The definition and criteria for the diagnosis of the metabolic syndrome have been discussed intensively worldwide. However, the concept of the term ‘metabolic syndrome’ is well known and relates to the clustering of the following metabolic risk factors: impaired glucose tolerance, high blood pressure, elevated TAG levels, low HDL-cholesterol levels, insulin resistance and central adiposity. The last two factors have been considered to contribute to the occurrence of all the others(Reference Alberti, Eckel and Grundy1, Reference Simmons, Alberti and Gale2). The ‘metabolic syndrome’ concept reminds health professionals about the importance of assessing each related risk factor. This would allow an early detection of risk factors and the establishment of strategies for the reduction of incidence of CVD and diabetes.
It is well recognised that an excessive supply of energy to the host associated with low expenditure plays an important role in the development of obesity(Reference Albala, Vio and Kain3). The enlargement of fat depots, particularly abdominal obesity, favours the development of insulin resistance(Reference Després and Lemieux4). The gastrointestinal tract is the site of absorption of nutrients that provide energy to the host. The interactions between dietary molecules, physiological conditions (digestive fluids, hormones secretion, motility, absorption rate) and microbiota within the gastrointestinal tract influence metabolic homeostasis(Reference Gastaldelli, Natali and Vettor5).
The gut microbiota has been suggested to be involved in the control of body weight and regulation of insulin resistance(Reference Bäckhed, Ding and Wang6, Reference Cani, Neyrinck and Fava7). The mechanisms proposed by which the microbiota would interact with the metabolism of the host include influence on gut-hormone production, intestinal permeability and endotoxaemia, and the provision of additional energy through SCFA(Reference Blaut and Klaus8, Reference Diamant, Blaak and de Vos9).
SCFA are organic fatty acids with one to six carbons. The most important SCFA for human metabolism are acetic, propionic and butyric, which arise mainly from bacterial fermentation in the gut(Reference Wong and Jenkins10). They can provide energy and are possibly involved in lipid and glucose metabolism in the host(Reference Wong and Jenkins10), suggesting that they may have an impact on the occurrence of metabolic risk factors.
In fact, an increased concentration of SCFA in faeces has been related to higher body weight gain in an animal model(Reference Turnbaugh, Ley and Mahowald11) and in obese humans(Reference Schwiertz, Taras and Schäfer12). In both studies, differences in gut microbiota composition were also reported. This has led to the hypothesis that differences in gut microbiota composition between the lean and the obese might contribute to a higher capacity to harvest energy from the diet by an increased production of SCFA, subsequently leading to weight imbalance. However, associations between faecal SCFA with different metabolic risk factors have not yet been reported. Hence, the aim of the present study was to determine the concentration of SCFA in faeces of lean and obese women and analyse whether associations between SCFA and variables included in the concept of the metabolic syndrome, such as abdominal obesity, glucose, insulin, TAG and HDL levels, are present.
Experimental methods
Subjects
The recruitment of female volunteers occurred using written announcements. Women interested in participating in the study were interviewed by phone. The subjects were selected based on the following criteria: age over 18 years, not pregnant or breast-feeding, free of any liver, thyroid or gastrointestinal disease, not taking any kind of supplements or medications in the last 6 months, except oral contraceptives, being lean (BMI 18·5–24·9 kg/m2) or obese (BMI>30 kg/m2). The subjects were taken into the study after they provided a written informed consent. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committee of the Federal University of Viçosa (protocol number 001/2010, Federal University of Viçosa).
A total of twenty lean females (BMI 21·5 (sd 1·39) kg/m2) and twenty obese females (BMI 35·04 (sd 3·98) kg/m2) of similar age (mean age of the lean and obese groups 28·5 (sd 7·6) v. 30·7 (sd 6·5), P= 0·33) participated in the study. According to a recent brief medical history, all subjects were healthy.
The subjects were evaluated at the Laboratory of Energetic Metabolism and Body Composition. After fasting for 10 h, they were weighed (wearing light clothes). Their body composition was analysed by tetra polar bio-impedance (BodySystems®). Blood pressure was measured and blood samples were collected for biochemical analyses. Fresh faecal samples were collected and then immediately frozen at − 20°C.
Biochemical analysis
Blood samples were analysed at the Laboratory of Clinical Analysis of the Health Division at the Federal University of Viçosa. The biochemical assessments included total cholesterol and lipoproteins (enzymatic colorimetric method), aspartate and alanine aminotransferases (kinetic colorimetric method), fasting plasma glucose (enzymatic colorimetric method of glucose-oxidase) (all the kits used were purchased from Bioclin/Quibasa) and insulin through the electrochemiluminescence method using the Modular Analytics E170 e Elecsys 2010 (Roche Diagnostics®). LDL concentration was estimated by the Friedwald formula(Reference Friedewald, Levy and Fredrickson13). The homeostasis model assessment (HOMA) index was used as an indicator of insulin resistance and calculated as follows(Reference Matthews, Hosker and Rudenski14):
Insulin resistance was diagnosed based on HOMA>2·71 according to Geloneze et al. (Reference Geloneze, Repetto and Geloneze15).
Food intake
Volunteers were instructed to fill in three food records on non-consecutive days and including one weekend day. Food records were analysed using the software DietWin Professional®, and the mean value from the 3 d was used for subsequent statistical comparison of the main macronutrients and total fibre intake between the groups and its correlation with SCFA concentration.
Faecal SCFA analysis
The extraction of SCFA was based on the method of Smiricky-Tjardes et al. (Reference Smiricky-Tjardes, Grieshop and Flickinger16). Briefly, around 800 mg of frozen faeces were weighed and homogenised with the addition of 1 ml of m-phosphoric acid solution (25 %). For each sample, this step was performed in duplicate. After incubation at room temperature for 30 min, the samples were centrifuged (Refrigerated microcentrifuge, HERMLE Z 216MK; Hermle Labortechnik) at 17 319 g for 30 min at 4°C. Then, the supernatants were transferred to a new Ependorf tube. After a second centrifugation, the supernatants were collected and subsequently frozen at − 20°C. Before analysis, a third centrifugation and supernatant collection were performed. The final volume of supernatants from each duplicate was mixed together and homogenised. Butyric, propionic and acetic acids were measured by GC (model CG-17A; Shimadzu®) equipped with a flame ionisation detector and capillary Nukol column (30 m × 0·25 mm; Supelco®). N2 was used as the carrier gas and the flux in the column was 1·0 ml/min. The temperatures of the injector and detector were set at 220 and 250°C, respectively. Initial column temperature was 100°C sustained for 5 min, rising at 10°C/min until it reached 185°C. Next, the samples were injected (1 μl) through a Hamilton® syringe (10 μl) in split system 5. The total run time was 33·5 min. Concentrations were given as parts per million. In order to transform the results from parts per million to percentage of faecal mass, we considered that the concentration in parts per million represents the mass of the specific SCFA in 2 ml of the solution, which in turn reflects the mass present in approximately 1600 mg of faeces (the exact weight of faeces was used for calculation). The results were represented as per 100 mg of faeces (% w/w).
Statistical analysis
Statistical analyses were performed using the software Sigma Plot for Windows version 11.0 (Systat® Software). To assess if all the variables differed between the obese and lean groups, two different tests were used. Normally distributed variables were analysed by Student's t test; otherwise, the Mann–Whitney test was used. Throughout the paper, the data are expressed as median (minimum–maximum). Spearman's correlation test was performed to measure the degree of correlation between each SCFA concentration and other metric variables. The level of significance was considered to be 5 %.
Results
Subjects’ characteristics
As shown in Table 1, both obese and lean groups had normal blood pressure levels, but obese women presented higher medians of systolic and diastolic blood pressure (P< 0·05) compared to lean subjects. Weight, BMI, waist circumference and adiposity were significantly higher in the obese group (P< 0·05). All the obese volunteers showed waist circumference greater than 80 cm. The biochemical variables are presented in Table 2 and it can be seen that subjects of both groups are normoglycaemic and normolipidaemic. However, fasting glucose was higher in the obese group (P= 0·027) as compared to lean subjects. The higher fasting insulin and HOMA index (P< 0·001) indicate the presence of insulin resistance in the obese group, which was detected in 45 % of obese women. The obese group had a lower level of HDL compared to the lean group (P= 0·001). In addition, 90 % of obese women (n 18) had HDL levels lower than 1·295 mmol/l. The ratios of total cholesterol:HDL and LDL:HDL were statistically higher in the obese group compared to the lean group (P< 0·05).
Table 1 Clinical characteristics of the study subjects (Median and minimum (min)–maximum (max) values)
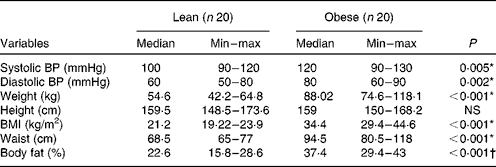
BP, blood pressure.
* Mann–Whitney.
† Student's t test.
Table 2 Biochemical characteristics of lean and obese women (Median and minimum (min)–maximum (max) values)
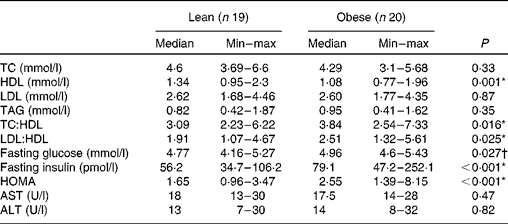
TC, total cholesterol; HOMA, homeostasis model assessment; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
* Mann–Whitney.
† Student's t test.
Food intake
The obese group presented a higher intake of energy, carbohydrates and lipids (P< 0·05) as compared to the lean group. The total intake of fibre was similar between both the groups assessed (P= 0·42). The median value of protein intake was slightly higher in the obese group (P= 0·058) than in the lean subjects (Table 3). No correlation was found between macronutrients and faecal SCFA concentration (data not shown).
Table 3 Habitual macronutrient, fibre and energy intake of the lean and obese groups (Median and minimum (min)–maximum (max) values)
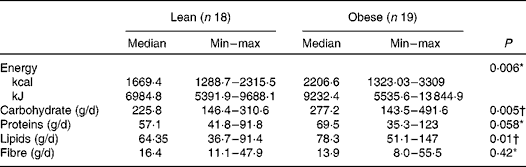
* Mann–Whitney.
† Student's t test.
Faecal SCFA
A higher percentage of butyric, acetic and propionic acids was observed in the faeces of the obese group (P< 0·05), as shown in Table 4. Also, a higher proportion of acetic acid was recorded in both groups. Additionally, the proportion of acetic:propionic:butyric acids, taking into account the median values, was also similar: 1·4:1:1 in the lean group and 1·3:1·1:1 in the obese group.
Table 4 Concentration (% w/w) of faecal SCFA from the lean and obese groups (Median and minimum (min)–maximum (max) values)
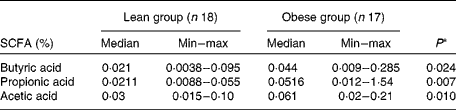
* Mann–Whitney.
Correlation tests were run for all variables analysed in the study, but only those which presented a statistically significant correlation (P< 0·05) were included in Table 5. Body fat, waist circumference, blood pressure, insulin and HOMA index were positively correlated with SCFA concentrations, while on the contrary, HDL was inversely correlated with butyric and acetic acids (P< 0·05).
Table 5 Correlation coefficient (ρ) from comparison of faecal SCFA concentration with anthropometric, blood pressure and biochemical variables
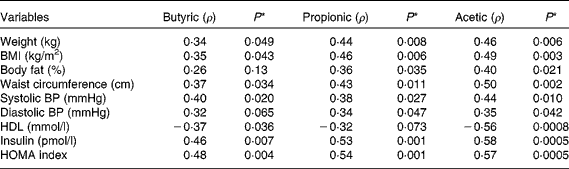
BP, blood pressure; HOMA, homeostasis model assessment.
* Spearman correlation test.
Discussion
Our present study is the first to investigate the association of faecal SCFA with clinical variables relevant to the diagnosis of the metabolic syndrome. The presence of these specific relationships suggests that SCFA modulation might be a target for weight management and abdominal obesity reduction, which are believed to improve metabolic risk factors and help in the prevention of metabolic diseases(Reference Wing, Lang and Wadden17).
In our study, we found that higher waist circumference was associated with an increase in SCFA concentration. Waist circumference has been considered a useful preliminary tool for the prediction of abdominal adiposity and metabolic syndrome screening(Reference Alberti, Eckel and Grundy1, Reference Janssen, Heymsfield and Allison18). The amount of intra-abdominal fat is related to a reduced insulin-stimulated glucose uptake rate in the skeletal muscle and in all fat depots(Reference Virtanen, Iozzo and Hällsten19), suggesting that reduction of waist circumference is important for proper glucose metabolism and the loss of central fat depots.
We found that propionic and acetic acids positively correlated with waist circumference, weight, BMI and body fat percentage, demonstrating that SCFA might contribute to increased fat depots. Higher faecal concentrations of propionate and acetate induced by colonisation of germ-free mice with specific bacteria-producing SCFA resulted in higher weight and fat gain(Reference Samuel, Shaito and Motoike20). Acetate and especially propionate are signalling molecules for the GPR41 receptor, the activation of which increases host adiposity(Reference Samuel, Shaito and Motoike20). The loss of GPR41 is associated with reduced efficiency to harvest energy from the diet. The interaction between the GPR41 receptors and SCFA results in increased absorption of SCFA used as a substrate for lipogenesis in the liver(Reference Samuel, Shaito and Motoike20). This shows that inhibition of SCFA activation of GPR41 could be a potential therapeutic target for control of fat gain. To reinforce this potential, acetate and propionate were also showed to inhibit lipolysis in cell culture, and thus favour lipid accumulation(Reference Hong, Nishimura and Hishikawa21).
Insulin levels and HOMA index are considered as markers of the metabolic syndrome related to fat accumulation(Reference Arner22). In our study, a positive correlation of insulin levels and HOMA with the SCFA analysed was observed. The secretion of insulin can be activated by the stimulation of the receptor GPR40 in pancreatic cells. Long-chain NEFA can interact with GPR40 receptor, induce an increase in intracellular Ca2+ and amplify glucose-stimulated insulin secretion in a cell culture model. On the contrary, acetic and butyric acids do not induce insulin secretion(Reference Itoh, Kawamata and Harada23). However, in overweight individuals the increase of acetate in blood, induced by lactulose intake, results in a slight increase in blood insulin, perhaps sufficient to decrease NEFA in plasma(Reference Ferchaud-Roucher, Pouteau and Piloquet24) through the stimulation of lipoprotein lipase(Reference Otarod and Goldberg25). Although this action helps to reduce plasma NEFA, it can favour higher body weight and fat percentage. The role of SCFA in insulin secretion is thus controversial and needs further investigation.
In the present study, negative correlations were found between butyric and acetic acids and HDL. The influence of SCFA on HDL metabolism is not well established. Lower carbohydrate intake in a low-energy diet resulted in weight loss and an increase in HDL concentration(Reference Sacks, Bray and Carey26). On the other hand, a reduction in carbohydrate intake is associated with lower butyric acid production(Reference Duncan, Belenguer and Holtrop27). Based on these studies, one may assume a negative correlation between HDL and butyrate and acetate. However, the exact mechanism still needs to be discovered.
The quantity and proportion of different SCFA found in the gut reflect the amount and type of substrate in the diet (especially carbohydrates resistant to digestion, carbohydrates which escape absorption in the small intestine and also proteins), gut microbiota composition and activity, and transit time of the ingested food(Reference Wong and Jenkins10, Reference Macfarlane and Macfarlane28). However, the specific mechanisms that could explain higher faecal SCFA concentrations in obese subjects are not yet clarified. Whether it is a result of an increased metabolic activity of specific bacterial groups, or a general increased intake of dietary substrates, still needs to be further investigated. The bacterial colonisation of the large intestine depends on the availability of dietary molecules that escape digestion and absorption by the host cells. The amount and type of such ‘non-digestible’ molecules, mainly carbohydrates, can influence the composition and metabolic activity of specific bacterial groups within the large-intestine environment(Reference Flint, Bayer and Rincon29). The microbiome of obese individuals is enriched with several carbohydrate metabolic pathways(Reference Turnbaugh, Hamady and Yatsunenko30), which possibly could lead to increased production of SCFA. In our study population, higher dietary intake (energy, carbohydrates, lipids) was observed in obese women. In fact, higher dietary intake contributes to higher weight, waist circumference and body fat percentage. Thus, it is important to emphasise that although significant correlations were observed, our study design does not support a relationship of causality between SCFA and the clinical variables analysed. Higher SCFA could reflect an increased capacity of microbiota of obese subjects to harvest more energy from the diet, contributing directly to weight gain, adiposity and indirectly to insulin resistance. More studies are needed to assess if the higher concentrations of faecal SCFA are just a consequence of obesity or if they could directly contribute to the development of obesity and its metabolic complications.
Taken together, our results suggest that a higher faecal concentration of SCFA is associated with metabolic risk factors and thus may have an impact on waist circumference, adiposity, blood pressure, HOMA index, HDL and insulin levels. Thus, the modulation of faecal SCFA levels could be a target for metabolic homeostasis. However, the mechanisms explaining how SCFA can contribute to metabolic alterations need to be further investigated.
Acknowledgements
The present study was supported by FAPEMIG. T. F. S. T. is the recipient of a CAPES grant. T. F. S. T., J. B. and M. C. G. P. participated in the design and protocol of the study. T. F. S. T. carried out the study, S. C. C. F. helped with the statistical analyses and C. L. L. F. F. participated in the SCFA assays. T. F. S. T. and L. G. wrote the manuscript. None of the authors had a personal or financial conflict of interest.







