The management of paediatric obesity using multidisciplinary interventions combining nutritional strategies, psychological approaches and physical activity programs has previously been shown as effective for weight loss(Reference Dâmaso, De Piano and Campos1–Reference Caranti, Inoue and Lira3). However, the benefits are difficult to maintain over time, leading to frequent weight regain in the following months and years(Reference Rank, Wilks and Foley4,Reference Reinehr, Widhalm and l'Allemand5) . Interestingly, although mainly considered for its effect on increasing energy expenditure, physical activity has been shown to impact both components of energy balance. Some authors have indeed highlighted the importance of physical activity to also indirectly influence energy intake (EI) and appetite sensations(Reference Blundell, Gibbons and Caudwell6–Reference Casanova, Beaulieu and Finlayson8). Beneficial appetitive responses to physical activity have been recently highlighted in children and adolescents with obesity(Reference Thivel, Finlayson and Blundell9). When performed at moderate-to-high intensity, physical exercise has been shown to favour a transient anorexigenic effect, decreasing energy intake without affecting appetite feelings in adolescents with obesity(Reference Thivel, Rumbold and King10). More recently, physical exercise has also been shown to counteract the compensatory appetitive responses that are observed in response to similar energy deficits induced by dietary restrictions, supporting a negative energy balance(Reference Thivel, Doucet and Julian11–Reference Fillon, Pélissier and Beaulieu13).
Although the available literature has mainly explored the effect of acute exercise alone or in comparison to similar energy deficits induced by dietary restriction, Hägele et al. recently examined appetitive responses to different levels of exercise energy expenditure matched with raised levels of dietary energy intake, producing different levels of ‘energy flux’(Reference Hägele, Büsing and Nas14). Briefly, energy flux in this context corresponds to the total volume of energy consumed through energy intake and expenditure, independent of energy balance status (i.e. deficit, maintenance or surplus of energy). Thus, a high-energy flux (HEF) is determined by a high level of EI where the level of energy expenditure is also high(Reference Bosy-Westphal, Hägele and Müller15). In healthy adults, Hägele et al. showed that HEF was associated with lower subsequent ad libitum intake, an increase in Glucagon-like peptide-1 (GLP-1) concentrations and a decrease in acylated ghrelin, insulin concentrations, hunger and desire to eat(Reference Hägele, Büsing and Nas14). A HEF has also been shown to induce a greater increase in post-exercise and 24 h fat oxidation(Reference Burton, Malkova and Caslake16,Reference Nas, Büsing and Hägele17) . In a pilot study, Paris et al. (2016) showed that following weight loss, a higher energy flux was associated with a higher basal metabolic rate and fat oxidation, lower perception of hunger and greater satiety in adults with obesity, compared with a similar energy balance achieved through a lower energy flux(Reference Paris, Foright and Werth18). The authors then suggested that a higher energy flux might help in limiting the metabolic adaptations and in reducing hedonic-driven food intake following weight loss, which are often associated with weight regain. To date, only one study has investigated the effects of the level of energy flux in adolescents with normal weight. The authors reported that a HEF may be more effective in reducing body fat gain over a 3-year follow-up period, in part because of its association with higher resting metabolic rate(Reference Hume, Yokum and Stice19). While all these studies have been conducted in adults or lean adolescents, it seems important to examine the potential role of energy flux on appetite regulation in paediatric obesity in order to inform weight management interventions.
In that context, the aim of this study was to compare the short-term effects of low, moderate or high-energy flux on food and macronutrient intake, appetite sensations and food reward in adolescents with obesity. We hypothesised that a HEF would be associated with lower ad libitum food intake, lower appetite sensations and lower liking and wanting for high fat foods compared with a low-energy flux (LEF).
Methods
Participants
Sixteen adolescents (aged 12–16 years, Tanner stages 3–5, 5 males) with obesity (as defined by Cole et al. 2000)(Reference Cole, Bellizzi and Flegal20) were included in the study and recruited from the Local Pediatric Obesity Center (Tza Nou, La Bourboule, France). The inclusion criteria to participate in this study were (1) adolescents aged between 12 and 16 years; (2) to have obesity based on sex- and age-specific World Obesity Federation cut-off points (Cole et al. 2000, 2012); (3) to be free of any medication that could interact with the results of the study; (5) not to be involved in any energetic restriction or weight loss program through physical activity at the time of inclusion or during the last 6 months; (7) to be free of any alcohol or tobacco use and (8) to not present any contraindications to physical activity and) to practice less than 2 h of physical activity per week according to the International Physical Activity Questionnaire. Parents or legal guardians were informed of the purpose of the study, and written informed parental and child consent was obtained. The NEXT project was approved by the relevant Human Research Ethics Authority (RBHP 2021 BOIRIE 2-2021-A02867-34) and registered as a clinical trial (NCT05365685).
Study design
After an inclusion visit with a paediatrician to ensure the eligibility of the adolescents to complete the whole study, their resting energy expenditure was evaluated and all of them performed a maximal aerobic test on a bicycle ergometer to determine their peak oxygen consumption (VO2peak). Their body composition was assessed by dual-energy X-ray absorptiometry. Afterwards, the adolescents completed three 14-h experimental sessions (separated by at least 7 d) in a randomised crossover design: (i) low-energy flux (LEF); (ii) moderate energy flux (MEF); and (iii) high-energy flux (HEF). During the LEF session, the adolescents did not perform any physical activity and their breakfast and lunch were calibrated at respectively 500 and 700 kcal. In MEF and HEF sessions, the energy intake of breakfast was identical and calibrated at 500 kcal (as in the LEF session) and lunches were increased by additional 250 (MEF) or 500 kcal (HEF) compared with the LEF condition. In MEF and HEF sessions, adolescents performed a moderate intensity cycling exercise (65 % of their individual VO2peak) during the afternoon, inducing an energy expenditure of either 250 (MEF) or 500 kcal (HEF), and whose duration was individually calibrated. Regardless of the conditions, adolescents were not allowed to consume food or beverages (except water) in-between meals. Ad libitum food intake was measured at dinner (07.00) as the primary outcome. Subjective appetite sensations taken at regular time intervals throughout the day and food reward measured before and after lunch and before dinner in all three conditions (as secondary outcomes). The study design is presented in Fig. 1.
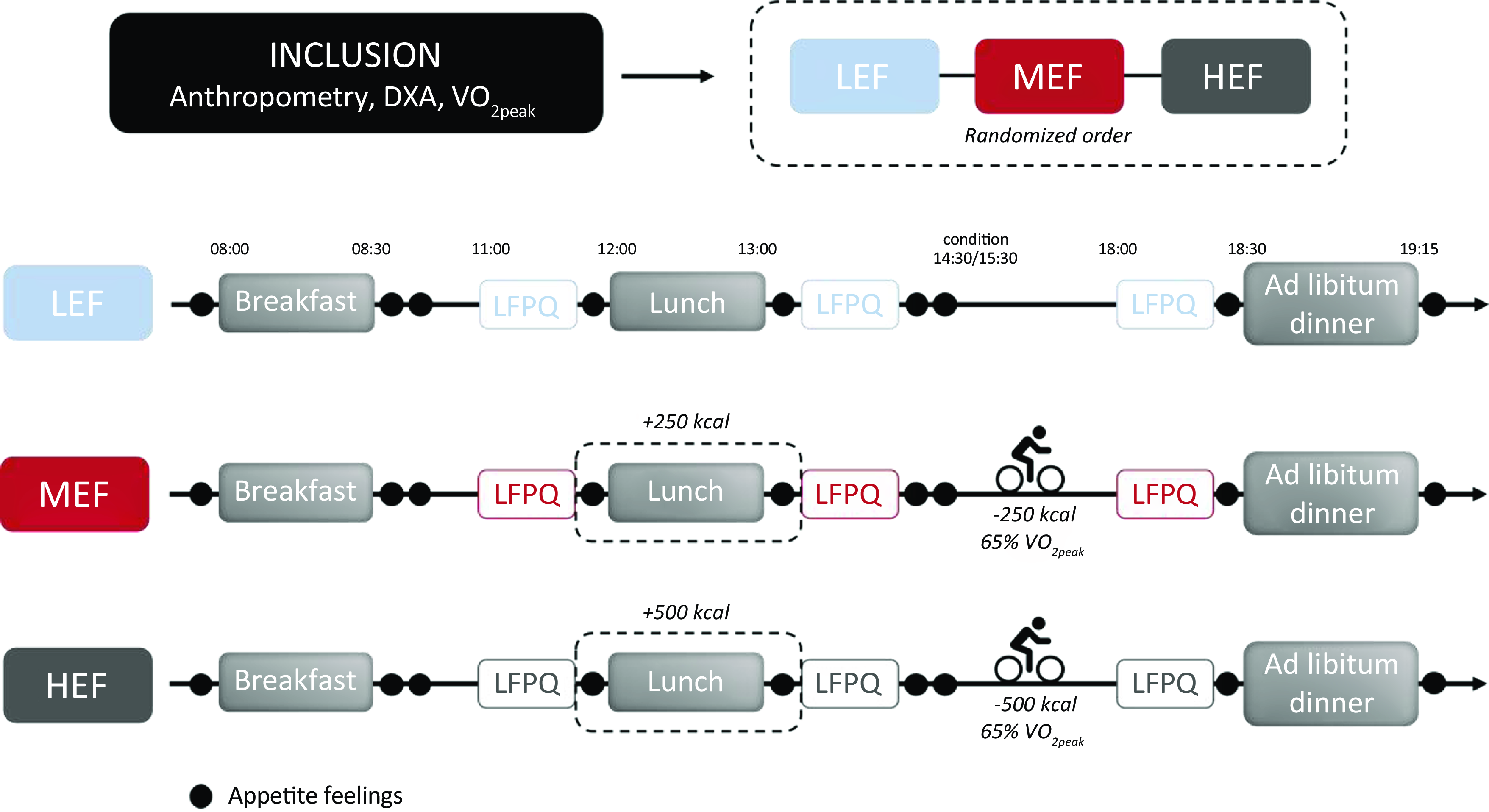
Fig. 1. Design of the study. HEF, high-energy flux; LEF, low-energy flux; LFPQ, Leeds Food Preference Questionnaire; MEF, moderate energy flux.
Anthropometric and body measurements
Body weight was measured using a digital scale, and height was obtained using a standard wall-mounted stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Body composition (fat mass, lean mass and bone mineral mass) was assessed by dual-energy X-ray absorptiometry (DXA) following standardised procedures (QDR-4500A, Hologic, Inc.).
Resting and maximal aerobic capacity
Resting and maximal aerobic capacity were assessed in the Department of Sport Medicine, Functional and Respiratory Rehabilitation (Clermont-Ferrand University Hospital). The adolescents were first asked to remain quiet at rest in a supine or semi-supine position for 20 min while wearing a face mask measuring their gas exchanges by indirect calorimetry. The last 10 min of this 20 min period were used to calculate their resting energy expenditure. Then, their maximal aerobic capacities were determined during a maximal incremental cycling test supervised by a specialised medical investigator(Reference Rowland21). The test started with 3 min at an initial power of 30 watts for girls and 40 watts for boys, followed by an increment of 15 watts per minute. During this maximal aerobic capacity test, electrocardiogram, heart rate, blood pressure and gas exchanges (oxygen consumption (VO2), carbon dioxide production (VCO2) and ventilation (VE)) were continuously assessed (BreezeSuite Software, Medgraphics Cardiorespiratory Diagnostics). Adolescents were encouraged by the experimenters to perform their best and maximum effort. The criteria for cessation of exercise were those determined by Rowland (1996): (i) to reach more than 90 % of theoretical maximal heart rate (210–0·65 × age); (ii) respiratory exchange ratio (VCO2/VO2) above 1·1 or (iii) a plateau of VO2. The VO2peak was defined as the highest VO2 value averaged over 30 s. For this test, the adolescents had not performed any physical activity in-between breakfast and the measure (this procedure has been previously detailed by Fillon et al. 2023; Pélissier et al. 2022).
Energy expenditure
During MEF and HEF sessions, adolescents performed a single bout of moderate intensity cycling exercise (65 % of their individual VO2peak). The heart rate corresponding to 65 % of their individual VO2peak from the maximal exercise test was determined and used to regulate the intensity of the exercise bout. Based on the results of the maximal aerobic capacity test, the duration was individually calculated to generate a total energy expenditure of 250 (MEF) or 500 kcal (HEF), maintaining a continuous intensity using a heart rate monitor (Polar V800). During LEF session, adolescents had to remain inactive and could not engage in any physical activity during the day.
Energy intake
During the three experimental sessions, adolescents received their breakfast at 08.00 a.m. and lunch at 12.00 p.m. In all three conditions, a 500 kcal breakfast was served in accordance with the nutritional recommendations for their age (total energy content and macronutrient composition)(Reference Pradalié22). Lunch was set at 750 kcal for LEF, 900 kcal for MEF and 1200 for HEF, in order to increase the energy intake of 250 (in MEF) or 500 kcal (in HEF). To note, the quantity of carbohydrates was increased to keep an equivalent proportion of macronutrients similar in all the sessions. The distribution of macronutrients for each condition is shown in Table 1 in Supplementary Materials. Dinner was served as an ad libitum buffet, and food consumption was measured by members of the investigation team who weighed each food item before and after the meal (this methodology has been previously used and validated in other studies)(Reference Thivel, Doucet and Julian11–Reference Fillon, Pélissier and Beaulieu13,Reference Thivel, Genin and Mathieu23) . The components of the meals were chosen on the basis of food preference and dietary habits questionnaires filled out during the inclusion visit. Foods indicated as ‘preferred’ by the participants were not offered during these sessions in order to avoid any influence of their palatability on the participants’ consumption. Similarly, foods that were indicated as liked but not regularly consumed were not presented so as not to create occasional consumption. Considering the answers from all the adolescents, a common buffet was elaborated and composed of white ham; turkey; eggs; French been, mashed potatoes, cheese, yoghurt, compote, and bread. Importantly, the adolescents were not informed about the main purpose of the study and that their energy intake (EI) was assessed. The ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail) nutritional composition was used to calculate energy and macronutrients intake (quantity and proportion) (Ciqual Table, ANSES 2020). Relative EI (REI) at dinner was calculated according to the following formula used in several previous studies(Reference Pélissier, Julian and Beaulieu12,Reference Fillon, Pélissier and Beaulieu13,Reference Masurier, Mathieu and Fearnbach24,Reference Miguet, Fillon and Khammassi25) : REI (kcal) = EI (kcal) – EE (kcal). The energy expenditures induced by the respective exercises were used to calculate REI during MEF and HEF sessions. Regarding the LEF session, resting energy expenditure assessed before the maximal aerobic test was used to estimate REI according to the MEF and HEF exercise durations. Since the exercises did not have the same duration on MEF and HEF, two REI were calculated for LEF: REI_1 (using the exercise duration of MEF) and REI_2 (using the exercise duration of HEF).
Appetite sensations
Appetite sensations were taken at regular intervals throughout the experimental days (before, immediately after and +30 min after breakfast; before, immediately after, +30 min and +60 min after lunch as well and before and immediately after dinner) using visual analogue scales (VAS) of 150 mm(Reference Flint, Raben and Blundell26,Reference Drapeau, King and Hetherington27) . Adolescents reported their sensations of hunger, satiety, desire to eat (DTE) and prospective food consumption (PFC) with 0 corresponding to ‘Not at all’ and 150 to ‘A lot’. AUCs were calculated for lunch (Lunch+60 min AUC) and the day (total AUC) using the trapezoid method. The satiety quotient (SQ) for hunger, satiety, DTE and PFC were calculated as follows : SQ (mm/kcal) = ((pre-meal rating (mm)) – (post-meal rating (mm))/energy content of the meal (kcal)) × 100(Reference Drapeau, King and Hetherington27).
Food reward and hedonic responses
Adolescents completed the Leeds Food Preference Questionnaire (LFPQ) 30 min before lunch, just after lunch and 30 min before dinner. This questionnaire was developed to measure different components of food reward, liking, and wanting(Reference Finlayson, King and Blundell28). Adolescents were asked to answer a series of questions about their food preferences (food choice) by selecting, in a 10-min computer exercise, the foods they preferred from a number of pictorial items. In the same way, they were asked during this exercise to estimate, using a 100 mm visual analogue scale, how much they would like to eat certain foods divided into four categories: (i) savoury and high-fat food; (ii) savoury and low-fat food; (iii) sweet and high-fat food; and (iv) sweet and low-fat food. The two questions used were: (i) ‘How pleasant would it be to taste this food now?’ (explicit liking); and (ii) ‘How much do you want to eat this food now? Frequency and speed of image selection were registered and enabled to measure implicit wanting. We obtained 2 scores, the ‘fat bias’ and the ‘sweet bias’, for each food reward component. The fat bias score was calculated by subtracting low-fat scores from high-fat scores, and the sweet bias score by subtracting savoury scores from sweet scores. If the score was positive for the fat or the sweet bias, there is a preference for high-fat relative to low-fat food and sweet relative to savoury food, respectively(Reference Oustric, Thivel and Dalton29). A French version of the LFPQ (LFPQ-fr), recently developed and validated according to the recommended cultural validation process(Reference Oustric, Thivel and Dalton29), was used in this work (Thivel et al. submitted).
Statistical analysis
Randomization was performed using a randomization list predetermined by the study biostatistician (BP) using permuted blocks and remained confidential until after database lock. Sample size was estimated according to (i) the CONSORT 2010 statement, extension to randomized pilot and feasibility trials and (ii) Cohen’s recommendations, which define effect-size bounds as small (ES: 0·2), medium (ES: 0·5) and large (ES: 0·8, ‘grossly perceptible and therefore large’) and (iii) previous works reported in literature(Reference Thivel, Finlayson and Blundell9,Reference Thivel, Doucet and Julian11,Reference Masurier, Mathieu and Fearnbach24,Reference Fearnbach, Silvert and Keller30–Reference Thivel, Chaput and Adamo33) . As reported in prespecified sample size estimation section of our protocol, for a two-sided type I error at 0·017 (correction due to multiple comparisons between conditions), at least 14 patients were needed in order to highlight an effect size greater than 1 (i.e. minimal differences between conditions equals one standard-deviation), especially for energy Intake, for an 80 % statistical power and an intra-individual correlation coefficient fixed at 0·5. It was proposed to include 18 participants to take into account unavailable data (such as lost to follow-up, etc.). Continuous data were expressed as mean ± standard deviation (sd). The assumption of normality was assessed using the Shapiro-Wilk test. The comparisons between conditions were carried out using random-effects models for cross-over designs considering the following effects: i) condition, period, sequence, and their interaction as fixed effects and ii) participant as random-effect to model between and within subject variability. Effect sizes were calculated and interpreted as small (ES: 0·2), medium (ES: 0·5) and large (ES: 0·8, ‘grossly perceptible and therefore large’). The normality of residuals estimated from these models was analyzed as aforementioned. When appropriate, a logarithmic transformation was applied to access the normality of dependent variables. The statistical analyses were performed using Stata software version 15 (Stata Corp). Statistical tests were two-sided with the type-I error set at 5 %, applying a Sidak’s type I error correction to consider multiple comparisons.
Results
The descriptive characteristics of the study sample are presented in Table 1 for 14 (4 males) of the 16 adolescents initially enrolled because 2 of them did not eat the entire calibrated lunch of the first experimental day which affects the total EI and therefore the energy flux over the day. The whole data analysis was conducted on these 14 participants. They had a mean age of 12·6 ± 1·0 years, an average BMI of 39·9 ± 9·2 kg/m2 and a BMI percentile above the 97th percentile (98·9 ± 0·4). Regarding their body composition, their fat-free mass was 64·6 ± 18·8 kg, and their fat mass was 38·8 ± 4·2 %. The adolescents had a mean relative VO2peak of 20·2 ± 5·0 ml/min/kg. The duration of the exercise bout was on average 37 ± 7 min and 73 ± 14 min in respectively MEF and HEF condition, and the targeted heart rate (HR) was 145 ± 11 beats per minute.
Table 1. Descriptive characteristics of the adolescents (n 14)
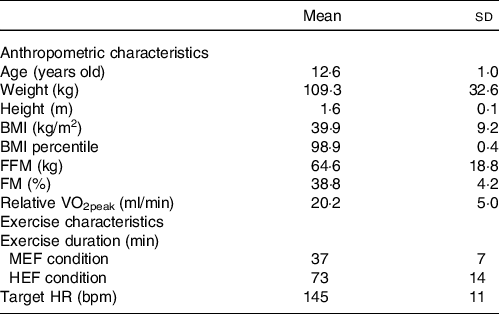
BMI, body mass index; FM, fat mass; FFM, fat-free mass; HEF, high-energy flux; HR, heart rate; LEF, low-energy flux; MEF, moderate-energy flux; VO2peak, peak oxygen consumption.
Energy and macronutrient intake
Figure 2 displays all the detailed results regarding dinner and REI. Results showed significantly higher ad libitum EI at dinner in LEF (1541 ± 423 kcal) compared with HEF (1357 ± 442 kcal; P = 0·008; ES: −0·71 (–1·23, −0·19)) while no difference was found in MEF (1463 ± 407 kcal) compared with the two other sessions (Fig. 2). REI calculated at dinner was higher in LEF (1475 ± 420 kcal for REI_1 and 1408 ± 417 for REI_2) compared with MEF (1213 ± 407 kcal; P = 0·003; ES: −0·80 (–1·33, −0·28) and P = 0·023; ES: −0·61 (–1·13, −0·08) respectively) and HEF (964 ± 463 kcal; P < 0·001; ES: −1·42 (–1·94, −0·80) and P< 0·001; ES: −1·23 (–1·76, −0·871) respectively). Also, we found a significant difference in REI at dinner between MEF and HEF (P = 0·019; ES: 0·62 (0·10, 1·15)). Total daily EI was significantly higher in HEF (3122 ± 430 kcal) compared to LEF (2766 ± 413 kcal; P< 0·001; ES: 1·44 (0·91, 1·96)) and MEF (2959 ± 394 kcal; P = 0·011; ES: −0·68 (–1·20, −0·15)) and in MEF compared to LEF (P = 0·004; ES: 0·76 (0·24, 1·28)). Regarding macronutrient intake, there was no significant difference in dinner macronutrient distribution between condition (Table 2). The absolute consumption of carbohydrates at dinner was found higher during MEF and HEF compared with LEF (P = 0·047; ES: −0·53 (–1·05, 0·006) and P< 0·001; ES: −0·93 (–1·46, 0·41) respectively) while no other significant difference was obtained. Differences in total daily energy intake difference from the LEF condition as a percentage of the increased energy expenditure induced by exercise calculated for each subject. These differences are presented in Figure S1 that shows an important inter-individual variability When considering the means for the whole sample, a higher but not significant percentage difference was obseved for the MEF compared to the HEF condition.
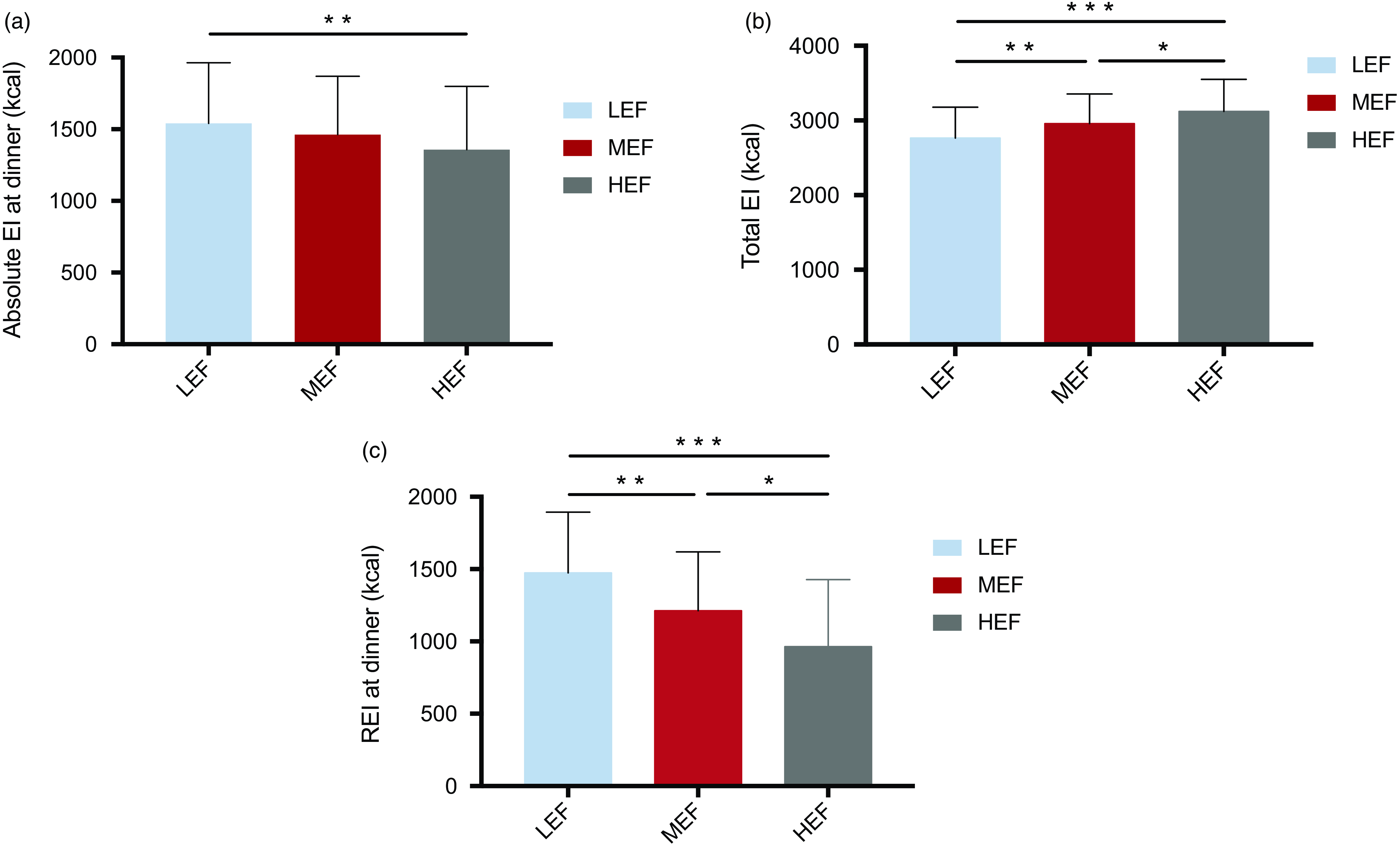
Fig. 2. Absolute and relative dinner and total ad libitum energy intake in response to the three conditions. EI, energy intake; REI, relative energy intake.
Table 2. Macronutrient intake in response to the three conditions
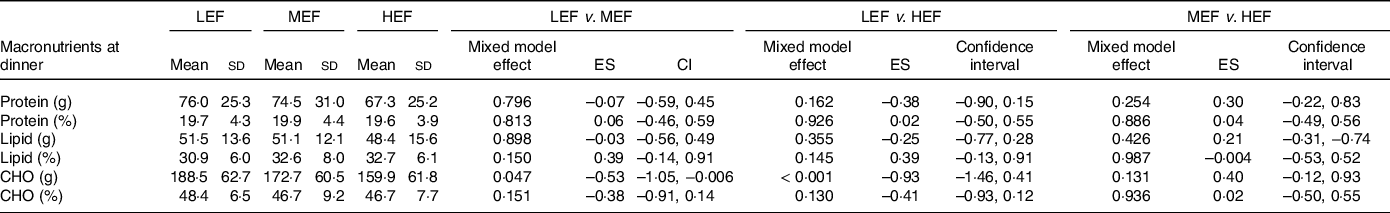
CHO, carbohydrates; ES, effect size; FM, fat mass; FFM, fat-free mass; HEF, high-energy flux; HR, heart rate; LEF, low-energy flux; MEF, moderate energy flux.
Subjective appetite feelings
As shown in Fig. 3 total AUC for hunger and DTE were lower in HEF (10 488 ± 4226 and 11 693 ± 4493 mm2 respectively) compared with both LEF (13 924 ± 5849 and 16 461 ± 5601 mm2, P < 0·001 respectively) and MEF condition (12 837 ± 4395 and 15 805 ± 5775 mm2, P = 0·038 and P< 0·001 respectively). Similar results were obtained for the PFC total AUC with a significant difference between HEF (12 119 ± 5252 mm2) and LEF condition (15 580 ± 5613 mm2, P = 0·004). For hunger, DTE and PFC, lunch+60 min AUC were significantly lower in HEF (5597 ± 3345, 6462 ± 3287 and 6617 ± 3980 mm2 respectively) than in LEF (8696 ± 4913, 10 719 ± 4815, and 9710 ± 4686 mm2 respectively, P < 0·001 for all), and MEF sessions (7418 ± 3726, 9416 ± 4373 and 8066 ± 4356 mm2 respectively, P = 0·039; P < 0·001, and P = 0·046 respectively). Considering lunch+60 min AUC for fullness, there was also a significant difference between HEF (7068 ± 4527 mm2) and LEF (4697 ± 3385 mm2, P = 0·021). Regarding hunger, fullness, and PFC, no difference was observed for the SQ at lunch and dinner between conditions. Dinner SQ for DTE was significantly lower in HEF than in LEF (P = 0·028).
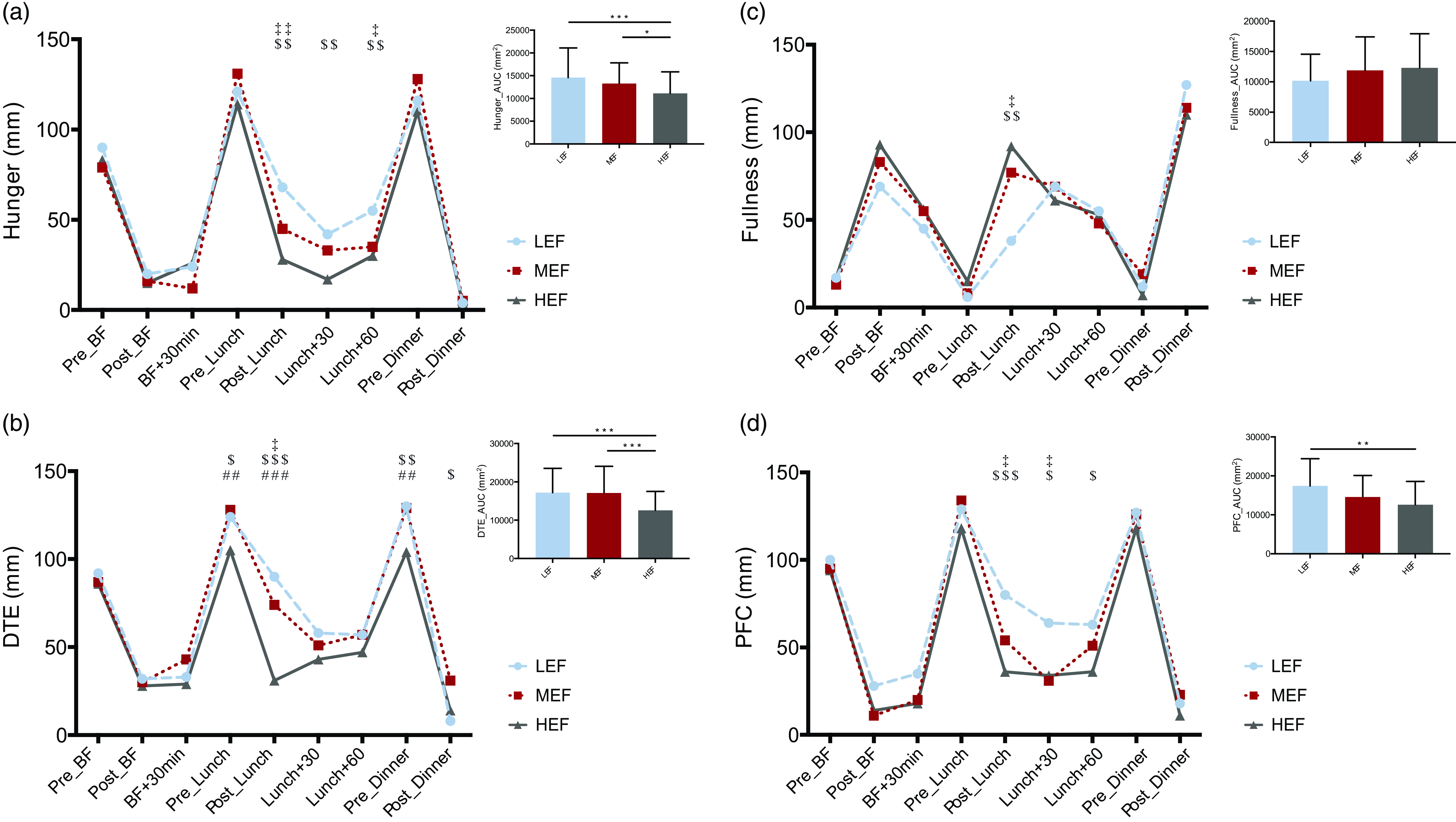
Fig. 3. Daily subjective appetite sensations and total area under the curve (AUC) for hunger, desire to eat (DTE), fullness and prospective food consumption (PFC) in response to the three conditions. (‡), LEF vs. MEF; (#), MEF vs. HEF; ($), LEF vs. HEF; (‡,#,$), P<0.05; (‡‡,##,$$), P<0.01; (‡‡‡,###,$$$), P<0.001.
Food reward
As detailed in Table 3, the components of food reward were not significantly different between conditions except for food choice where sweet bias was higher in HEF (7·6 ± 9·5 mm) compared to LEF (2·3 ± 12·7 mm; P = 0·005).
Table 3. Food reward on the three experimental conditions
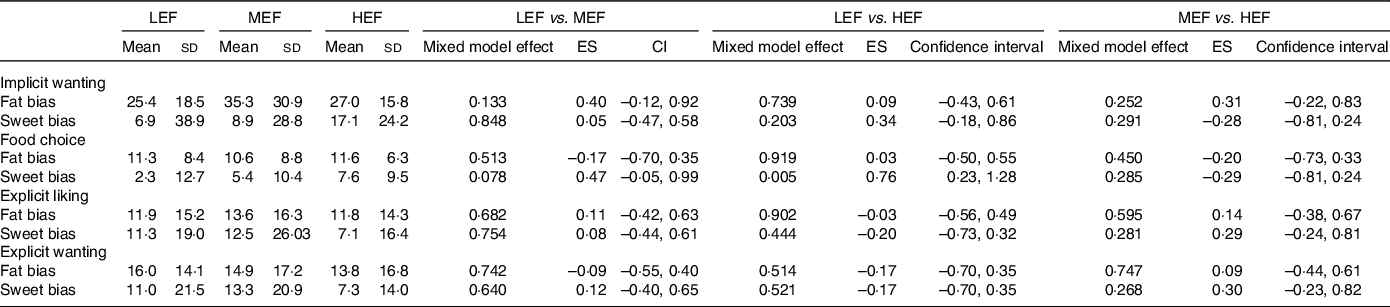
ES, effect size; HEF, high-energy flux; LEF, low-energy flux; MEF, moderate energy flux.
Discussion
In youth with overweight and obesity, there is a clear need to better understand the regulation of energy balance and especially the related behavioral and physiological compensatory mechanisms that appear after weight loss that favor weight regain. To our knowledge, this study is the first to explore the effect of energy flux on appetite and food intake in adolescent with overweight/obesity, which aimed to compare the short-term effects of different levels of energy flux (low, moderate, or high) on subsequent food and macronutrient intake, appetite sensations and food reward in this population. According to our hypotheses, our results suggest a benefit of higher energy flux via lower absolute and relative energy intake during an ad libitum test meal as well as lower subjective hunger, desire to eat and prospective food consumption over the day. We also found no differences in liking or wanting for high fat foods, but a higher bias for sweet foods was observed in the HEF condition. Overall, these findings provide preliminary evidence that comports with previously published studies performed in normal weight adolescents and adults that may suggest better regulation of appetite with higher energy flux, but more comprehensive, longer term studies are needed to substantiate the relationship between energy flux and appetite control(Reference Hägele, Büsing and Nas14,Reference Hume, Yokum and Stice19) .
According to our results, a higher energy flux resulted in lower energy intake at a subsequent ad libitum test meal in adolescents with obesity, compared to low and medium ones. These results are in line with previous observations in similar studies conducted in lean adults(Reference Hägele, Büsing and Nas14,Reference Nas, Büsing and Hägele17) . In their work with healthy adults, Hägele and colleagues for instance observed a lower ad libitum energy intake after 3 experimental days spent at higher energy flux compared to medium or low flux. Interestingly, in this last work the authors observed this lower energy intake independently of the nature of the individual’s energy balance (neutral, +25 % or –25 % EB)(Reference Hägele, Büsing and Nas14). Moreover, while the increase in energy expenditure induced on MEF compared with LEF was similar to what has been previously suggested as insufficient to impact appetite control (250 kcal)(Reference Pélissier, Julian and Beaulieu12), the findings observed during the HEF condition (500 kcal) and subsequent food intake reduction reinforce the observations in previous works that suggest the need for a minimal degree of energy deficit to positively affect appetite and food intake in youth suffering obesity(Reference Thivel, Doucet and Julian11). Overall, these results provide tentative to support for the importance of physical exercise in the management of short-term energy balance rather than emphasizing reduced food consumption which remains difficult in the context of an obesogenic food environment(Reference Mayer, Roy and Mitra34,Reference Melby, Paris and Sayer35) , via induced energy expenditure, positive cardio-respiratory and muscular effects(Reference Janssen and LeBlanc36), and a potential benefit in regulation of food intake(Reference Thivel, Rumbold and King10). Indeed, a low energy flux seems difficult to sustain in the long term for most people because, as explained by Swift et al. (Reference Swift, McGee and Earnest37), the high food availability environment results in high energy intake, which is the principal driver of the return to a HEF. Altogether, these results may suggest a potential benefit of emphasizing physical activity in the management of pediatric obesity through a more efficient coupling of energy intake and expenditure above a certain threshold of energy flux conducive to body weight maintenance(Reference Foright, Presby and Sherk38). However, longer term studies are needed to attribute the effects on energy intake to the energy flux with more confidence, given the evidence that metabolic adaptations occur over time frames longer than that used in this study.
Additionally, we observed lower overall daily sensations of hunger, DTE, and PFC as well as a lower pre-test meal DTE during the HEF condition relative to both LEF and MEF, suggesting adaptations in appetite control as a potential mechanism for the aforementioned effects on energy intake.
These findings are also in line with previous studies showing decreased hunger and DTE during HEF sessions in adults with normal weight(Reference Hägele, Büsing and Nas14). Our results could be explained by the fact that exercise has a suppressant effect on appetite that may lead to greater meal-induced satiety and better appetite regulation(Reference Blundell, Gibbons and Caudwell6,Reference Bosy-Westphal, Hägele and Müller15,Reference Hopkins and Blundell39,Reference King, Caudwell and Hopkins40) . Furthermore, based on the findings of Paris et al. (Reference Paris, Foright and Werth18) weight-reduced adults with obesity reported a decrease in subjective hunger ratings and an increase in satiety following four experimental days of HEF compared to the four days of low flux. Since a high perceived hunger sensation has been shown to predict greater weight regain(Reference Elfhag and Rössner41), the reduction thereof could contribute to limiting or preventing weight regain that is typically observed after weight loss interventions(Reference Sumithran and Proietto42). Altogether these results indicate, in line with previous studies(Reference Mayer, Roy and Mitra34,Reference Granados, Stephens and Malin43) that exercise, even when combined with higher energy intake, may improve the short-term regulation of appetite in adolescents with obesity.
In line with our expectations, we observed that lower energy flux was associated with a greater preference for high-fat/energy foods over low-fat/energy food stimuli. Low energy flux has been postulated as a metabolic state associated with lower appetite control where there may be a greater susceptibility to hedonic inputs from foods and reward driven eating, in contrast to HEF where satiety signaling could be improved and appetite is more tightly regulated(Reference Beaulieu, Hopkins and Blundell44). Contrary to expectations however, our results showed a significantly higher food choice sweet bias after HEF compared to LEF while there were no other statistical differences between conditions in bias for sweet foods. In addition, there was a dissociation between wanting and liking with an increase in wanting but a decrease in liking, following energy intake. Overall, these observed trends may indicate that, although both components are important, the measurement of liking seems to be even more important for understanding the energetic adaptations that occur following modulation of energy flux. The short-term nature of the study could partly explained these results, suggesting that liking may be more likely to change in the short term compared to wanting, which is also linked to increase in choice, as this is part of the implicit wanting metric. In this sense, a longer-term intervention and observation period might be better suited to highlight changes in wanting. Future studies are needed to further explore changes in food reward responses as a function of energy flux, with particular consideration of, as indicated by Fillon et al. (Reference Fillon, Pélissier and Beaulieu13) potentially influential factors such as the degree of adiposity and intervention duration.
The results of this study must be interpreted in light of some limitations. First, although based on previously measured resting metabolic rate and maximal aerobic capacities, the energy expenditure induced during the experimental session was estimated using heart rate records, while the use of indirect calorimeters (and ideally metabolic chambers) would have been more accurate to obtain more precise results. Second, the present work compared different levels of energy flux generated through the course of a single day, with appetitive responses mainly assessed on the basis of a single subsequent meal, thus longer term research is needed to evaluate the effects of energy flux on appetitive responses with more confidence. It could also be interesting to conduct these future studies particularly after a weight loss period to test the efficacy of exercise-induced HEF as a weight regain prevention tool. Indeed, some studies have suggested that the appetitive responses to exercise may occur over few days(Reference Beaulieu, Hopkins and Blundell44). However, research has previously demonstrated transient metabolic and appetitive adaptations can occur after acute exercise, thus it is feasible that higher energy flux may also have similar acute and short-term effects(Reference Moghetti, Bacchi and Brangani45). Finally, our study lacked information regarding the usual free-living daily energy balance and energy flux of the adolescents, thus it is not possible to compare the energy intake volumes observed here to their usual dietary habits. It is important to note that it remains extremely and resource intensive to properly track daily free-living energetic and nutritional behaviors in addition to the high inter-individual variability in appetitive responses to exercise shown in Supplementary Fig. 1. However, future research would undoubtedly benefit from a longer time frame and multiple assessments to mitigate such a limitation.
Conclusion
The findings of the present study suggest that a higher energy flux might lower ad libitum energy intake at a subsequent test meal in adolescents with obesity via lower total daily hunger, DTE, PFC and preference for high-fat foods, indicative of enhanced appetite control. Increasing energy expenditure through exercise may be more effective in improving short-term appetite control than energetic restriction alone, often considered the main target in the management of obesity. As recently reviewed by Bosy-Westphal et al. (Reference Bosy-Westphal, Hägele and Müller15), unlike exercise, small increase in energy flux induced by short sleep duration or increased mental work can lead to an increase in energy intake and thus to a deregulation of the energy balance. Future studies comparing different levels of energy flux over a longer time frame are needed to better understand and characterise the appetitive responses to energy balance manipulations that may inform design of interventions that improve sustainable weight loss interventions and fight the childhood obesity pandemic.
Acknowledgements
We are grateful to all the adolescents who participated in the study and to the Nutrition Obesity Ambulatory Hospital (UGECAM) for their support.
This work has been supported by the Challenge 3 Mobility of the CAP2025 I-Site project of UCA as well as by the Health in Motion Chaire of UCA and the French University Institute IUF.
D. T, L. I., V. J., M. D. and Y. V.: Conceptualisation; J. S., A. F., H. M., C. D. and V. J.: data curation, investigation; B. P., M. D., D. T. and J. S.: formal analysis; A. F., L. I., D. T. and V. J.: methodology, project administration; J. S. and D. T.: writing and G. S. F. and L. I.: review and editing.
The authors have no conflict of interest to disclose.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523001824









