grain is milled to bruised grain which then undergoes a two-step fermentation process, first a yeast fermentation and second a lactic acid fermentation. During the fermentation process starch is reduced thus the proportion of refined grain is diminished. After drying the WG base is enriched with further nutrients to the final WG product. Accordingly, the fibre composition of WG is similar to the natural one of whole-grain wheat. WG contains all parts of the whole grain, i.e. germ, bran and endosperm with the compounds that are embedded in the fibre matrix such as minerals and phytochemicals. In contrast, MR is not based on one starting substance but represents a mixture containing added dietary fibre components with the main part being inulin. Inulin is present in a number of plant species which are often eaten as vegetablesReference van Loo, Coussement, de Leenheer, Hoebregs and Smits19. For industrial food inulin is commonly extracted from chicory roots (Cichorium intybus, Compositae)Reference Roberfroid20.
Favourable effects of cereal dietary fibre with regard to carbohydrate metabolism were shown in various studies. For example, two prospective cohort studies showed that diabetes incidence was inversely correlated with the consumption of dietary fibre derived from cereals but not with the consumption of fibre derived from vegetables and legumesReference Meyer, Kushi, Jacobs, Slavin, Sellers and Folsom9, Reference Montonen, Knekt, Jarvinen, Aromaa and Reunanen11. Moreover, insulin resistance was inversely correlated with consumption of cereal dietary fibre but not with fibre stemming from vegetables in the Framingham Offspring CohortReference McKeown, Meigs, Liu, Saltzman, Wilson and Jacques13. These results parallel the findings in our study which, after statistical adjustment for the amount of body weight lost, show an improvement of insulin resistance after a diet rich in wheat whole-grain fibre but not after a diet rich in vegetable-derived fibre.
Generally, cereal grains consumed in developed countries are subjected to some sort of refining omitting bran and germ. Consequently, in refined products a substantial amount of nutrients and phytochemicals located in the fibre matrix are lost and the relative concentration of starch is highReference Slavin7. In prospective studies an inverse association of incident type 2 diabetes with increasing intake of whole-grain food but no positive association with refined-grain intake was foundReference Meyer, Kushi, Jacobs, Slavin, Sellers and Folsom9, Reference Fung, Hu and Pereira10, and the risk for type 2 diabetes increased as the ratio of refined:whole-grain food intake increasedReference Liu, Manson, Stampfer, Hu, Giovannucci and Colditz21. In the Iowa Women's Health Study, fibre from whole grain, but not refined grain, was inversely associated with all-cause mortality in older womenReference Jacobs, Pereira, Meyer and Kushi22. Moreover, two randomized controlled studies showed that fasting insulin decreased significantly after consumption of whole-grain food instead of refined-grain foodReference Jang, Lee, Kim, Park and Lee14, Reference Pereira, Jacobs, Pins, Raatz, Gross and Slavin15. In our study also fasting serum insulin decreased beyond the amount of body weight lost after consumption of a diet rich in cereal whole-grain fibre but not after consumption of a diet rich in refined forms of fibre.
Apart from the distinction with respect to fibre composition, dietary fibre can be classified as soluble or insolubleReference Brown, Rosner, Willett and Sacks23. WG contains 85 % insoluble and 15 % soluble fibre. In contrast, 66 % of dietary fibre of MR consists of inulin which is a soluble fibreReference Schneeman24. In prospective cohort studies insoluble fibre consumption, but not soluble fibre consumption was inversely associated with the incidence of type 2 diabetesReference Meyer, Kushi, Jacobs, Slavin, Sellers and Folsom9, Reference Montonen, Knekt, Jarvinen, Aromaa and Reunanen11. Moreover, in a randomized cross-over study consumption of whole grain consisting of 72 % of insoluble dietary fibre resulted in a significant decrease of fasting insulin concentrations and insulin resistance in overweight and obese adultsReference Pereira, Jacobs, Pins, Raatz, Gross and Slavin15. Recently it was shown that increased insoluble dietary fibre intake for 3 d significantly improved whole body insulin sensitivity in overweight and obese womenReference Weickert, Mohlig and Schofl25. Our data agree with these results, as adjusted fasting serum insulin and insulin resistance improved after diet with WG providing mainly insoluble dietary fibre. The mechanisms, however, by which insoluble dietary fibre improves carbohydrate metabolism remain to be clarified in future studies.
Specifically soluble fibre has been reported to be beneficial in lowering glucose and insulin responses due to its physical action in the gastrointestinal tractReference Jenkins, Axelsen, Kendall, Augustin, Vuksan and Smith26. Soluble dietary fibre delays gastric emptying and slows intestinal absorption and digestion of carbohydrates. This effect has been attributed primarily to the viscosity of soluble fibre which forms a gel-like substance in the gastrointestinal tract and was abolished when hydrolyzed non-viscous fibre was usedReference Jenkins, Wolever and Leeds27. In our study consumption of a diet rich in the soluble fibre inulin did not affect insulin resistance after elimination of the amount of body weight lost which is probably due to the fact that the highly soluble inulin does not increase the viscosity of the aqueous phase. Therefore its effect on glycaemia and insulin response is likely to be modest compared with more viscous dietary fibreReference Schneeman24. Our finding correspond with the results of previous randomized controlled trials demonstrating no effect of inulin consumption on insulin sensitivity, fasting blood glucose and fasting serum insulin in obese or in normal weight subjects, respectivelyReference Jackson, Taylor, Clohessy and Williams28, Reference Balcazar-Munoz, Martinez-Abundis and Gonzalez-Ortiz29.
Both products investigated differ not only with respect to their composition of dietary fibre but at the same time with respect to their GI. Whereas the GI of the starch-reduced WG is very low, the one of MR, reflecting its higher sugar content, is slightly higher. One has to admit, however, that even though the GI of WG and MR differ, both values are in the low range and therefore could hardly represent the main cause for the different effect on fasting serum insulin and insulin resistance. When considering the amount of carbohydrates of WG and MR in a serving however, the difference between both products becomes more pronounced. For WG the GL representing the arithmetic product of the GI and carbohydrate contentReference Salmeron, Manson, Stampfer, Colditz, Wing and Willett30 per serving was low, whereas the one for MR lay in the medium rangeReference Denyer, Dickinson and Brand-Miller18. As in the Framingham Offspring Cohort a diet with a high GL was associated with insulin resistanceReference McKeown, Meigs, Liu, Saltzman, Wilson and Jacques13 it is tempting to speculate that the low GL of WG has contributed to its beneficial effect on insulin resistance. Nevertheless, it is worth mentioning that two large cohort studies have failed to demonstrate any relationship between GL and insulin resistanceReference Lau, Faerch and Glumer31, Reference Liese, Schulz and Fang32.
Both treatments differed with respect to total cholesterol which after adjustment for the amount of body weight lost decreased only after diet with MR but not with WG. The discrepancy between WG and MR may result from the low content of soluble fibre in the whole-grain wheat product in contrast to MR rich in soluble fibre. This result is in accordance with studies showing cholesterol-lowering properties in products rich in soluble dietary fibre, but not in those rich in insoluble dietary fibreReference Brown, Rosner, Willett and Sacks23. Moreover, our findings correspond with the results from Jenkins et al. Reference Jenkins, Kendall and Augustin33 who did not find a cholesterol-lowering effect in subjects with type 2 diabetes who consumed breakfast cereals and breads high in wheat bran. Likewise, the consumption of wheat bran did not influence cholesterol in subjects with the metabolic syndromeReference Vuksan, Sievenpiper and Owen34.
With regard to the effect of inulin on cholesterol concentrations in human subjects conflicting results have been foundReference Williams and Jackson35. Significant lower cholesterol concentrations after inulin ingestion were observed in normolipidaemic young menReference Brighenti, Casiraghi, Canzi and Ferrari36 and in subjects with hypercholesterolaemiaReference Balcazar-Munoz, Martinez-Abundis and Gonzalez-Ortiz29, Reference Davidson and Maki37. In contrast, after intake of inulin cholesterol did not decrease in healthy and moderately hypercholesterolaemic subjects, respectivelyReference Jackson, Taylor, Clohessy and Williams28, Reference Letexier, Diraison and Beylot38. However, as stated earlier, for those physiologic responses of fibre that are mediated through the small intestine, the effect of the non-viscous inulin is likely to be minimalReference Schneeman24. Probably further mechanisms may be involved in the reduction of plasma cholesterol such as mediation by SCFA produced by the gut microflora during the fermentation of inulinReference Kaur and Gupta39.
Total daily energy consumption was less than the target of 7120 kJ/d in both treatment groups. Since it is unusual for basically unsupervised dieters to exceed study requirements one cannot completely rule out that participants may have not recorded all ingested energy. In any case, subjects both during diet with WG or MR experienced a substantial weight loss which we attributed primarily to the energy restriction rather than to an effect of the dietary products per se.
Both the diets with WG and MR were safe and well tolerated. The incidence of gastrointestinal side effects was acceptable and did not differ between WG and MR, with one exception. During diet with WG constipation was a conspicuous side effect. This is somewhat surprising, since the hardly fermentable wheat bran is particularly laxative40. Hence, it might be useful to recommend an adequate hydration accompanying consumption of WG, in particular for subjects unaccustomed to whole-grain products.
In summary, our data demonstrate that while losing weight during a hypo-energetic diet on the basis of a wheat whole-grain product metabolic risk factors for type 2 diabetes in obese subjects with elevated fasting blood glucose were improved, even beyond the metabolic effects originating from the amount of body weight lost. The beneficial effect of WG probably relied on synergetic product properties, i.e. high content of insoluble cereal dietary fibre derived from double-fermented wheat, low amount of digestible carbohydrates and low GI resulting in a low GL per meal. In conclusion, a diet based on WG may potentially reduce the incidence of type 2 diabetes in subjects at high risk of developing the disease.
Acknowledgements
This study was funded by Cargill GmbH. Klaus Rave, Kerstin Roggen, Sibylle Dellweg and Tim Heise had no financial or personal interest in the company sponsoring this research. Heike tom Dieck was employed at Cargill Food Ingredients GmbH.








