Most researchers agree that the current obesity epidemic predominately results from the combination of energy-dense diets and sedentary lifestyles(Reference Martínez-González, Martinéz and Hu1–Reference Koh-Banerjee, Chu and Spiegelman4). The obesity trend is thus considered to be caused by a combination of factors, and is found in most social groups, in both men and women of all ages(Reference Lissner, Johansson and Qvist5–Reference Sanchez, Norman and Sallis8). However, information about specific factors contributing to weight gain and the development of obesity seems to be either inconclusive or contradictory, primarily since most factors are inter-related and difficulties arise when attempting to isolate the effect of one single factor(Reference Newby, Muller and Hallfrisch9–Reference Swinburn, Caterson and Seidell12). In addition, due to confounding, it is difficult to estimate the true impact of the various risk factors for weight gain.
Meal and snack patterns, including the daily eating frequency, are thought to influence weight status as well as development of chronic diseases(Reference Drummond, Crombie and Cursiter13–Reference Fábry, Fodor and Geizerová17). However, several authors have disputed the fact that the eating frequency in itself is related to adverse health effects(Reference Bellisle, McDevitt and Prentice18–Reference Hampl, Heaton and Taylor19). The daily eating frequency is likely to be associated with different food choices as well as lifestyle and socio-economic factors; it may thus be accompanied by changes in dietary quality. Overweight and obese individuals have been found to have different eating habits in comparison with normal-weight individuals, including morning anorexia and consumption of larger food amounts in the afternoon and evening as well as a higher eating frequency(Reference Bertéus Forslund, Torgerson and Sjöström14, Reference Ma, Bertone and Stanek20–Reference Greenwood and Stanford22). In addition, several studies have shown sex differences in eating patterns(Reference Bertéus Forslund, Torgerson and Sjöström14, Reference Kushner and Choi23, Reference Westenhoefer24). Women are often reported to have lower intake of fat, and higher intakes of fruits and vegetables and dietary fibre. However, women are also more likely to find healthy eating more important, and are more likely to restrain their eating behaviour(Reference Neumark-Sztainer, Sherwood and French25).
Sex and several psychosocial and behavioural characteristics are related to energy misreporting, including incomplete recordkeeping by subjects(Reference Maurer, Taren and Teixeira26). Therefore it is essential to examine the influence of potential confounding factors on self-reported energy and food intake in dietary research. Previous studies investigating diet–disease associations in the Malmö Diet and Cancer (MDC) cohort have shown that it is important to consider past changes in food habits and energy misreporting in sensitivity analysis(Reference Mattisson, Wirfält and Aronsson27–Reference Sonestedt, Gullberg and Wirfält29).
The aim of the present study was to examine the associations between daily eating frequency, BMI and waist circumference. In order to identify factors that correlate with eating frequency we examined the association between daily eating frequency and the intake of total energy, selected nutrients and food groups and lifestyle factors. In addition, an important part of this investigation was to evaluate the potential confounding associated with possible reporting errors. An improved understanding of the potential health effects of eating frequency as well as the association between eating frequency and diet quality could be important for future public health advice and strategies dealing with the current obesity epidemic.
Subjects and methods
Study design
This cross-sectional study examined data from the MDC cohort, a population-based prospective study set in Malmö, Sweden. In 1991, the MDC source population was defined as all individuals living in the city of Malmö and born between 1926 and 1945. In May 1995, the cohort was extended to include all women born between 1923 and 1950 and all men born between 1923 and 1945. With this extension, 74 138 individuals constituted the source population. Inadequate Swedish language skills and mental incapacity were the only exclusion criteria. The MDC study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committee at the Medical Faculty, Lund University (LU 51-90). Written informed consent was obtained from all subjects. Details of the recruitment procedures and the cohort are described elsewhere(Reference Berglund, Elmståhl and Janzon30, Reference Manjer, Carlsson and Elmstahl31). Briefly, the participants were invited by mail or advertisements in the local media and primary health-care centres. The participants visited the MDC screening centre twice. During the first visit, participants were instructed on how to register meals in a menu book and how to fill out the questionnaires that were completed at home. Anthropometric measurements and blood collection were conducted on location by nurses. During the second visit (approximately 10 d later) the questionnaires were checked and a dietary interview was conducted by trained interviewers. In October 1996, when recruitment was closed, 28 098 participants had completed all baseline examinations.
Study participants
The present study includes individuals that joined the MDC study from January 1993 until March 1994. These participants were, in addition to the other diet history information, requested to give a general overview of their eating occasions during an ordinary day. A total of 3009 subjects (1355 men and 1654 women) aged between 47 and 68 years are included in the study.
Eating frequency assessment
The questionnaire used for assessment of the daily eating frequency included registration of the number and types of meals and snacks eaten during an ordinary day, and how many times per week these meals or snacks usually were eaten. If weekends differed significantly from weekdays the participants were requested to describe two different days (for further details on registration of meals, see Appendix). The self-reported information was carefully checked by trained nutritionists during the diet history interview. Based on the participants' self-reported information on number of eating occasions (including both snacks and meals) during a week, the average daily eating frequency was calculated. In the present study we excluded non-nutrient contributing snacks, i.e. coffee and tea. The eating frequencies were aggregated into: ‘three or fewer’, ‘four to five’ or ‘six or more’ eating occasions per d.
Dietary assessment
In the MDC study, food and nutrient intakes were assessed using an interview-based modified diet history method. This methodology combines a 7 d menu book collecting descriptions of prepared meals, nutrient supplements and cold beverages (including alcoholic beverages) with a 168-item dietary questionnaire covering regularly eaten foods other than prepared meals during the previous year. The consistency of the information provided was carefully checked during a 45 min interview so that the questionnaire and menu book did not overlap. The average energy and nutrient intakes were computed by compiling the reported food intake from the two methodologies and applying this information to the MDC Food and Nutrient Database, mainly originating from the database PC Kost2-93 of the National Food Administration in Uppsala, Sweden(Reference Callmer, Riboli and Saracci32). Data on the validity(Reference Elmståhl, Riboli and Lindegärde33, Reference Riboli, Elmståhl and Saracci34) and reproducibility(Reference Elmståhl, Gullberg and Riboli35) of the method have been published.
Dietary variables
The present study examined the associations between eating frequency and total energy intake (EI) (MJ), nutrient densities and intake of selected food groups. The energy percentage (E%) from fat, carbohydrates and protein was computed using non-alcohol energy. Similarly, the micronutrient densities were expressed as intake per MJ of non-alcohol energy. In addition, alcohol was examined both as percentage of total energy and as alcohol habits (categorical variable, see below). The nutrient variables examined in the present study were: fibre (g/MJ), Fe (mg/MJ), Ca (mg/MJ), Mg (mg/MJ), β-carotene (mg/MJ), ascorbic acid (mg/MJ), folate (μg/MJ) and vitamin E (mg/MJ). The food groups examined were: fruits and vegetables; meat, eggs and poultry; low-fat meat; fish and shellfish; milk and dairy products; low-fat dairy; bread and cereals; high-fibre bread and cereals; confectionery foods (the sum of sweets, added sugar, jam, and chocolate); soft drinks; cakes and pastries.
Energy misreporting and past food habit change
Mattisson et al. (Reference Mattisson, Wirfält and Aronsson27) have previously defined low-, adequate- and high-energy reporters in the MDC cohort using the approach described by Goldberg et al. (Reference Goldberg, Black and Jebb36) and later refined by Black(Reference Black37). Energy misreporting was defined as having an EI:BMR ratio outside the 95 % CI limits of the calculated physical activity level. The physical activity level of each individual was carefully calculated using all available information in the MDC cohort. The estimation used information from the questionnaire concerning hours and intensity of leisure-time physical activity, hours of household work, and hours and intensity of occupational activities. Hours of sleeping, time for self-care, and ‘passive’ time were estimated(Reference Mattisson, Wirfält and Aronsson27). Individuals with EI:BMR below the lower 95 % confidence limit were classified as under-reporters of EI. Individuals with EI:BMR within the confidence limits were classified as adequate-energy reporters and individuals with EI:BMR above the upper 95 % confidence limit were classified as over-reporters of EI.
In addition, past food habit change was dichotomised (yes or no) based on the questionnaire item ‘Have you substantially changed your dietary habits because of illness or another reason?’
Socio-economic and lifestyle variables
Information on socio-economic and lifestyle factors was collected from the structured lifestyle questionnaire. The participants were divided into four categories to describe educational level according to the number of years of education completed (i.e. ≤ 8 years, 9–10 years, 11–13 years, or>13 years). Socio-economic status was classified according to the Swedish population census as ‘blue-collar workers’, ‘white-collar workers’ and ‘employers/self-employed’ (in the present study including high-positioned white-collar workers)(38). Leisure-time physical activity was assessed by questions adapted from the Minnesota Leisure Time Physical Activity Questionnaire(Reference Taylor, Jacobs and Schucker39, Reference Richardson, Leon and Jacobs40). The overall leisure-time physical activity score was categorised into quartiles based on the population distribution. The smoking habits of the participants were defined as current smokers (including irregular smokers), former smokers or never-smokers. Alcohol habits were classified as zero, low, medium or high consumption. Participants reporting no consumption of alcohol during the previous year in the socio-economic and lifestyle questionnaire and that in addition did not report any alcohol intake in the 7 d menu book were classified as zero alcohol consumers. For all other participants low, medium and high alcohol consumption level was set at alcohol intakes of < 15, 15–30, and>30 g/d for women, and at < 20, 20–40, and>40 g/d for men, based on the information collected in the menu book.
Anthropometric variables
Nurses measured weight, height and waist circumference. BMI was calculated from weight (kg) over the square of height (m2). Relative weight categories (i.e. BMI < 18·5; 18·5 to < 25; 25 to < 30; ≥ 30 kg/m2) were used to classify individuals as underweight, normal weight, overweight, or obese, according to WHO recommendations(41). Waist circumference was transformed into dichotomised variables to assess central obesity. Waist circumference associated with increased risk of metabolic and CVD was set to ≥ 80 cm (women) and ≥ 94 cm (men); waist circumference associated with a greatly increased risk of metabolic and cardiovascular disease was set to ≥ 88 cm (women) and ≥ 102 cm (men), according to WHO recommendations(41).
Statistical analysis
All analyses investigated men and women separately. The study population characteristics were assessed using the χ2 test and ANOVA. Since energy misreporting and past food habit change was differentially associated with eating frequency, further analysis included only men (n 892) and women (n 1024) classified as adequate energy reporters and reporting no past food habit change. Total EI and relative nutrient intakes across daily eating frequency were expressed as mean values with their standard errors, and mean differences examined using ANOVA while adjusting for age. Some nutrient variables were log transformed to normalise the distribution before analysis. Differences in intake from selected food groups across daily eating frequency were examined using ANOVA adjusting for age and total EI.
The mean differences in BMI and waist circumference across categories of daily eating frequency was examined while adjusting for potential confounders. The risk of overweight/obesity and central obesity associated with the daily eating frequency was estimated using logistic regression with six or more meals per d as the reference, while adjusting for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity and total EI. Other logistic regression models were in addition adjusted for nutrient and food group variables. OR and 95 % CI were calculated. Two dichotomous variables identified overweight (i.e. BMI 25 to < 30 kg/m2) and obese (i.e. BMI ≥ 30 kg/m2) individuals respectively, with normal weight (i.e. BMI 18·5 to < 25 kg/m2) as the reference category. The risk associated with a large waist circumference used similar variables (i.e. 94 to < 102 and ≥ 102 cm in men; 80 to < 88 and ≥ 88 cm in women) using subjects with healthy waist circumferences (i.e. < 94 cm for men and < 80 cm for women) as reference. Significance level was set at P < 0·05. The SPSS statistical computer package (version 17; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
The study population characteristics are presented in Table 1 and associations between daily eating frequency and lifestyle and socio-economic factors are presented in Table 2. On average, men reported a daily eating frequency of 4·8 (sd 1·2) with a range of 1–13 (median 4·7). Women reported a daily eating frequency of 5·0 (sd 1·1) with a range of 1–9 (median 5·0). The majority of men and women reported eating between four to five meals per d; however, men were more likely than women to report three or fewer meals per d, while women were more likely to report an eating frequency of six or more meals per d (P < 0·0001). Men and women with a low daily eating frequency (i.e. three or fewer) were more likely to be younger, current smokers, high alcohol consumers, and less physically active compared with subjects reporting a high daily eating frequency (i.e. six or more). These individuals were also more likely to under-report EI, but less likely to have changed their food habits substantially in the past compared with more frequent eaters (Table 2).
Table 1 Study population characteristics and differences between men and women

Table 2 Study population characteristics across categories of daily eating frequency in men (n 1355) and women (n 1654)
(Percentages)

* χ2 Analysis of sex by eating frequency.
The mean relative energy and nutrient intakes across categories of daily eating frequency are shown in Table 3. The analysis excluded energy misreporters and individuals with past food habit change. Total EI (MJ), E% from carbohydrates, and relative fibre intake increased significantly with the daily eating frequency, while E% from fat, protein, and alcohol decreased. E% from fat did not differ significantly for men with different eating frequency. Nutrient densities of ascorbic acid, folate and Fe were highest for women reporting six or more meals per d. In men, nutrient density of Mg was higher for subjects with lower daily eating frequency (three or fewer and/or four to five meals per d). There were no significant differences in intakes of the various food groups apart from a higher intake of cake and pastries for women with a high eating frequency (P = 0·036).
Table 3 Relative nutrient intakes across categories of daily eating frequency in men (n 892) and women (n 1024) adjusting for age†
(Mean values with their standard errors)

* Significance level tested with log-transformed variables.
† Misreporters of energy intake and subjects reporting past food habit change are excluded from the analysis.
The associations between daily eating frequency and the different overweight/obesity variables are shown in Tables 4–6. Mean BMI and waist circumference among men and women across daily eating frequency did not differ significantly when adjusting for potential confounders, and excluding subjects who were classified as misreporters of EI and reported a change of food habits in the past. The regression model adjusting for lifestyle confounders including total EI (Table 5, model 1) showed that men with a low daily eating frequency were more likely to be obese (i.e. BMI ≥ 30 kg/m2; P for trend = 0·037) and have a large waist circumference (i.e. ≥ 102 cm; P for trend = 0·029) compared with men with a high daily eating frequency (i.e. six or more). Results in women failed to reach statistical significance. The daily eating frequency was not significantly associated with the likelihood of being overweight or having a waist circumference of 94 to < 102 cm in men or a waist circumference of 80 to < 88 cm in women. The interaction between obesity, eating frequency, and sex was non-significant.
Table 4 BMI and waist circumference (WC) among men (n 892) and women (n 1024) in categories of daily eating frequency adjusting for potential confounders*
(Mean values and 95 % confidence intervals)
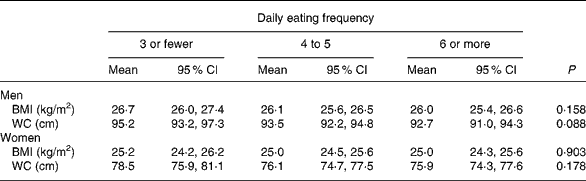
* Adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, total energy intake, non-alcohol energy percentage from fat, non-alcohol energy percentage from carbohydrates, fibre intake (g/MJ), fruit and vegetables, confectionery foods, soft drinks, and low-fat meat and dairy. Misreporters of energy intake and subjects reporting past food habit change are excluded from the analysis.
Table 5 Associations between overweight, obesity, and moderate or severe central obesity (estimated separately) and daily eating frequency in men (n 892)*
(Odds ratios and 95 % confidence intervals)

* Misreporters of energy intake and subjects reporting past food habit change are excluded from the analysis.
† Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, and total energy intake.
‡ Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, total energy intake, non-alcohol energy percentage from fat and fibre intake.
§ Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, total energy intake, non-alcohol energy percentage from fat, fibre intake, fruit and vegetables, confectionery foods, soft drinks, cakes and pastries and low-fat meat and low-fat dairy products.
∥ Normal-weight (BMI 18·5 to < 25 kg/m2) men (n 338) were used as the reference.
¶ Men with a waist circumference less than 94 cm (n 496) were used as the reference.
Table 6 Associations between overweight, obesity, and moderate or severe central obesity (estimated separately) and daily eating frequency in women (n 1024)*
(Odds ratios and 95 % confidence intervals)

* Misreporters of energy intake and subjects reporting past food habit change are excluded from the analysis.
† Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity and total energy intake.
‡ Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, total energy intake, non-alcohol energy percentage from fat and fibre intake.
§ Model adjusted for age, education, socio-economic status, smoking, alcohol consumption, leisure-time physical activity, total energy intake, non-alcohol energy percentage from fat, fibre intake, fruit and vegetables, confectionery foods, soft drinks, cakes and pastries and low-fat meat and low-fat dairy products.
∥ Normal-weight (BMI 18·5 to < 25 kg/m2) women (n 546) were used as the reference.
¶ Women with a waist circumference less than 80 cm (n 704) were used as the reference.
Several regression models were considered in order to adjust for diet composition. However, the inclusion of both fat and carbohydrate densities was the only combination that attenuated results to the degree of borderline significance (results not shown). However, the strong correlation (correlation coefficient = 0·94) between fat and carbohydrate intake (E%) indicates that including both in the model might be superfluous. Adjusting for various food groups thought to be important in the development of obesity (including dietary fibre, fruit and vegetables, confectionery foods, soft drinks, and cakes and pastries, high- or low-fat meat, and high- or low-fat dairy) attenuated the results. However, men with a low eating frequency still had an increased risk of general (P = 0·044) and central obesity (P = 0·043) (Table 5, model 3).
Discussion
The results suggest that a high daily eating frequency is associated with an overall healthy lifestyle in both men and women. A high daily eating frequency was associated with higher leisure-time physical activity, non-smoking, lower alcohol consumption, and lower E% from fat and alcohol and higher E% from carbohydrates and higher relative fibre intake. Men with a low eating frequency showed an increased risk of general and central obesity, which remained when adjusting for lifestyle and dietary factors. Results for women showed similar but non-significant tendencies.
Participants in the MDC cohort are in many aspects representative of the source population; differences between participants and non-participants in the MDC study have been described elsewhere(Reference Manjer, Carlsson and Elmstahl31). A common source of bias in studies on diet and obesity is misreporting of food intake. ‘Social desirability’ may influence both what subjects actually eat and what they report eating. Obese individuals tend to under-report their dietary intake(Reference Lafay, Basdevant and Charles42–Reference Heitmann and Lissner45), but whether the type of under-reporting in the present study is selective under-reporting of certain foods or general under-reporting of all foods is difficult to determine. Cross-sectional studies in particular are often hindered by the effects of misreporting by subjects as well as change in food habits over time. The present study attempted to overcome these potential sources of bias by excluding both energy misreporters and subjects reporting to have changed their food habits substantially in the past. Excluding these individuals reduces the likelihood of including those prone to under-report eating occasions as well as those with restrained eating. In addition, the eating frequency was defined as all eating occasions during an ordinary day (including both meals and snacks), which, together with the careful checking of self-reported diet information during interview, reduces the likelihood of misclassification of eating frequency.
An important finding is that the daily eating frequency was linked to differences in dietary quality. A high daily eating frequency was associated with higher E% from carbohydrates and relative intake of fibre, and lower E% from protein and fat. Individuals with a high daily eating frequency may be more likely to consume healthy snacks (for example, sandwiches and fruits) in between main meals (i.e. cooked meals). The high relative fibre intake together with higher density of ascorbic acid and folate also suggest a higher consumption of fruit and vegetables for women with a higher daily eating frequency. A higher E% from protein may indicate consumption of primarily cooked meals (including meat) for those reporting a low daily eating frequency.
The present study suggests that there may be a favourable impact of increasing eating frequency in regards to preventing general and central obesity. Although the associations seen among men in the present study (Table 5) remained significant when adjusting for lifestyle and dietary confounders (including total EI), it is possible that residual confounding of both lifestyle factors and/or energy or dietary intake could contribute to the results. Thus, the present results may rather indicate that individuals with a more active lifestyle, resulting in higher energy expenditure and intake, are likely to consume a more ‘healthy’ diet, which results in reduced body fatness and waist circumference. In addition, the cross-sectional design makes it impossible to determine if reverse causation (i.e. obese individuals eat fewer meals per d as a strategy for weight loss) is an underlying factor. A previous study within the MDC cohort suggests that individuals (especially men) with fibre-rich food patterns are less likely to be centrally obese(Reference Wirfält, Hedblad and Gullberg46). In the present study a high fibre intake was the clearest diet-quality indicator associated with a high eating frequency among men, and thus, consistent with the study by Wirfält et al. (Reference Wirfält, Hedblad and Gullberg46), likely to predict a healthy waist circumference. However, in the present study it is difficult to determine if a high daily eating frequency in itself has beneficial health effects. Evidence is inconclusive in the literature regarding a possible metabolic effect of eating frequency. It has previously been hypothesised that a high eating frequency has an impact on appetite control, and improves factors such as dietary induced thermogenesis, lipidaemia and glycaemia(Reference Farshchi, Taylor and Macdonal47–Reference LeBlanc, Mercier and Nadeau49). In addition, a study by Titan et al. (Reference Titan, Bingham and Welch50) showed an inverse relationship between serum lipid concentrations and eating frequency in a free-living population. A plausible explanation for the observed sex differences in the present study is a low discriminating power due to very few women reporting three or fewer meals per d. The association with a high fibre intake also stresses the possible benefits of high-fibre food (including fruit and vegetables).
In conclusion, the present study suggests that a high eating frequency in the context of an overall healthy lifestyle and a higher intake of low-fat and high-fibre foods may be inversely associated with the likelihood of general and central obesity in middle-aged men. Since body-fat mass, as well as the distribution of body fat, are risk factors of diabetes, CVD and several forms of cancer(Reference Hu51–Reference Abu-Abid, Szold and Lausner53), our findings should be of interest to public health professionals involved in chronic disease prevention. Because the present cross-sectional study cannot differentiate cause from effect, the observed association between eating frequency, diet and body composition warrants further investigation, particularly prospective research and intervention studies.
Acknowledgements
The present investigation was supported by the Ernhold Lundström Foundation, Lund University and Region Skåne.
I. H. conducted the study and wrote the manuscript. All authors contributed to the interpretation of the results and to the finalisation of the manuscript.
There are no conflicts of interest.
Appendix
Presented is an example of how the meal order during a typical week was self-reported by the participants in the Malmö Diet and Cancer study, including instructions to participants. The daily eating frequency was calculated from the total number of meals per week.
Meal intake
Describe in broad terms the meals including snacks that you usually eat/drink during one day. Note the name of the meal, the time of the meal, what it consists of, and how many times per week you usually eat this meal. If Saturday and Sunday are significantly different from weekdays, you can report two meal orders.
For an example, see the Table below.









