Depression is a major cause of illness burden to societies worldwide, with increasing effects on personal suffering, work disability, and use of healthcare resources( Reference Wittchen, Jacobi and Rehm 1 ). The 1-year prevalence of depressive disorders in Finland has been estimated to be about 8 % in women and 5 % in men( Reference Pirkola, Isometsä and Suvisaari 2 ).
The pathogenesis of depression is thought to involve multiple interacting risk factors, covering biological( Reference Belmaker and Agam 3 ), psychological and psychosocial factors( Reference Kendler, Gardner and Prescott 4 – Reference Kendler, Gatz and Gardner 6 ), for example maladaptive consequences on personality and behaviour of being exposed to adverse childhood experiences, losses, and other stressful life events in biologically vulnerable persons. The possible pathophysiological mechanisms of depression have been linked to functional and structural brain abnormalities( Reference Palazidou 7 ). They are mostly related to genetic vulnerability, the malfunction of noradrenergic and serotonergic neurotransmitter systems that modulate brain areas concerning mood, thinking, sleep, appetite and behaviour, as well as to the role of dysregulation of the stress hormone cortisol( Reference Belmaker and Agam 3 ) and the immune response system( Reference Bufalino, Hepgul and Aguglia 8 ). Depression is a disorder with a high prevalence( Reference Baumeister and Harter 9 ) and a frequently recurrent and chronic course( Reference Kessler, Berglund and Demler 10 ). For this reason, research is needed to identify potential biological, psychological and environmental risk factors in order to prevent its prevalence.
Vitamin D has many physiological roles( Reference DeLuca 11 ). In addition to its classic role in bone metabolism, vitamin D may also have many potential non-skeletal functions. This is supported by the finding that vitamin D receptors are expressed not only in cells related to Ca and P metabolism, but also in other cells and tissues in the human body( Reference DeLuca 11 ). Vitamin D appears to play an important role in brain development and function, and may be directly involved in the autocrine or paracrine regulation of the brain( Reference Eyles, Smith and Kinobe 12 , Reference Eyles, Burne and McGrath 13 ).
Some recently published larger cross-sectional studies have demonstrated an inverse association between serum 25-hydroxyvitamin D (25(OH)D) concentration and depression. For example, a Norwegian population-based study on 10 086 individuals has indicated that low serum 25(OH)D concentrations are associated with depressive symptoms measured by the Hopkins Symptoms Check List 10( Reference Kjaergaard, Joakimsen and Jorde 14 ). In addition, one study conducted in a sample of 12 594 US men and women showed that low serum 25(OH)D concentration is associated with depressive symptoms, measured by the Center for Epidemiologic Studies-Depression (CES-D) scale, particularly in subjects with a history of depression( Reference Hoang, Defina and Willis 15 ). Similar results have also been found in a large, nationally representative sample of 7970 young adults in the USA assessed by the Diagnostic Interview Schedule( Reference Ganji, Milone and Cody 16 ). Furthermore, an inverse association has been demonstrated in three studies on older adults where depression or depressive symptoms were measured using the CES-D scale and the Diagnostic Interview Schedule( Reference Hoogendijk, Lips and Dik 17 ), the Geriatric Depression Scale (GDS10)( Reference Stewart and Hirani 18 ) and the Beck Depression Inventory (BDI)( Reference Lee, Tajar and O'Neill 19 ).
It has been shown that serum 25(OH)D is associated with a large number of sociodemographic, lifestyle and metabolic health-related variables( Reference Jääskeläinen, Knekt and Marniemi 20 ). This, and the finding of a lack of association with depression after controlling for potential confounding factors in two studies( Reference Pan, Lu and Franco 21 , Reference Zhao, Ford and Li 22 ), underlines the need for large-scale studies with a comprehensive set of serum 25(OH)D determinants. The objective of the present study was to assess the cross-sectional associations between serum 25(OH)D concentration and the prevalence of depression after comprehensively controlling for potential confounding factors in a representative sample of the Finnish adult population. To ensure that there is a specific association between serum 25(OH)D and depression, we also evaluated the association between serum 25(OH)D and anxiety disorder.
Subjects and methods
Study population
The present cross-sectional study was based on the Health 2000 Survey, which was carried out in Finland from 2000 to 2001( Reference Heistaro 23 ). The Health 2000 Survey included interviews, self-administered questionnaires and a comprehensive health examination. The study population consisted a two-stage stratified cluster sample representing the adult population aged 30 years and over living in mainland Finland. The sample frame was regionally stratified according to five university hospital regions. In the first stage of sampling, eighty healthcare districts were sampled as a cluster. In the second stage, a random sample of individuals from each of the eighty healthcare districts was drawn from a national population register. The study population of the Health 2000 Survey consisted of 8028 individuals, of whom 85 % participated in a health examination. The present study included 2524 men and 2847 non-pregnant women aged 30–79 years whose serum 25(OH)D concentrations were determined and whose depressive symptoms and diagnoses of depressive and anxiety disorders were assessed.
Written informed consent was obtained from all the participants of the survey. The present study was approved by the Ethical Committee for Research in Epidemiology and Public Health at the Hospital District of Helsinki and Uusimaa in Finland.
Methods
Current depressive symptoms and depressive and anxiety disorders
Current depressive symptoms were measured using the BDI( Reference Beck, Ward and Mendelson 24 ), and the cut-off point ≥ 10 points( Reference Beck, Steer and Garbin 25 ) indicated the presence of symptoms (1404 cases). Diagnoses of depressive disorder (354 cases), major depressive disorder (271 cases), dysthymic disorder (131 cases) and anxiety disorder (222 cases) were assessed using a computerised version of the Munich-Composite International Diagnostic Interview, allowing the estimation of diagnoses for mental disorders during the past 12 months, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)( 26 , Reference Wittchen, Lachner and Wunderlich 27 ).
Serum 25-hydroxyvitamin D assay
Serum samples were collected from the subjects between September 2000 and March 2011. Serum 25(OH)D concentrations were determined based on serum samples stored at − 70°C using RIA (DiaSorin) between January 2001 and November 2002. The inter-assay CV for 25(OH)D concentration measurements was 7·80 % at a mean concentration of 47·3 nmol/l. The laboratory where the analyses were carried out participated in an external quality control programme run by Labquality Limited. Serum 25(OH)D concentration was categorised according to quartiles (7–33, 34–43, 44–55 and 56–134 nmol/l). Serum 25(OH)D concentration less than 50 nmol/l was considered as a cut-off point of vitamin D deficiency( Reference Holick 28 , Reference Ross, Taylor, Yaktine and Del Valle 29 ).
Sociodemographic and lifestyle factors and chronic diseases
Interviews and self-administered questionnaires provided information on marital status (unmarried, married or cohabiting, divorced and widow/widower), education ( < 7, 7–12 and >12 years), leisure-time physical activity (no, moderate ≥ 4 h/week and vigorous ≥ 3 h/week), smoking status (never, former smoker and current smoker) and alcohol consumption (male 0, 1–199 and ≥ 200 g/week; female 0, 1–99 and ≥ 100 g/week)( Reference Heistaro 23 ). Currently perceived economic problems were also determined using a questionnaire on a 5-point ordinal scale that asked whether the household had to make considerable or major compromises in consumption. Likewise, currently perceived lack of social support was determined by asking the respondent whether there was no one close by to get help or support from in time of exhaustion, when in need of practical help or emotional support. Perceived health was determined in the interview based on a 5-point scale that ranged from good to poor. Also, chronic diseases were self-reported in the interview (stroke, coronary artery disease, diabetes and cancer) or diagnosed in the health examination (musculoskeletal disorder).
Diet and vitamin D supplementation
Data on diet were collected using a self-administered FFQ that was designed to estimate average food intakes over the preceding year( Reference Männistö, Virtanen and Mikkonen 30 , Reference Paalanen, Männistö and Virtanen 31 ). Participants recorded their average consumption of food items and prepared dishes in nine frequency categories ranking from ‘never or seldom’ to ‘at least six times per d’. Average food consumption and intakes of nutrients per d were calculated using software developed at the National Institute for Health and Welfare (Finessi) and the National Finnish Food Composition Database (Fineli®)( Reference Reinivuo, Hirvonen and Ovaskainen 32 ). The FFQ also included questions about the regular use of vitamin D supplements, which included single-ingredient vitamin D supplements and multivitamin supplements including vitamin D.
Dietary index
A dietary index (modified Alternate Healthy Eating Index, mAHEI) was created based on the definition of the nine-item Alternate Healthy Eating Index( Reference McCullough, Feskanich and Stampfer 33 , Reference Sääksjärvi, Knekt and Lundqvist 34 ). The mAHEI was adapted to the Finnish diet and included seven components (vegetables, fruits and berries, legumes, nuts, seeds and soyabeans, white:red meat ratio, rye, polyunsaturated:saturated fat ratio, and trans-fat intake). The components were divided into quintiles and, with the exception of trans-fat intake, received scores in ascending order, such that the lowest quintile received 1 point and the highest quintile 5 points. Alcohol consumption and multivitamin use were excluded because alcohol consumption was considered an independent lifestyle factor in the present study and habitual multivitamin use is not recommended in Finland. Scores of all components were summed to the total score that ranged from 7 (lowest) to 35 (highest). A higher score indicated a healthier diet.
Lifestyle index
A lifestyle index was created based on the following five factors: BMI; physical activity; smoking status; alcohol consumption; diet( Reference Hu, Manson and Stampfer 35 ). A healthy lifestyle was defined as a BMI < 25·0 kg/m2, regular leisure-time physical activity at least approximately 30 min/d (moderate ≥ 4 h/week or vigorous ≥ 3 h/week), no current smoking, alcohol consumption ranging from 1 to 99 g/week in women or 1 to 199 g/week in men, and an above-median total score on the mAHEI index (21 points)( Reference Laaksonen, Knekt and Rissanen 36 ). For each factor, participants who met the criteria for a healthy lifestyle received 1 point, while those who did not meet the criteria were scored 0. Thus, the total score ranged from 0 to 5, with higher scores suggesting a healthier lifestyle than lower scores.
Metabolic health-related factors
Weight, height and waist circumference were measured with subjects wearing light clothing and without shoes, and BMI (kg/m2) was calculated( Reference Heistaro 23 ). Blood pressure was measured twice by the auscultation method, and the mean value of the two measurements was used. Fasting blood samples were taken and stored at − 70°C. Serum fasting glucose and TAG concentrations were analysed by enzymatic methods (Triglycerides GPO PAP, Glucose and Hexokinase; Olympus System Reagent) and serum HDL-cholesterol by a direct method (HDL-C Plus; Roche Diagnostics GmbH). The positive metabolic syndrome was defined according to the harmonisation definition( Reference Alberti, Eckel and Grundy 37 ) as the presence of any three of the following five risk factors: elevated waist circumference ( ≥ 94 cm in men and ≥ 80 cm in women); elevated serum TAG ( ≥ 1·7 mmol/l); reduced HDL-cholesterol ( < 1·0 mmol/l in men and < 1·3 mmol/l in women); elevated mean blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or antihypertensive drug treatment); elevated serum fasting glucose level ( ≥ 5·6 mmol/l).
Statistical analyses
The associations between serum 25(OH)D concentration and potential confounding and effect-modifying factors were estimated using linear models. Multivariate logistic regression was used to evaluate the cross-sectional association between serum 25(OH)D concentration and the prevalence of depressive disorder. Relative odds (OR) and 95 % CI between quartiles of serum 25(OH)D were calculated. Test for trend was performed using the likelihood ratio test, with serum 25(OH)D included as a continuous variable in the model. The population attributable fraction (PAF) was assessed based on the estimated OR. The PAF estimated the proportion of cases (e.g. depressive disorder) in a given population that would theoretically not have occurred if all of the individuals had low-risk target values of the risk factors (i.e. serum 25(OH)D concentration at least 50 nmol/l) of interest instead of their true values( Reference Laaksonen, Härkänen and Knekt 38 ). This was done by combining information about the prevalence of the risk factor in the population with estimates of the strength of the association between the risk factor and the outcome. The rest of the factors in the model retained their values. In the calculation of PAF, a causal relationship between the risk factors and the outcome was assumed. Two-sided 95 % CI of the PAF were estimated by the delta method.
We defined three main-effects models. Model 1 (OR only) included age, sex and month of serum sampling for serum 25(OH)D determinations. Model 2 (OR only) further included education and four lifestyle factors (BMI, leisure-time physical activity, smoking status and alcohol consumption). Model 3 (OR and PAF) was additionally adjusted for the components of the metabolic syndrome excluding waist circumference (i.e. blood pressure, serum HDL-cholesterol, serum TAG and fasting glucose). Waist circumference was excluded because BMI was already included as a lifestyle factor, both of which are measures of obesity.
Possible modification by sex, age, marital status, healthy lifestyle index, healthy dietary index (mAHEI), the metabolic syndrome, perceived economic problems, perceived social support, anxiety disorder, chronic diseases and perceived health on the prevalence of depressive disorder was studied by including an interaction term between serum 25(OH)D and the potential effect-modifying factor in model 3 one at a time.
Statistical analyses were carried out using SAS 9.2 (SAS Institute, Inc.)( 39 ).
Results
Participants with higher serum 25(OH)D concentrations (highest quartile range 56–134 nmol/l) were somewhat older, generally married or cohabiting, more educated and with no economic problems compared with others (Table 1). Participants with higher serum 25(OH)D concentrations also had a healthier lifestyle by being leaner, performing more leisure-time physical activity, smoking less often and consuming less alcohol. Their vitamin D intake from diet and supplements was higher and eating habits were healthier. In addition, their metabolic health was better, showing lower blood pressure and lower serum TAG and serum fasting glucose concentrations, and higher serum HDL-cholesterol concentrations. They also perceived their health to be better. Lower serum 25(OH)D values were more often measured during the winter season.
Table 1 Descriptive variables of the total study population by quartiles of serum 25-hydroxyvitamin D (25(OH)D) concentrations (Mean values and standard deviations or percentages, adjusted for age (continuous) and sex)

mAHEI, modified Alternate Healthy Eating Index; BDI, Beck Depression Inventory.
* 7–33, 34–43, 44–55 and 56–134 nmol/l.
† One to thirty-seven participants were excluded because of missing data.
‡ 367 participants were excluded because of missing data on diet.
§ Normal blood pressure: systolic blood pressure < 130 mmHg, diastolic blood pressure < 85 mmHg and no antihypertensive medication.
∥ At least one of the following: stroke (self-reported); coronary artery disease (self-reported); diabetes (self-reported); cancer (self-reported); musculoskeletal disorder (diagnosed).
An inverse association was found between serum 25(OH)D concentration and depressive disorder during the past 12 months after adjustment for age, sex and month for serum 25(OH)D measurements. The relative odds (OR) of the disease comparing the highest with the lowest quartile of serum vitamin D concentration was 0·56 (95 % CI 0·40, 0·78; P for trend < 0·001; Table 2). After further adjustment for potential confounding sociodemographic and lifestyle factors, the OR was slightly changed (OR 0·66, 95 % CI 0·47, 0·93; P for trend = 0·007). Further adjustment for the indicators of metabolic health did not notably alter the results (OR 0·65, 95 % CI 0·46, 0·93; P for trend = 0·006). The results for major depressive disorder (OR 0·68–0·74), dysthymic disorder (OR 0·40–0·63) and current depressive symptoms according to the BDI (OR 0·69–0·82) were quite similar; however, in models 2 and 3, the association between serum 25(OH)D concentration and current depressive symptoms attenuated to a non-significant suggestive association. However, anxiety disorders showed a different picture with no association in the different models, the OR varying from 0·92 to 1·22 between the three models.
Table 2 Depression, depressive symptoms and anxiety disorder by quartiles of serum 25-hydroxyvitamin D (25(OH)D) concentration (Odds ratios and 95 % confidence intervals)

BDI, Beck Depression Inventory.
* 7–33, 34–43, 44–55 and 56–134 nmol/l.
† Age (continuous), sex and month of blood sampling (September to March).
‡ Further included education ( < 7, 7–12 and >12 years), BMI (kg/m2, continuous), leisure-time physical activity (no, moderate ≥ 4 h/week and vigorous ≥ 3 h/week), smoking (never, former smoker and current smoker) and alcohol consumption (male 0, 1–199 and ≥ 200 g/week; female 0, 1–99 and ≥ 100 g/week).
§ Further included blood pressure (optimal, normal, high normal and hypertension), serum HDL-cholesterol (mmol/l, continuous), serum TAG (mmol/l, continuous) and serum fasting glucose (mmol/l, continuous).
The inclusion of an interaction term between serum 25(OH)D and potential effect-modifying factors in model 3 one at a time (sex, age, marital status, healthy lifestyle index, healthy dietary index (mAHEI), the metabolic syndrome, perceived economic status, perceived social support, diagnosed anxiety disorder, chronic disease and perceived health) showed that the strength of the association between serum 25(OH)D concentration and the prevalence of depression varied between several subgroups (Table 3). Higher serum 25(OH)D concentrations were associated with a lower prevalence of depressive disorder especially among men (OR 0·40, 95 % CI 0·22, 0·73), and among young and divorced individuals. Similarly, participants who had an unhealthy lifestyle or poor dietary habits, or suffered from the metabolic syndrome, showed a stronger association. By contrast, for participants who received social support or were free from anxiety disorder or chronic disease, higher serum 25(OH)D concentration was related to a lower risk of depressive disorder.
Table 3 Depressive disorder between the highest and lowest quartiles of serum 25-hydroxyvitamin D (25(OH)D) concentration in the categories of potential effect-modifying factors (Odds ratios and 95 % confidence intervals*)

mAHEI, modified Alternate Healthy Eating Index.
* Model: age (continuous); sex; month of blood sampling (September to March); education ( < 7, 7–12 and >12 years); BMI (kg/m2, continuous); leisure-time physical activity (no, moderate ≥ 4 h/week and vigorous ≥ 3 h/week); smoking status (never, former smoker and current smoker); alcohol consumption (male 0, 1–199 and ≥ 200 g/week; female 0, 1–99 and ≥ 100 g/week); blood pressure (optimal, normal, high normal and hypertension); serum HDL-cholesterol (mmol/l, continuous); serum TAG (mmol/l, continuous); serum fasting glucose (mmol/l, continuous).
† Depressive disorder cases in the total population.
‡ OR and 95 % CI for the fourth quartile of serum 25(OH)D with the first quartile as a referent group.
§ BMI, leisure-time physical activity, smoking and alcohol consumption were excluded from the model.
∥ BMI, blood pressure, serum HDL-cholesterol, TAG and fasting glucose were excluded from the model.
¶ At least one of the following: stroke (self-reported); coronary artery disease (self-reported); diabetes (self-reported); cancer (self-reported); musculoskeletal disorder (diagnosed).
Serum 25(OH)D appeared to be strongly associated with depressive disorder in terms of the PAF (Table 4). If a causal relationship between serum 25(OH)D and depressive disorder is assumed, 19 (95 % CI 4, 31) % of all cases could have been avoided if serum 25(OH)D concentration of each individual had been at least 50 nmol/l. The association was non-significant for major depressive disorder (PAF 15 %, 95 % CI − 3, 29 %) and dysthymic disorder (PAF 23 %, 95 % CI − 5, 43 %) and weaker for depressive symptoms (PAF 10 %, 95 % CI 1, 18 %). Anxiety disorders did not show any reduction at higher concentrations of serum 25(OH)D (PAF − 3 %, 95 % CI − 22, 12 %).
Table 4 Population attributable fractions (PAF)* for diagnoses of depression, anxiety and depressive symptoms at a serum 25-hydroxyvitamin D (25(OH)D) concentration of at least 50 nmol/l (Odds ratios and 95 % confidence intervals)
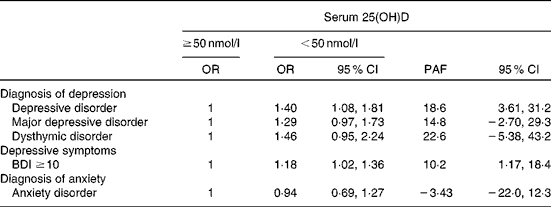
BDI, Beck Depression Inventory.
* Model: age (continuous); sex; month of blood sampling (continuous); education ( < 7, 7–12 and >12 years); BMI (kg/m2, continuous); leisure-time physical activity (no, moderate ≥ 4 h/week and vigorous ≥ 3 h/week); smoking (never, former smoker and current smoker); alcohol consumption (male 0, 1–199 and ≥ 200 g/week; female 0, 1–99 and ≥ 100 g/week); blood pressure (optimal, normal, high normal and hypertension); serum HDL-cholesterol (mmol/l, continuous); serum TAG (mmol/l, continuous); serum fasting glucose (mmol/l, continuous).
Discussion
Findings
The present cross-sectional study suggests that a higher serum 25(OH)D concentration is associated with a reduced risk of depressive disorder and depressive symptoms in a representative sample of the Finnish adult population. This association remained significant after adjustment for potential confounding factors related to sociodemographic status, lifestyle and metabolic health. Conversely, there was no association between serum 25(OH)D concentration and anxiety disorder.
The present results confirm the findings of previous cross-sectional studies indicating that serum 25(OH)D is inversely associated with the diagnosis of depression( Reference Ganji, Milone and Cody 16 ) or depressive symptoms( Reference Kjaergaard, Joakimsen and Jorde 14 , Reference Hoang, Defina and Willis 15 ). In contrast, two cross-sectional studies among US adults( Reference Zhao, Ford and Li 22 ) and elderly Chinese( Reference Pan, Lu and Franco 21 ) have reported a lack of association between serum 25(OH)D and depression after controlling for potential confounders (e.g. sociodemographic factors, lifestyle and chronic diseases). However, in the present study, adjustment for a large number of potential confounding factors only slightly attenuated the association, thereby reducing the possibility that the association is caused by confounding factors. In addition, few randomised trials and cohort studies have reported contradictory results. In one small randomised double-blind trial among overweight and obese Norwegians, it has been indicated that 1 year of supplementation with vitamin D at high doses reduced depressive symptoms, as measured by the BDI( Reference Jorde, Sneve and Figenschau 40 ). However, in a randomised double-blind trial on postmenopausal US women, 2 years of supplementation with vitamin D did not reduce depressive symptoms, as measured by the Burnam scale( Reference Bertone-Johnson, Powers and Spangler 41 ). Also, a few cohort studies, most of them based on some subpopulation of age, sex or disease, investigating the prediction of vitamin D status on the incidence of depression have given contradictory results, showing either an inverse association( Reference Milaneschi, Shardell and Corsi 42 , Reference May, Bair and Lappe 43 ) or no association( Reference Chan, Chan and Woo 44 ). Recently, a study based on depressed individuals has shown an inverse association of serum 25(OH)D concentrations with the severity of depressive symptoms and the risk of developing depressive disorder during a 2-year follow-up( Reference Milaneschi, Hoogendijk and Lips 45 ).
A potential role of vitamin D in the pathophysiology of depression and the exact biological mechanisms behind this association remain mainly unknown, but some plausible hypotheses have been presented. Because receptors of vitamin D and vitamin D-activating enzyme 1α-hydroxylase are present in human brain regions (e.g. prefrontal cortex and hippocampus) known to be associated with the pathophysiology of depression( Reference Eyles, Smith and Kinobe 12 , Reference Eyles, Burne and McGrath 13 ) it is possible that vitamin D may play a role in this process. It has been suggested that vitamin D deficiency may contribute to disruptions in the neuroendocrine and central nervous systems( Reference Garcion, Wion-Barbot and Montero-Menei 46 ). These disruptions may lead to further dysregulation of neurotransmission and neurotransmitter metabolism and signalling, neuroprotection, neuroimmunomodulation, and the modulation of glucocorticoid-related actions in hippocampal cells, potentially leading to depression( Reference Garcion, Wion-Barbot and Montero-Menei 46 , Reference Obradovic, Gronemeyer and Lutz 47 )
The present study investigating the interactions between serum 25(OH)D and selected potential effect-modifying factors showed, in contrast to two previous studies( Reference Kjaergaard, Joakimsen and Jorde 14 , Reference Milaneschi, Shardell and Corsi 42 ), that the association between serum 25(OH)D concentration and the prevalence of depression was stronger among men than among women. We also found that vitamin D protects against depression in the younger age group (30–59 years), but not among older adults. One explanation for the weaker association in the older age group might be that in the present data, low vitamin D concentrations are more common among younger individuals( Reference Jääskeläinen, Knekt and Marniemi 20 ). Another explanation might be the small number of depressive disorder cases (n 56) among older adults. We also made an interesting finding that the higher the serum 25(OH)D concentration, the lower the risk of depression, particularly among those who were divorced, had an unhealthier diet or lifestyle or had the metabolic syndrome. Assuming that there really is a causal relationship between vitamin D and depression, this may suggest that vitamin D provides protection against depression, with currently unknown mechanisms, especially for individuals with poor socio-economic status, lifestyle choices and metabolic health. Conversely, among individuals free from chronic diseases or anxiety disorder, the association between serum 25(OH)D concentration and the risk of depression was stronger than among those suffering from these diseases.
Methodological issues
The study has several strengths. First, the results are based on a large representative sample of the Finnish adult population, allowing the estimation of the PAF. Second, depression and anxiety were assessed with widely used and validated instruments. Third, we used serum 25(OH)D concentration as the indicator of vitamin D status. Serum 25(OH)D concentration reflects vitamin D obtained from both diet and supplements and through subcutaneous synthesis stimulated by exposure to UVB radiation from sunlight. Fourth, we took into account a large variety of potential confounding and effect-modifying factors, which were determined by reliable methods( Reference Heistaro 23 ). Finally, we compared the results according to depressive and anxiety disorders. Serum 25(OH)D concentration was not associated with anxiety disorders, which reduces the possibility that vitamin D is generally associated with mental health. It may support the hypothesis that there is a biological mechanism for the association between serum 25(OH)D concentration and the prevalence of depression.
There are also some limitations. First, due to the cross-sectional nature of the study, any conclusions about causality cannot be drawn. It is possible that low serum 25(OH)D concentration may be a result of depression. Individuals suffering from depression may spend less time outdoors or have a poor diet low in natural (e.g. fish) or fortified (milk products and margarine) vitamin D. Second, despite the large number of potential confounding and effect-modifying factors considered in the present study, it is possible that residual confounding still remains. Finally, there are also some limitations relating to serum 25(OH)D measurements. Serum 25(OH)D concentration was measured only once, thus reflecting only recent exposure and providing no information on intra-individual seasonal variation. Furthermore, the possibility that the concentrations may have declined during storage, although only a small decline has been reported in the analyses based on frozen serum samples( Reference Antoniucci, Black and Sellmeyer 48 , Reference Ocke, Schrijver and Obermann-de Boer 49 ), cannot be ruled out. It should also be noted that mean serum 25(OH)D concentration reported in the present study is an underestimate of the full-year average, because the present data do not include any values for the summer months. In addition, the RIA used may have underestimated serum 25(OH)D concentrations( Reference Perna, Haug and Schöttker 50 ).
Conclusions
In conclusion, the present study suggests a protective effect of vitamin D against depressive disorder, but not against anxiety disorder, even after adjustment for a large number of potential confounding factors. If this association is causal, our finding may be of importance for public health policy, especially in populations with low concentrations of serum vitamin D. For example, in Finland, the annual costs due to depression are about 1 billion euros( Reference Sillanpää, Andlin-Sobocki and Lönnqvist 51 ). In the case of vitamin D providing protection against depression, elevation of serum 25(OH)D concentration to a level above 50 nmol/l in the total population would result in the avoidance of every fifth prevalent case with depressive disorder and in subsequent savings. Because the hypothesis of the inverse association between serum 25(OH)D concentration and the prevalence of depression is still mainly based on observational studies with a cross-sectional design, and because the determinants of vitamin D are not yet fully understood, large-scale cohort studies and experimental evidence are needed before any firm conclusions can be drawn.
Acknowledgements
The present study was financially supported by the Academy of Finland (grant no. 138876), the Yrjö Jahnsson Foundation and the Juho Vainio Foundation.
The author's contributions are as follows: T. J. and P. K. contributed to the study concept and design; P. K. was responsible for the supervision of the study; T. J., P. K., J. S., S. M. and T. P. assisted with the implementation of the data; T. J. wrote the draft of the manuscript and performed the statistical analyses; T. J., P. K., J. S., S. M., T. P., K. S., N. E. K., N. K. and O. L. were involved in the interpretation of the data and critical revision of the manuscript.
There are no conflicts of interest.






