The maintenance of a stable internal environment is the main target of all physiological processes( Reference Cravo, Lopes and Pedrino 1 , Reference Cannon 2 ), which is positively correlated with the regulation of ionic concentrations in the intracellular and extracellular compartments. Among the different types of inorganic salts present in the body fluids, NaCl is the most predominantly consumed salt and Na concentration is directly related to the maintenance of body fluid homeostasis( Reference Cravo, Lopes and Pedrino 1 , Reference Cannon 2 ). Changes in Na concentrations result in an osmotic flux between the intracellular and extracellular compartments. Na influx or efflux affects the concentrations of all the other components in these compartments. Therefore, it is not surprising that many homeostatic mechanisms exist to maintain plasma Na concentrations with a limited rate of variation.
The regulation of blood pressure (BP) involves complex mechanisms, including local, hormonal, neuronal and renal regulation, that, working together, are responsible for the redistribution of blood through changes in peripheral vascular resistance and cardiac output. Experimental evidence has demonstrated that acute increases in plasma Na concentrations trigger a set of autonomic( Reference Morita, Matsuda and Tanaka 3 – Reference May, McAllen and McKinley 6 ), humoral( Reference Pettersson, Hedner and Ricksten 7 – Reference Blanch, Freiria-Oliveira and Murphy 12 ) and renal responses. These adjustments comprise a set of integrated responses aimed at promoting increases in BP( Reference Colombari, Colombari and Lopes 13 – Reference Toney and Stocker 15 ), renal vasodilation( Reference Colombari, Colombari and Lopes 13 , Reference Pedrino, Monaco and Cravo 14 , Reference Fujita, Matsuda and Shibamoto 16 , Reference Colombari and Cravo 17 ) and consequently natriuresis, re-establishing normal volaemic conditions.
Dysfunctions in the control of plasma Na concentrations can lead to pathologies, among which hypertension predominates. In fact, epidemiological and experimental evidence has shown that a high-Na diet is a significant risk factor for the development of arterial hypertension( Reference Horan, Blaustein and Dunbar 18 , Reference Simons-Morton and Obarzanek 19 ).
Recently, a group of researchers have described an experimental model for inducing hypertension, combining increases in angiotensin II concentrations and Na circulation (angiotensin II salt hypertension) (‘Neurogenic Cardiovascular Diseases Consortium’). In this model, normotensive animals are maintained on high-NaCl diets (2 % NaCl) and subjected to chronic subcutaneous angiotensin II administration to induce an increase in BP levels in 3 d( Reference Pedrino, Rossi and Schoorlemmer 20 , Reference Pedrino, Freiria-Oliveira and Almeida Colombari 21 ). These same researchers further demonstrated that subpressor doses of angiotensin II do not lead to significant changes in BP, thus requiring the combination of a high-Na diet to induce hypertension. Consistent with these findings, experimental studies( Reference Horan, Blaustein and Dunbar 18 , Reference Simons-Morton and Obarzanek 19 ) have demonstrated a positive correlation between salt consumption and BP elevation.
Some epigenetic studies suggest that diseases developed in adulthood are related to certain conditions to which the individual is exposed during the initial stages of life, including prenatal phases. Bao et al. ( Reference Bao, Threefoot and Srinivasan 22 ) demonstrated that the risk of developing hypertension during adulthood is related to BP levels during the initial stages of life. Li et al. ( Reference Li, Wang and Cao 23 ) showed that high BP levels during childhood are positively associated with systolic and diastolic BP levels in later life. In addition, Vidonho et al. ( Reference Vidonho, da Silva and Catanozi 24 ) found offspring born to mothers maintained on high-Na diets during pregnancy and lactation to have higher BP levels in adulthood.
Although several studies have demonstrated the importance of prenatal phases to hypertension development, no study has been carried out to evaluate the contribution of postnatal phases to the development of this pathology. Therefore, the aim of the present study was to evaluate the effects of increased Na consumption in postnatal phases on mean arterial pressure (MAP) and heart rate (HR), barosensitivity, changes in water and Na intakes induced by Na depletion, and haemodynamic changes induced by hypernatraemia in adult rats.
Materials and methods
Animals
Experiments were carried out in 21-d-old male Wistar rats, obtained from the Central Animal House of the Universidade Federal de Goiás. In total, thirty rats were used as control animals and twenty-eight rats from four litters were used as experimental animals. Rats were randomly selected from each litter in the control and experimental groups. Rats were housed in an acclimatised room (22–24°C) and given free access to food and water as described previously (0·4 % NaCl; AIN-93M( Reference Reeves, Nielsen and Fahey 25 )). All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee of the Universidade Federal de Goiás (process no. 051/2010) and were performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
The experimental group (high-Na diet) was provided hypertonic saline (HS; 0·3 m-NaCl; Sigma-Aldrich) for drinking instead of water, while the control group (normal-Na diet) was provided tap water. Both groups were given food regularly throughout the experiments. High-Na diet feeding was started on the 21st day of life and was stopped after 60 d. After this treatment, tap water was provided to both groups for 15 d (recovery period).
Determination of plasma and urinary sodium concentrations and plasma osmolarity
During the last week of the treatment and recovery periods, rats were individually acclimatised in metabolic cages with free access to their respective drinking supplies to enable urine collection and urinary volume measurement. The average values of the 24 h urinary volume of the control and experimental groups were calculated and recorded during the last 7 d of the treatment and recovery periods. Blood and urine samples (0·5 ml each) were collected from control and experimental rats at the end of the treatment and recovery periods. To determine the haematocrit, blood samples were collected with the aid of a glass capillary and centrifuged. Blood samples were centrifuged for 5 min at 6000 g . Plasma was separated and stored at − 20°C. Plasma and urinary Na concentrations were measured using a flame photometer (model 910M; Analyser). Plasma osmolality was measured as freezing point depression (Micro-Osmette 5004; Precision Systems).
Water and sodium intake test
Adult rats from the experimental and control groups were submitted to a water and Na intake test using furosemide (FURO). To carry out this test, after the last week of the recovery period, rats were maintained in metabolic cages over a 24 h period, with free access to water and 0·3 m-NaCl in graduated burettes. After this period, rats were administered a subcutaneous dose of FURO (10 mg/kg body weight; Lasix®, Sanofi Aventis). Then, food was removed from the cages and rats were given access to water to prevent dehydration. After 24 h of FURO administration, HS and water were provided in graduated burettes and their intakes were measured every 30 min over the course of 2 h. Rats were not given access to food throughout the test period.
Mean arterial pressure and heart rate recordings in unanaesthetised animals
After the recovery period, rats were anaesthetised with ketamine (116 mg/kg body weight, intraperitoneal; Sespo) and xylazine (20 mg/kg body weight, intraperitoneal; Rhobifarma) and submitted to a surgical procedure to implant a polyethylene (PE) tube (PE-10 connected to a PE-50) in the abdominal aorta through the right femoral artery for recording BP. The right femoral vein was catheterised for drug administration (phenylephrine and sodium nitroprusside), and the cannulas were led subcutaneously to exit between the scapulae. A prophylactic antibiotic was administered (penicillin, 60·000 IU/kg body weight, intramuscular; Sigma-Aldrich), and rats were kept in individual cages. Later, 24 h after the surgery (recovery period described as a standard procedure by several authors( Reference Alderman 26 – Reference Johnson and Thunhorst 30 )), the pulsatile arterial pressure was obtained by connecting the arterial cannula to a pressure transducer attached to an amplifier (ETH-200; CB Sciences). Pulsatile pressure was recorded continuously with a PowerLab System device (ADInstruments). MAP and HR were determined from the pulsatile signal using Chart software (version 5.5.6; ADInstruments). The MAP and HR recordings were performed in unanaesthetised animals, with free movement within their cages, for 60 min. At the end of the baseline recordings of MAP and HR, the baroreflex test was performed.
Baroreflex test
After the recovery period, to evaluate baroreflex integrity, intravenous infusion (0·1 ml) of phenylephrine (10, 15 and 20 μg/ml; adrenergic agonist; Sigma-Aldrich) and sodium nitroprusside (100, 150 and 200 μg/ml; nitric oxide donor; Sigma-Aldrich) was performed after the baseline recordings of MAP and HR. The baroreflex index (BI) was evaluated as a mean index, calculated as the ratio of changes in HR:changes in MAP induced by phenylephrine and sodium nitroprusside infusions. The mean index is expressed as beats per min (bpm) per mm of mercury, as described previously( Reference Pedrino, Rossi and Schoorlemmer 20 , Reference Harthmann, De Angelis and Costa 31 ).
Determination of haemodynamic changes induced by acute hypernatraemia
After anaesthetic induction with halothane (2 %; Cristália Ltda) in 100 % O2, polyethylene tubes were inserted into the femoral artery and vein for MAP recordings and venous infusions, respectively, as described above. The anaesthesia was maintained with urethane (1·2 g/kg body weight, intravenous; Sigma-Aldrich). After vascular cannulation, tracheostomy was performed to reduce airway resistance. Through retroperitoneal incisions, miniature probes (Transonic Systems, Inc.) were implanted around the renal artery to record renal blood flow (RBF).
RBF was recorded by transit-time flowmetry. A miniature probe (1RB; Transonic Systems, Inc.) was implanted around the left renal artery and attached to a flowmetry T206 device (Transonic Systems, Inc.) to determine the flow in absolute values (ml/min). The signals obtained were sent to the data acquisition and analysis PowerLab System (ADInstruments). Data were digitised at 1000 samples/s. Changes in the RBF were calculated as a percentage of the baseline value (%RBF). Renal vascular conductance (RVC) was determined as the ratio of RBF:MAP. RVC changes were calculated as a percentage of the baseline value (%RVC).
Na overload was induced through HS infusion (3 m-NaCl; Sigma-Aldrich( Reference Morris and Alexander 9 )). The infusion was realised by a cannula implanted into the right atrium through the right jugular vein. Administration was performed for 60 s, at a dose of 1·8 ml/kg body weight. Changes in MAP, HR, RBF and RVC were recorded for 10 min before and 60 min after the HS infusion.
Data analysis
The GraphPad Prism 5 software was used for performing the data analysis and drawing the graphs. In all the experiments, the independent variable was the treatment and the dependent variable was the measured response. The results are expressed as means with their standard errors. Daily water, HS and food intakes, plasma and urinary Na concentrations, urinary flow, Na excretion rate and osmolality were analysed using a one-way ANOVA. The baseline values of MAP, HR, BI and haemodynamic parameters were analysed using the unpaired t test. The weekly average values of water/saline/food intakes and body weight, induced water and Na intakes, and variations in MAP, HR, %RBF and %RVC induced by HS infusion were analysed using a two-way repeated-measures ANOVA followed by Fisher's least significant difference post hoc test. A P value < 0·05 was considered to denote a significant difference.
Results
Daily food, water and hypertonic saline intakes of the control and experimental groups during the treatment and recovery periods
At the end of the treatment period, during which HS solution was provided to the experimental group and tap water was provided to the control group, the daily fluid intake of experimental rats (n 11; 116·7 (sem 26·2) ml, P< 0·05) was significantly higher than that of control rats (n 12; 36·6 (sem 1·6); Table 1). At the end of the recovery period, during which tap water was provided to both groups, no difference was observed in daily water intake between experimental rats (39·0 (sem 2·2) ml) and control rats (41·0 (sem 2·7); Table 1). At the end of the treatment and recovery periods, the daily food intake values of both groups were similar (Table 1). However, the body weight of experimental rats was lower than that of control rats at the end of the treatment period (193·8 (sem 18·8) v. 303·6 (sem 6·9) g, respectively, P< 0·05; Table 1) and at the end of the recovery period (244·0 (sem 12·5) v. 335·0 (sem 8·6) g, respectively, P< 0·05; Table 1).
Table 1 Daily water or hypertonic saline intake, food intake, body weight, urinary volume, urinary sodium concentration, urinary flow, sodium excretion rate, plasma sodium concentration, plasma osmolarity and haematocrit of control and experimental rats during the treatment and recovery periods (Mean values with their standard errors)
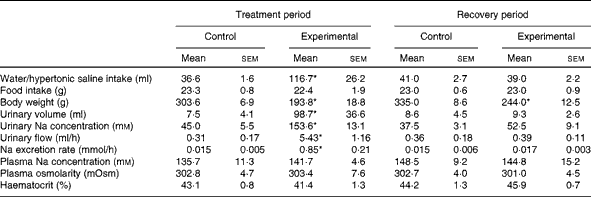
* Mean value was significantly different from that of the control group in the respective period (P< 0·05).
Consistent with the results given in Table 1, Fig. 1 demonstrates that the weekly HS intake of experimental rats was higher than the weekly water intake of control rats during the treatment period. However, during the recovery period, the absolute values of water intake of both groups were similar. There was a decrease in the weekly food intake of the experimental group over the treatment period when compared with that of the control group. This was not observed during the recovery period. The average values of the weekly body weight of experimental rats were lower than those of the weekly body weight of control rats during the treatment and recovery periods.

Fig. 1 Average values of weekly water or hypertonic saline intake (a) and food intake (b) and body weight (c) during the treatment and recovery periods. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control (●) group (P< 0·05). ○, Experimental group.
The values of water/HS intake, food intake, urinary volume, urinary flow and Na excretion rate per 100 g body weight of the control and experimental groups during the treatment and recovery periods are given in Table 2. The absolute values of Na excretion rate and daily water and food intakes of experimental rats were similar to those of control rats during the recovery period (Table 1). Experimental rats exhibited increases in water and food intakes and Na excretion rate per 100 g body weight when compared with control rats during the recovery period.
Table 2 Values of water/hypertonic saline intake, food intake, urinary volume, urinary flow and sodium excretion rate per 100 g body weight of control and experimental rats (Mean values with their standard errors)
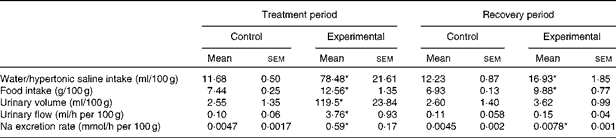
* Mean value was significantly different from that of the control group in the respective period (P< 0·05).
Effects of high sodium intake during postnatal phases on urinary volume and sodium concentrations
Significant differences were observed in diuresis between control (n 6) and experimental (n 6) rats during the treatment period (7·5 (sem 4·1) v. 98·7 (sem 36·6) ml, respectively, P< 0·05; Table 1). However, these differences were not observed during the recovery period (control: 8·6 (sem 4·5) ml v. experimental: 9·3 (sem 2·6) ml, respectively; Table 1). The urinary Na concentrations of experimental rats were significantly higher than those of control rats during the treatment period (153·6 (sem 13·1) v. 45·0 (sem 5·5) mm, respectively, P< 0·05; Table 1). These differences in urinary Na concentrations between the groups were not observed during the recovery period (experimental: 52·5 (sem 9·1) mm; control: 37·5 (sem 3·1) mm; Table 1).
Effects of high sodium intake during postnatal phases on plasma sodium concentrations, plasma osmolarity and haematocrit
As can be seen from Table 1, there were no differences in plasma Na concentrations between the control and experimental groups, either during the treatment period (135·7 (sem 11·29) mm (n 7) v. 141·7 (sem 4·61) mm (n 6), respectively) or during the recovery period (148·5 (sem 9·19) mm (n 7) v. 144·8 (sem 15·23) mm (n 6), respectively). Similarly, the plasma osmolarity values of control and experimental rats were similar both during the treatment period (302·8 (sem 4·67) mOsm (n 6) v. 303·4 (sem 7·63) mOsm (n 6), respectively) and during the recovery period (302·7 (sem 4·14) mOsm (n 6) v. 301·0 (sem 4·53) mOsm (n 6), respectively). No differences were observed in the haematocrit between the groups either during the treatment period (control: 43·1 (sem 0·8) % (n 8) v. experimental: 41·4 (sem 1·3) % (n 9)) or during the recovery period (control: 44·2 (sem 1·3) % (n 8) v. experimental: 45·9 (sem 0·7) % (n 9)).
Effects of high sodium intake during postnatal phases on changes in water and sodium intakes induced by sodium depletion
The FURO treatment induced changes in water intake in both groups (Fig. 2(a)). However, the experimental group (n 9) exhibited greater water intake than the control group (n 9) (10 (sem 1·2) v. 8·4 (sem 0·8) ml after 120 min, respectively, P< 0·05; F= 0·5172). As expected, Na depletion induced an increase in Na intake in both the control group (12·1 (sem 0·6) ml after 120 min; Fig. 2(b)) and the experimental group (7·8 (sem 1·1) ml after 120 min, P< 0·05; F= 4·016; Fig. 2(b)). However, the increase in Na intake induced by Na depletion was of lower magnitude in the experimental group (Fig. 2(b)).

Fig. 2 Cumulative water intake (a) and 0·3 m-sodium intake (b) in response to subcutaneous administration of furosemide (10 mg/kg body weight) in control (●) and experimental (○) rats. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that recorded at time 0 (P< 0·05). † Mean value was significantly different from that of the control group (P< 0·05).
Effects of high sodium intake during postnatal phases on the baseline values of mean arterial pressure and heart rate in unanaesthetised animals
Unanaesthetised experimental rats (n 6) had higher MAP (121·7 (sem 7·3) mmHg, P< 0·05; Fig. 3(a)) and HR (408·1 (sem 7·5) bpm, P< 0·05; Fig. 3(b)) levels when compared with unanaesthetised control rats (n 6; MAP: 98·6 (sem 3·0) mmHg and HR: 372·7 (sem 12·4) bpm; Fig. 3). These results clearly indicate that high Na intake during postnatal phases induces a chronic increase in MAP in unanaesthetised rats.

Fig. 3 Mean arterial pressure (MAP) (a) and heart rate (HR) (b) in unanaesthetised rats from the control (■) and experimental (□) groups. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). bpm, Beats per min.
Effects of high sodium intake during postnatal phases on baroreflex index
Both phenylephrine and sodium nitroprusside infusions induced a decrease in the BI of experimental rats (n 8) when compared with that of control rats (n 9) (Fig. 4). Phenylephrine infusion resulted in a BI of − 1·20 (sem 0·2) bpm/mmHg in experimental rats and a BI of − 1·83 (sem 0·04) bpm/mmHg in control rats (P< 0·05; Fig. 4(a)). Sodium nitroprusside infusion resulted in a BI of 0·85 (sem 0·22) bpm/mmHg in experimental rats and a BI of 2·12 (sem 0·2) bpm/mmHg in control rats (P< 0·05; Fig. 4(b)).

Fig. 4 Baroreflex index (BI) of control (■) and experimental (□) rats induced by phenylephrine (a) and sodium nitroprusside (b) infusions. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). bpm, Beats per min.
Effects of high sodium intake during postnatal phases on cardiovascular adjustments induced by acute sodium overload
The baseline values of cardiovascular parameters of anaesthetised control and experimental rats are given in Table 3. The values of MAP, RBF and RVC were similar in both groups, while the HR value was significantly higher in the experimental group.
Table 3 Baseline values of mean arterial pressure, heart rate, renal blood flow and renal vascular conductance of anaesthetised control and experimental rats (Mean values with their standard errors)
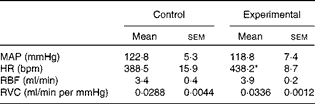
MAP, mean arterial pressure; HR, heart rate; bpm, beats per min; RBF, renal blood flow; RVC, renal vascular conductance.
* Mean value was significantly different from that of the control group.
Na overload induced a transient pressor response, with a peak at 10 min (Δ 11·5 (sem 3·0) mmHg), in the control group (n 8), whereas it induced a greater response in the experimental group (n 5) (Δ 18·7 (sem 1·7) mmHg, P< 0·05; F= 1·213; Fig. 5(a)). In the control group, RBF promptly increased after Na overload (Δ 37·3 (sem 6·0) % 10 min after infusion; Fig. 5(c)) and remained elevated until 60 min after the infusion (Δ 50·8 (sem 4·1) %; Fig. 5(c)); furthermore, this increase was greater than that observed in the experimental group (RBF: Δ 30·8 (sem 11·0) % 60 min after infusion, P< 0·05; F= 1·984; Fig. 5(c)). In addition, HS infusion was also able to induce a greater increase in RVC in the control group than in the experimental group during all the experimental periods (control: Δ 149 (sem 4·0) % v. experimental: Δ 27·9 (sem 12·3) % 60 min after the infusion; P< 0·05; F= 1·375; Fig. 5(d)).

Fig. 5 (a) Mean arterial pressure (MAP), (b) heart rate (HR), (c) renal blood flow (RBF) and (d) renal vascular conductance (RVC) in anaesthetised rats from the control (●) and experimental (○) groups exposed to hypertonic NaCl conditions (3 m, 1·8 ml/kg body weight during 60 s). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that recorded at time 0 (P< 0·05). † Mean value was significantly different from that of the control group (P< 0·05).
HS infusion resulted in a decreased HR in both groups (control: Δ − 18·9 (sem 7·9) bpm v. experimental: Δ − 16·0 (sem 4·2) bpm, 10 min after the infusion; Fig. 5(b)); however, the HR of experimental rats remained lower than normal levels 60 min after HS infusion (Δ − 25·0 (sem 10·2) bpm 60 min after the infusion, P< 0·05; F= 0·8036; Fig. 5(b)).
Discussion
Studies carried out in animals( Reference Vidonho, da Silva and Catanozi 24 ) and human subjects( Reference Bao, Threefoot and Srinivasan 22 , Reference Li, Wang and Cao 23 ) suggest that diseases that develop in adulthood are related to certain conditions to which the individual is exposed during the initial stages of life. Despite abundant evidence highlighting the importance of prenatal phases to hypertension development, the relevance of postnatal phases to this condition is unclear. A number of interesting findings were recorded in the present study: (1) high Na intake during postnatal phases chronically increases arterial BP and HR while decreasing baroreflex sensitivity; (2) an increase in Na intake during the postnatal period causes hypersensitivity to Na appetite induced by Na depletion as well as a lesser renal vasodilation induced by hypernatraemia; (3) high Na intake promotes an increase in diuresis, natriuresis and fluid intake during treatment; (4) high Na intake during postnatal phases promotes an increase in natriuresis and water and food intakes in need-free conditions in adult male rats.
Salt-dependent hypertension is strongly dependent on sympathoexcitation( Reference Osborn, Fink and Sved 32 ). Moreover, lesions in hypothalamic regions related to cardiovascular and hydroelectrolytic regulation, such as the anteroventral region of the third ventricle, subfornical organ and paraventricular nucleus of the hypothalamus, prevent or reduce increases in arterial pressure observed in salt-dependent hypertension models( Reference Brody, Johnson, Martini and Ganong 33 ). Another study has demonstrated that an increase in salt consumption potentiates sympathetic excitation and elevates arterial pressure by stimulating glutamatergic input to the rostral ventrolateral medulla( Reference Adams, Madden and Sved 34 ). The constant stimulation of these regions by Na overload leads to the stimulation of the rostral ventrolateral medulla, resulting in increased sympathetically mediated vasomotion and in pressor responses similar to those observed in the present study. Thus, chronic Na overload could change intrinsic neuronal properties and, consequently, increase the excitation of preganglionic sympathetic neurons( Reference Adams, Madden and Sved 34 ). It is not unreasonable to propose that hypothalamic and medullary cardiac presympathetic neurons may also be recruited during Na overload in the early stages of life, which would explain the positive chronotropy observed in the experimental group. Another plausible hypothesis is that the animals fed the high-Na diet became hypervolaemic, with increased blood volume being detected by stretch receptors located in the atria. Thus, the HR is increased to prevent congestive heart failure (Bainbridge reflex)( Reference Jones 35 ). However, we did not observe any differences in the haematocrit of both groups, either during the treatment period or during the recovery period (Table 1).
During the recovery period, experimental rats exhibited a higher Na excretion rate and higher daily water and food intakes per body weight when compared with control rats (Table 2). The results of the present study indicate that postnatal Na overload permanently alters the excretion patterns and spontaneous intake patterns in adulthood. These results differ from those reported by Macchione et al. ( Reference Macchione, Caeiro and Godino 36 ), who found that offspring born to mothers given free access to a hypertonic NaCl solution during the perinatal period exhibited decreased water intake in need-free conditions in adulthood. It is worth noting that HS solution was provided to the rats after weaning in the present study, which would explain the differences when compared with the above-mentioned study. Taken together, the results of the present study indicate that high Na intake during the postnatal period can be more harmful than high Na intake during the perinatal period, as it promotes increases in spontaneous Na intake in adulthood, which is a risk factor for the development of several diseases( Reference Alderman 26 , Reference Brown, Tzoulaki and Candeias 27 , Reference Campese 37 , Reference Rodriguez, Bibbins-Domingo and Jin 38 ), such as hypertension( Reference Vidonho, da Silva and Catanozi 24 , Reference Stolarz-Skrzypek 28 , Reference He, Marrero and Macgregor 29 ).
FURO is a diuretic/natriuretic that reduces renal Na and water reabsorption, causing hypovolaemia. This hypovolaemia activates the neuroendocrine and autonomic components to maintain arterial pressure( Reference Johnson and Thunhorst 30 ). In this condition, the activation of the sympathetic nervous system is related to the increase in vascular tone, venous return, heart rate and contractility and, moreover, to the increase in renal water and Na reabsorption( Reference Jones 35 ). However, Na-seeking and drinking behaviours, in addition to thirst, are required to induce Na appetite and to reset the physiological levels of water and extracellular solutes after a deficit( Reference McKinley and Johnson 39 ). In the present study, rats fed the high-Na diet during early postnatal phases exhibited a Na appetite induced by FURO that was significantly lower than that induced in control rats. This disparity is probably due to an increase in Na sensitivity in these animals: even on ingesting the same quantity of Na, the experimental group achieved satiation before the control group and exhibited a decrease in Na intake. In fact, Macchione et al. ( Reference Macchione, Caeiro and Godino 36 ) showed that central structures involved in osmoreception are more activated in FURO-Na-depleted rats, suggesting a possible sensitisation of osmosentitive circuits due to perinatal manipulations, a finding that parallels the results of the present study.
Some studies suggest that diseases that develop in adulthood are related to specific conditions in the early stages of life( Reference Bao, Threefoot and Srinivasan 22 , Reference McKinley and Johnson 39 , Reference Barker 40 ). Nicolaidis et al. ( Reference Nicolaidis, Galaverna and Metzler 41 ) revealed that pregnant rats submitted to extracellular dehydration generated offspring with an increased Na appetite. Together, these results demonstrate that Na sensitivity in adulthood can be determined before birth through different maternal–fetal influences, including changes in hydromineral homeostasis. However, Nicolaidis et al. ( Reference Nicolaidis, Galaverna and Metzler 41 ) did not consider the possible role of postnatal phases in the determination of Na sensitivity in adulthood. Our findings corroborate and extend these findings reported by Nicolaidis et al. ( Reference Nicolaidis, Galaverna and Metzler 41 ), as the increase in Na intake induced in postnatal phases promoted the decrease of Na appetite induced by Na depletion in adult rats.
The results of the present study demonstrate that high Na intake during postnatal phases chronically increases arterial BP in adulthood. In contrast to these results, Ufnal et al. ( Reference Ufnal, Drapala and Sikora 42 ) found that the ingestion of a solid diet during early postnatal phases does not change the Na intake pattern or the MAP in adult animals. This might be because these authors used the food as a source of Na intake elevation and gave the animals free access to water, thus following a protocol different from that used in the present study. As the experimental animals were given free access to water, the ingested ions might have been diluted in spite of these animals ingesting greater amounts of Na. Due to this, it is likely that Na intake was not enough to significantly change the plasma osmolarity of the experimental animals in terms of cardiovascular or behavioural parameters. In the present study, by contrast, the increase in Na intake occurred through drinking, thus preventing the dilution of the ingested Na in the animals.
As can be seen from Fig. 4, rats fed the high-Na diet during the postnatal period exhibited a BI that was significantly lower than that of control rats, indicating a lower sensitivity of baroreceptors. This might be due to a greater stimulation during early periods, leading to neuronal adaptation. It has been reported that during continuous or repetitive stimulation (constant within a pattern), the sensitivity of a neural cell is redefined through adaptation( Reference Krueger-beck, Nogueira-neto and Neves 43 ). This continuous stimulation leads to an increase in the depolarisation threshold, but does not trigger new action potentials( Reference Judy and Farrell 44 ). In this case, the main hypothesis is that baroreceptors would be unable to detect and correct changes in BP, which, in the long term, would result in arterial hypertension. This hypothesis is supported by previous studies that have shown impairment of baroreflex function in some models of hypertension( Reference Judy and Farrell 44 , Reference Maliszewska-Scislo, Chen and Augustyniak 45 ), similar to the pressor changes observed in the present study.
It has previously been stated that transient pressor responses to Na overload may depend on increases in lumbar sympathetic activity and vasopressin secretion( Reference Hatzinikolaou, Gavras and Brunner 46 , Reference Crofton and Share 47 ). In the present study, the pressor response to Na overload was found to be greater in rats exposed to high-Na conditions (0·3 m-NaCl), but not in control rats. This is in good agreement with the results of another study showing an increase in the magnitude and duration of hypertensive responses to hypernatraemia under renal vasodilation blockade( Reference Sera, Colombari and Cravo 48 ). Moreover, it has been proposed that hypernatraemia initially induces a mechanism comprising secretion of vasoactive peptides and renal sympathoinhibition( Reference Pedrino, Monaco and Cravo 14 ), which decreases peripheral resistance and Na reabsorption, resulting in natriuresis. In case this mechanism is unable to induce adequate plasma Na concentrations, a widespread increase in sympathetic activity promotes pressor responses, causing increases in renal perfusion pressure, glomerular filtration rate and, consequently, natriuresis( Reference Toney, Chen and Cato 49 ). In this regard, the increase in hypertensive responses to hypernatraemia observed in animals exposed to high-Na conditions 10 min after HS infusion could be explained by a failure of the primary peptidergic and sympathoinhibitory mechanisms, resulting in a widespread increase in sympathetic activity and therefore in sustained arterial hypertension.
Although ontogenesis of essential hypertension is not completely clear, studies in both human and animal models indicate that an increase in Na intake is associated with this disease( Reference Li, Wang and Cao 23 , Reference Brooks, Haywood and Johnson 50 ). Despite being a controversial subject, several studies have demonstrated that an increase in daily Na intake itself does not promote an increase in arterial pressure in control animals. However, it is worth noting that high-Na diets were fed to adult animals in these studies( Reference Li, Wang and Cao 23 , Reference Brooks, Haywood and Johnson 50 ), which differs from the protocol used in the present study. Another study has demonstrated that mothers who have suffered episodes of vomiting and dehydration during the first 3 months of pregnancy give birth to children who have increased systolic pressure during adolescence( Reference Málaga, Arguelles and Díaz 51 ). Vidonho et al. ( Reference Vidonho, da Silva and Catanozi 24 ) found offspring born to mothers fed a high-Na diet (4 or 8 % NaCl) during pregnancy and lactation to have higher arterial pressure in adulthood. In addition, in the present study, the increase in Na intake (0·3 m-NaCl) during the postnatal period was found to promote increases in MAP in unanaesthetised adult rats. The results of the present study show that not only the uterine phases but also the postnatal phases determine arterial pressure in adulthood. Interestingly, the arterial hypertension in these animals was not reversed after removal of Na intake, indicating that the increase in Na consumption in postnatal phases promotes permanent changes in pressoric levels. To the best of our knowledge, no other study has shown that high Na intake during postnatal phases induces chronic increases in arterial BP in adult male rats. Finally, it should be emphasised that the present study was performed only in male animals to avoid possible hormonal influences on cardiovascular, renal and behavioural parameters analysed, as food intake and the motivation for food can be modified by the phases of the oestrous cycle( Reference Crofton and Share 47 , Reference Voznesenskaya and Tordoff 52 – Reference Cheikh Ismail, Al-Hourani and Lightowler 56 ).
Taken together, the results of the present study demonstrate that changes in hydromineral homeostasis during the initial stages of postnatal life can change the behavioural and cardiovascular adjustments induced by Na depletion and HS infusion. However, further studies are required to elucidate the mechanisms whereby such changes occur.
Acknowledgements
The present study was supported by Coordenadoria de Aperfeiçoamento em Pesquisa (CAPES; http://www.capes.gov.br; to M. C. S. M.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; no. 477832/2010-5; 483411/2012-4; http://www.cnpq.br; to G. R. P.) and Fundação de Amparo a Pesquisa do Estado de Goiás (no. 200910267000352; http://www.fapeg.go.gov.br; to G. R. P.). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors’ contributions are as follows: M. C. S. M., D. A. R., E. C., C. H. X. and G. R. P. conceived and designed the experiments; M. C. S. M., E. F. d. S., L. L. S. and Y. B. d. P. performed the experiments; M. C. S. M., A. H. F.-O., D. A. R., P. M. F., C. H. d. C. and G. R. P. analysed the data; D. A. R., E. C., C. H. X., P. M. F. and G. R. P. contributed the reagents/materials/analysis tools; M. C. S. M., A. H. F.-O., D. A. R., P. M. F., C. H. d. C., E. C., C. H. X. and G. R. P. wrote the article.
None of the authors has any conflicts of interest to declare.










