Excess dietary cholesterol consumption and oestrogen deficiency are two well-recognised independent factors inducing hepatic lipid accumulation( Reference Subramanian, Goodspeed and Wang 1 – Reference Kainuma, Fujimoto and Sekiya 3 ). There is also recent evidence that oestrogen deficiency and dietary cholesterol are two independent experimental variables that together potentiate hepatic lipid accumulation more than each one alone( Reference Kamada, Kiso and Yoshida 4 ). Several pathways have been identified as deregulated by either dietary cholesterol or female hormone deficiency including hepatic inflammation( Reference Subramanian, Goodspeed and Wang 1 , Reference Dimitrova-Shumkovska, Veenman and Ristoski 5 ), decreased biliary cholesterol secretion( Reference Treguier, Briand and Boubacar 6 ), stimulated de novo fatty acid synthesis( Reference Fungwe, Fox and Cagen 7 ) and impaired VLDL production( Reference Cote, Yasari and Pighon 8 , Reference Barsalani, Chapados and Lavoie 9 ), all contributing to the development of hepatic steatosis. Among them, VLDL secretion is crucial in preventing lipid accretion because the liver constantly takes up circulating lipids from both endogenous and exogenous sources( Reference Flamment, Kammoun and Hainault 10 , Reference Alger, Brown and Sawyer 11 ). However, molecular data supporting the defects in VLDL metabolism under the conditions of oestrogen deficiency and a hypercholesterolaemic diet are limited.
Although there are some physiological data supporting the concept that overconsumption of cholesterol induces the overproduction of hepatic lipoproteins( Reference Teramoto, Kato and Hashimoto 12 , Reference Chen, Song and Redinger 13 ), other evidence points towards a reduction in VLDL assembly. For instance, we( Reference Cote, Ngo Sock and Levy 14 ) and others( Reference Savard, Tartaglione and Kuver 15 ) found that a high-fat/high-cholesterol diet in rats suppressed the gene expression of microsomal TAG transfer protein (Mttp), a rate-limiting molecule for VLDL assembly and secretion. Dietary cholesterol has also been shown to increase cholesterol ester storage in hepatocytes and to reduce hepatic VLDL–TAG secretion, resulting in neutral lipid retention within the liver( Reference Alger, Brown and Sawyer 11 ). Similar to high cholesterol intake, oestrogen deficiency in ovariectomised (Ovx) animals or the blockage of oestrogen receptors results in a decrease of Mttp gene expression and impaired VLDL–TAG secretion( Reference Cote, Yasari and Pighon 8 , Reference Barsalani, Chapados and Lavoie 9 ).
Taking together, these findings suggest that the combination of ovariectomy and cholesterol consumption would disrupt VLDL assembly at the molecular level. To test this hypothesis, we measured the gene expression of several key molecular markers involved in the different steps of VLDL synthesis in Ovx rats fed with three diets rich in cholesterol. Besides Mttp, molecular markers of VLDL assembly included apoB-100, an essential structural protein that translocates into the luminal side of the endoplasmic reticulum( Reference Hussain, Bakillah and Nayak 16 , Reference Hussain, Shi and Dreizen 17 ), diacylglycerol acyltransferase 2 (Dgat2), involved in converting fatty acids into TAG, and acyl-CoA:cholesterol acyltransferase 2 (Acat2) that converts free cholesterol into cholesterol esters( Reference Cianflone, Yasruel and Rodriguez 18 ). We also targeted cell death-inducing DNA fragmentation factor alpha (DFFA)-like effector B (Cideb), a lipid droplet-associated protein contributing to further lipidation of lipoprotein particles after they exit the endoplasmic reticulum compartment( Reference Tiwari, Siddiqi and Siddiqi 19 , Reference Ye, Li and Liu 20 ), and small GTPase a (Sar1a), an intracellular vesicular trafficking protein that facilitates the movements of VLDL particles between the endoplasmic reticulum and the Golgi apparatus where they are secreted in the plasma.
Furthermore, we investigated the gene expression of the molecular markers for bile acid metabolism, a pathway that might also be involved in liver fat accumulation in Ovx rats under high dietary cholesterol feeding. These included farnesoid X receptor (Fxr), a nuclear receptor involved in hepatic bile acid metabolism( Reference Cote, Ngo Sock and Levy 14 ), and its target genes sterol 12α-hydroxylase (Cyp8b1), which converts cholesterol into bile acids( Reference Russell 21 ), and bile salt export pump (Bsep), which stimulates bile acid excretion from the liver( Reference Alrefai and Gill 22 ).
The aim of the present study was to identify novel mechanisms involved in hepatic steatosis induced by high dietary cholesterol consumption in Ovx rats. We found that in almost all the measured genes involved in VLDL assembly and bile acid metabolism, their transcripts were reduced by the combination of dietary cholesterol and oestrogen deficiency.
Experimental methods
Animal care
Female Sprague–Dawley rats (n 64) weighing 190–210 g were obtained from Charles River (St-Constant, PQ, Canada) and housed individually to monitor food intake in each animal. The 12 h light–12 h dark cycle started at 06.00 hours, and room temperature was maintained at 20–23°C. The animals had free access to food and water. Body weight and food intake were monitored twice per week. All the experiments in the present study were conducted according to the ARRIVE guidelines (BJN website; http://journals.cambridge.org/BJN) for animal research and the directives of the Canadian Council on Animal Care after institutional approval (CDEA: 12-108). The rat model and the number of rats (n 8 per group) used in the present experiment have been repeatedly shown to be appropriate.
Diets and surgery
At 1 week after their arrival, the rats were either sham-operated (n 32) or Ovx (n 32) according to the technique described by Robertson et al. ( Reference Robertson, Owens and Klindt 23 ). After surgery, the animals were injected with antibiotics (Tribrissen 24 %; 0·125 cm3/kg, subcutaneously) and analgesic (Carprofen; 4·4 mg/kg, subcutaneously) for 3 d. Thereafter, the Ovx and sham-operated rats were assigned one of the following four diets described in online supplementary Table S1.
Blood and tissue sampling
At 6 weeks after surgery, the rats were killed between 09.00 and 12.00 hours. Any remaining food was removed from the animal's cage at least 12 h before killing. Immediately after complete anaesthesia with isoflurane, the abdominal cavity was opened following the median line of the abdomen. Blood was collected into syringes treated with EDTA (15 %) and centrifuged (3000 rpm; 4°C; 10 min; Beckman GPR Centrifuge; Beckman Coulter). After blood collection, the liver median lobe was removed and freeze-clamped. The uterus, mesenteric, urogenital, retroperitoneal and subcutaneous fat deposits were removed and weighed (Mettler AE-100; Mettler Toledo). All tissues and plasma samples were stored at − 80°C until analyses.
Molecular analyses
Total RNA was extracted from the liver using RNA extraction Mini Kits (Invitrogen), according to the manufacturer's protocol. Thereafter, the RNA was treated with DNase (Invitrogen) to avoid genomic contamination. Total RNA (2 μg) was reverse-transcribed into complementary DNA using high-capacity complementary DNA reverse transcription kits (Applied Biosystems). RT samples were stored at − 20°C. The gene expression of the target genes was determined using assays designed with the Universal Probe Library. The primer sets and UPL probe numbers are presented in online supplementary Table S2. The ABI PRISM® 7900HT (Applied Biosystems) was used to detect the amplification level and programmed with an initial step of 3 min at 95°C, followed by forty cycles for 5 s at 95°C and 30 s at 60°C. All reactions were run in triplicate, and the average of threshold cycle (C
T) was used for quantification. The relative quantification of the target genes was determined using the ΔΔC
T method. Briefly, the C
T values of the target genes were normalised to that of an endogenous control gene (β-actin) (ΔC
T= C
T
target− C
T
β-actin) and compared with a calibrator (ΔΔC
T= ΔC
T Sample− ΔC
T Calibrator). Relative expression (RQ) was calculated using the Sequence Detection System 2.2.2 software (Applied Biosystems), and the formula is as follows:
![]() $$RQ = 2^{ - \Delta \Delta C _{T}} $$
.
$$RQ = 2^{ - \Delta \Delta C _{T}} $$
.
Liver and plasma lipid measurements
Liver TAG concentration was estimated from glycerol released after ethanolic KOH hydrolysis by a colorimetric method using commercial kits from Sigma. To measure liver cholesterol concentration, liver lipids were extracted using an adapted procedure developed by Folch et al. ( Reference Folch, Lees and Sloane Stanley 24 ). Liver homogenate in a chloroform–methanol solution (2:1) was filtered and rinsed with chloroform. Methanol and water (20 % of the filtrate volume each) were added to the filtered solution. After vortexing, the solution with water and methanol was centrifuged for 20 min at 2400 rpm. The lower phase was transferred to clean tubes and evaporated overnight at 30°C. The dried lipid residues were dissolved in 0·2 ml of Triton X100–isopropanol solution (10 %). Total cholesterol (TC) was measured with enzymatic kits (Wako). Plasma TC and TAG concentrations were measured using the COBAS INTEGRA 400 analyser (Roche Diagnostics).
Statistical analysis
Results are presented as means with their standard errors. Differences between means were tested for statistical significance (P< 0·05) using a two-way ANOVA for non-repeated measures with ovariectomy and diets as main factors. Fisher's least significant difference post hoc test was used in the event of a significant interaction effect (P< 0·05). For a significant diet effect without interaction, Fisher's least significant difference from a one-way ANOVA was used.
Results
Biometric parameters
In all the dietary interventions, Ovx rats had higher food intake (P< 0·001) and changes in body composition including higher final body weight, intra-abdominal fat pads (P< 0·001) and subcutaneous adiposity (P< 0·05) compared with sham-operated animals (Table 1). A lower uterus weight in Ovx rats confirmed total ovariectomy compared with sham-operated rats (Table 1). The high-fat (HF) and high-fat+0·25 % cholesterol (HFHC (0·25 %)) diets did not affect intra-abdominal and subcutaneous fat deposits, food intake, and final body weight in both Ovx and sham-operated rats. However, sham-operated and Ovx rats fed the high-fat+0·5 % cholesterol (HFHC (0·5 %)) diet had a higher food intake and final body weight (P< 0·01; Table 1).
Table 1 Biometric parameters and food intake (Mean values with their standard errors)
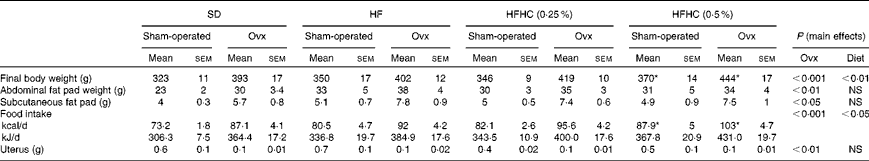
SD, standard diet; HF, high-fat diet; HFHC (0·25 %), high-fat+0·25 % cholesterol diet; HFHC (0·5 %), high-fat+0·5 % cholesterol diet; Ovx, ovariectomised.
* Mean value was significantly different from that of the SD (P< 0·05).
Plasma lipids
Plasma TAG concentrations were higher (P< 0·05) upon ovariectomy in all the dietary interventions (Fig. 1(a)). Surprisingly, the HFHC diet-fed rats exhibited lower plasma TAG concentrations (P< 0·001) than their corresponding standard diet (SD)-fed rats (Fig. 1(a)). In contrast, the effect of ovariectomy on plasma TC concentrations was largely influenced by the dietary interventions. After feeding the SD, plasma TC concentrations were higher by 64 % (P< 0·01) in Ovx rats than in sham-operated rats, but the differences between the Ovx and sham-operated rats were attenuated after feeding the HF and HFHC (0·25 %) diets (Fig. 1(b)). Surprisingly, increasing the dietary cholesterol content to 0·5 % (HFHC) reversed the effect of ovariectomy on plasma TC concentrations reported in SD-fed animals. Indeed, in response to the HFHC (0·5 %) diet, plasma TC concentration in Ovx rats was 28 % lower than that in the corresponding sham-operated rats (2·72 v. 3·74 mmol/l; P< 0·05). By comparison, plasma TC concentrations in response to the two HFHC diets increased more linearly in sham-operated rats than in Ovx rats, with increases reaching up to 56 % (0·25 % cholesterol) and 113 % (0·5 % cholesterol) compared with SD-fed animals (P< 0·01; Fig. 1(b)).

Fig. 1 Plasma and hepatic TAG and total cholesterol (TC) in sham-operated (![]() ) and ovariectomised (Ovx,
) and ovariectomised (Ovx, ![]() ) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the sham-operated rats: * P <0·05, ** P <0·01, *** P< 0·001 (ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the sham-operated rats: * P <0·05, ** P <0·01, *** P< 0·001 (ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
Liver lipids
As expected, Ovx rats had higher liver TAG concentrations (37–78 %; P< 0·001) in all the dietary interventions than the corresponding sham-operated rats (Fig. 1(c)). High cholesterol consumption in sham-operated rats was associated with 90 % (P< 0·01; 0·25 %) and 76 % (P< 0·05; 0·5 %) higher liver TAG content than that in SD-fed rats. Interestingly, ovariectomy and HFHC diets concomitantly induced a higher level of hepatic steatosis. In fact, liver TAG levels in HFHC diet-fed Ovx animals reached up to 151 % of the levels found in SD-fed Ovx rats and up to 270 % of the levels measured in SD-fed sham-operated rats (Fig. 1(c)). Liver TC content was not increased in Ovx rats compared with sham-operated rats when fed the SD and the HF diet (Fig. 1(d)). However, after feeding both HFHC diets, liver TC content in Ovx rats was 131 % (P< 0·001; 0·25 %) and 144 % (P< 0·001; 0·5 %) higher than the values measured in sham-operated rats (Fig. 1(d)). In response to the HFHC diets, hepatic TC levels in sham-operated rats were 170 % (P< 0·001; 0·25 %) and 280 % (P< 0·001; 0·5 %) of the levels reported in SD-fed sham-operated animals. In Ovx rats fed the two HFHC diets, liver TC content values reached up to 270–400 % (P< 0·01) of the values measured in SD-fed Ovx rats and 350–520 % (P< 0·001) of the values measured in SD-fed sham-operated rats, indicating a concomitant increase (Fig. 1(d)).
Molecular markers of VLDL assembly and secretion
The gene expression levels of Mttp and Dgat2, two key molecules in VLDL assembly, were lower (P< 0·001) in Ovx animals, regardless of the dietary interventions (Fig. 2(a) and (b)). In addition, the gene expression levels of Apob-100, Acat2, Sar1a and Cideb, also involved in VLDL synthesis and secretion, were lower (P< 0·001) in Ovx rats than in sham-operated rats in all the dietary interventions (Fig. 2(c)–(f)). In sham-operated and Ovx rats, both HFHC diets were associated with a lower gene expression level (P< 0·001) of Mttp (Fig. 2(a)). Considering the cumulative effects, the HFHC-fed Ovx rats showed a large decrease in the gene expression of Mttp such that the levels dropped as low as 31 % of those reported in SD-fed sham-operated rats.

Fig. 2 Hepatic gene expression of microsomal TAG transfer protein (Mttp), diacylglycerol acyl transferase 2 (Dgat2), Apob-100, acyl-CoA cholesterol acyl transferase 2 (Acat2), small GTP-binding protein a (Sar1a) and cell death-inducing DNA fragmentation factor alpha (DFFA)-like effector type B (Cideb) in sham-operated (![]() ) or ovariectomised (Ovx,
) or ovariectomised (Ovx, ![]() ) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the sham-operated rats (P< 0·001; ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the sham-operated rats (P< 0·001; ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
Molecular markers of hepatobiliary cholesterol and bile acid excretion
The gene expression of nuclear receptors Fxr and liver X receptor (Lxr) was lower (P< 0·05 and < 0·001) in Ovx rats than in sham-operated animals, in all the dietary interventions (Fig. 3(a) and (b)). In Ovx and sham-operated rats, both HFHC diets decreased the gene expression level of Fxr (P< 0·01), but the transcript level of Lxr was decreased only in HFHC (0·5 %)-fed rats (P< 0·01). Regardless of the dietary interventions, oestrogen deficiency was associated with lower transcript levels of hepatic Cyp8b1 and Bsep, suggesting that the synthesis and excretion of bile acids were decreased in Ovx rats (Fig. 3(c) and (d)). In addition, the two HFHC diets further decreased the gene expression level of Cyp8b1 (P< 0·001) in sham-operated rats as well as in Ovx rats, but the expression level of Bsep was not affected by these dietary interventions (Fig. 3(c)). Finally, the transcript levels of the canalicular cholesterol excretion transporters ATP-cassette binding protein G5/G8 (ABCG5/ABCG8) were not affected by ovariectomy. However, the gene expression of Abcg5/Abcg8 was 2- to 3-fold higher (P< 0·01) in both Ovx and sham-operated rats fed the HF diet than in those fed the SD.

Fig. 3 Hepatic gene expression of farnesoid X receptor (Fxr), liver X receptor (Lxr), sterol 12α-hydroxylase (Cyp8b1), bile salt export pump (Bsep), ATP-cassette binding protein G5 and G8 (Abcg5/Abcg8) in sham-operated (![]() ) or ovariectomised (Ovx,
) or ovariectomised (Ovx, ![]() ) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the sham-operated rats: * P <0·05, *** P< 0·001 (ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the sham-operated rats: * P <0·05, *** P< 0·001 (ovariectomy as the main effect). Mean value was significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
Molecular markers of hepatic cholesterol metabolism
The gene expression levels of sterol regulatory element-binding protein 2 (Srebp2), a key regulator of hepatic cholesterol content, and its target genes LDL receptor (Ldlr) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (Hmgcr) were down-regulated (P< 0·001) in Ovx rats compared with sham-operated rats after the SD and HF dietary interventions (Fig. 4(a) and (c)). In sham-operated rats, the HF diet did not affect the gene expression levels of Srebp2 and Ldlr, but stimulated (P< 0·05) the expression level of Hmgcr (Fig. 4(a) and (c)). However, the addition of cholesterol to the HF diet highly suppressed the gene expression levels of Srebp2 (P< 0·01), Ldlr (P< 0·001) and Hmgcr (P< 0·05) in sham-operated rats. However, the ingestion of the HFHC diets did not further decrease the gene expression levels of Srebp2, Ldlr and Hmgcr in Ovx rats (Fig. 4(c) and (d)).

Fig. 4 Hepatic gene expression of sterol regulatory element-binding protein-2 (Srebp2), LDL receptor (Ldlr) and hydroxy-3-methyl-glutaryl-CoA reductase (Hmgcr) in sham-operated (![]() ) or ovariectomised (Ovx,
) or ovariectomised (Ovx, ![]() ) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the sham-operated rats (P <0·001; ovariectomy as the main effect). Mean values were significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
) rats fed either a standard diet (SD), a high-fat (HF) diet, a HF+0·25 % cholesterol (HFHC (0·25 %)) diet or a HF+0·5 % cholesterol (HFHC (0·5 %)) diet. Values are means, with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the sham-operated rats (P <0·001; ovariectomy as the main effect). Mean values were significantly different from that of the SD: † P <0·05, †† P <0·01, ††† P <0·001 (diet as the main effect).
Discussion
The aim of the present study was to identify novel mechanisms involved in hepatic steatosis induced by a HFHC diet and oestrogen deficiency in rats. Although the relevance of the present data to postmenopausal women needs to be established, to our knowledge, the present study is the first to provide hepatic and plasma lipid profiles accompanied by molecular changes that together indicate a defect in VLDL assembly upon ovariectomy and/or HFHC diet consumption. Additionally, our molecular analyses revealed a second potential novel mechanism through which ovariectomy and HFHC diets may collectively enhance hepatic steatosis, namely defective hepatic bile acid secretion.
Ovariectomy and high-fat/high-cholesterol diets concomitantly repressed VLDL assembly-related enzymes
The present study corroborated the synergistic effect of high cholesterol consumption and the oestrogen deficiency state by ovariectomy on the development of hepatic steatosis. We found that liver TAG concentration was higher by 37 to 78 % in all Ovx rats compared with sham-operated rats fed the corresponding diets. More importantly, the highest liver TAG levels were observed in Ovx rats fed the HFHC diets, reaching up to 270 % of the level measured in SD-fed sham-operated rats. We observed that Ovx rats compared with sham-operated rats presented substantially lower gene expression levels of Acat2 and Dgat2, enzymes involved in the synthesis of cholesterol esters and TAG, respectively, which are the two components of the VLDL lipid core( Reference Willner, Tow and Buhman 25 , Reference Liu, Millar and Cromley 26 ). In addition, the gene expression of Mttp, a determinant enzyme that interacts with the N-terminus of apoB allowing initial lipid transfer into nascent VLDL particles, was lower in Ovx rats than in sham-operated rats( Reference Hussain, Bakillah and Nayak 16 ). Finally, Cideb, a molecule recently identified as critical in VLDL lipidation, and Sar1 GTPase, involved in VLDL transfer from the endoplasmic reticulum to the Golgi apparatus, were also lower in Ovx rats than in sham-operated rats( Reference Tiwari, Siddiqi and Siddiqi 19 ). Collectively, these results are consistent with the interpretation that VLDL assembly is disrupted upon ovariectomy.
Besides the effects of ovariectomy, it appears that HFHC feeding also disrupted VLDL assembly and secretion. The gene expression of Mttp measured in all HFHC-fed rats was decreased to the levels as low as 31 % of those reported in SD-fed sham-operated rats (Fig. 2(a)). Similar decreases in the gene expression of Mttp following the HFHC diets have been previously reported by us( Reference Cote, Ngo Sock and Levy 14 ) and others( Reference Savard, Tartaglione and Kuver 15 ). In addition, we found that plasma TAG levels were largely reduced in sham-operated and Ovx rats fed the HFHC diets. Since the liver provides the major source of plasma TAG under the fasted state, it has been reported that fasted plasma TAG level can be considered as an indirect marker for hepatic VLDL production( Reference Bjorkegren, Karpe and Milne 27 ). Therefore, lower levels of fasted plasma TAG found in sham-operated and HFHC-fed Ovx animals further support the possibility that HFHC feeding impairs liver VLDL secretion. Taken together, the present results suggest that impaired VLDL production contributes to hepatic lipid accumulation in Ovx rats as well as in all rats fed the HFHC diets. Moreover, disruption of VLDL production is a mechanism that may explain the high accumulation of TAG found in the liver of Ovx rats fed the HFHC diets compared with those fed the other diets.
Ovariectomy and high-fat/high-cholesterol diets down-regulated the gene expression of bile acid excretion
An additional plausible explanation for hepatic cholesterol accumulation in Ovx rats fed the HFHC diets is the decrease in the synthesis and excretion of biliary acids. This view is supported by the observation that animals fed the HFHC diets had a lower gene expression level of Fxr, the regulator of hepatic bile acid metabolism, and its target gene Cyp8b1. The specific role of hepatic Fxr is to initiate the expression of a complete gene network involved in the synthesis and excretion of bile acids in order to prevent bile acid hepatotoxicity. Since bile acids are synthesised from cholesterol in hepatocytes, bile acid synthesis is also an important pathway to remove cholesterol from the liver( Reference Vlahcevic 28 ). Furthermore, bile acid secretion exerts the driving force for biliary cholesterol excretion, another pathway for liver cholesterol output( Reference Oude Elferink, Paulusma and Groen 29 , Reference Oude Elferink and Groen 30 ). The role of bile acids on liver lipids and cholesterol metabolism has been enlightened by the generation of Fxr-null mice, showing massive accumulation of TAG and cholesterol in hepatocytes( Reference Sinal, Tohkin and Miyata 31 ). Dietary interventions, such as HFHC diets, have also been reported to repress the gene expression of hepatic Fxr ( Reference Cote, Ngo Sock and Levy 14 , Reference Savard, Tartaglione and Kuver 15 ). The present decrease in the gene expression levels of Fxr and Cyp8b1 in Ovx rats fed with the HFHC diets may thus be taken as an indication that bile acid metabolism may be reduced and, in turn, favours cholesterol accumulation in the liver.
Disruption in bile acid metabolism might also be involved in higher TC accumulation found in the liver of Ovx rats compared with sham-operated rats. Ovariectomy suppressed the transcription factors Fxr and Lxr as well as Cyp8b1 and Bsep, enzymes involved in bile acid synthesis and excretion, respectively. In line with these results, Czerny et al. ( Reference Czerny, Teister and Juzyszyn 32 – Reference Czerny, Teister and Juzyszyn 34 ) found a significant decrease in total bile production in Ovx rats, thus supporting the hypothesis that biliary metabolic pathways are disrupted in Ovx animals. Since disrupted biliary metabolic pathways restrain hepatic cholesterol output, the repression of key enzymes involved in bile acid excretion by ovariectomy as well as by HFHC diet consumption is consistent with the massive accumulation of liver TC in Ovx rats fed the HFHC diets.
Ovariectomy and high-fat/high-cholesterol diets repressed hepatic cholesterol regulatory molecules
Liver TC content was not changed in Ovx rats compared with sham-operated rats after the SD and HF diet interventions, corroborating previous reports( Reference Kamada, Kiso and Yoshida 4 , Reference Kato, Ogawa and Kishida 35 ). However, liver TC levels accumulated in both Ovx and sham-operated rats following the HFHC diets and to a larger extent in Ovx rats than in sham-operated rats, indicating a synergistic action of these two interventions (Fig. 1). Liver TC levels accumulated in these rats despite the fact that cholesterol synthesis, as supported by the transcript levels of Hmgcr, was reduced in Ovx rats and by the HFHC diets in sham-operated animals. An increase in TC content in the liver of Ovx rats has also been associated with a lower gene expression level of the transcription factor Srebp2 and its target gene Ldlr ( Reference Ngo Sock, Chapados and Lavoie 36 ). Accordingly, the mRNA levels of Srebp2 and Ldlr were decreased following ovariectomy and HFHC diet consumption in the present study. The lower expression of Ldlr may explain the hypercholesterolaemia observed in Ovx rats following the SD and HF dietary interventions. In contrast, the higher liver cholesterol levels found in Ovx rats compared with sham-operated rats fed the HFHC diets might be associated with a reduction in VLDL synthesis and secretion, as mentioned previously.
Ovariectomy and high-fat/high-cholesterol diets differently affected plasma lipid profile
As reported previously( Reference Lucas, Mahajan and Soung do 37 ), Ovx rats had significantly higher plasma TC concentrations than sham-operated rats following the SD. Unexpectedly, this difference between the Ovx and sham-operated animals was entirely abolished after consumption of the HF and HFHC diets. An intriguing outcome was that following the HFHC diets, the difference between the Ovx and sham-operated rats on plasma cholesterol concentration was even reversed, with higher levels being found in sham-operated rats than in Ovx rats. Given that these lower plasma cholesterol levels in Ovx rats following the HFHC diets are accompanied by higher liver TC levels, it may be deduced that ovariectomy led to the impaired mobilisation of cholesterol from the liver.
In summary, the results of the present study indicate that HFHC diets and ovariectomy concomitantly stimulated hepatic lipid and cholesterol accumulation. Molecular changes observed in VLDL assembly and bile acid regulation of key molecules suggest that HFHC diets and ovariectomy both affect hepatic lipid retention through a decrease in VLDL assembly and bile acid synthesis.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514002517
Acknowledgements
The present study was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC; 7594) and from the Canadian Institutes of Health Research (CIHR; T 0602 145.02).
The authors’ contributions are as follows: I. C. was responsible for the conception, design and acquisition of the data; N. A. C. was involved in the acquisition of the data; J.-M. L. contributed to the conception and design of the study. All authors interpreted the data, drafted/revised the manuscript for important intellectual content, and approved the final version.
The authors declare that they have no conflicts of interest.







