Colorectal cancer (CRC) is the third most common type of cancer and the second with the highest mortality rate among men and women worldwide, and in Brazil, it is the second with the highest incidence accompanied by high mortality rates(1,Reference Sung, Ferlay and Siegel2) . CRC is a neoplasm that includes morphological and pathological changes that affect the entire segment of the large intestine, and this process involves the appearance of pre-neoplastic lesions called aberrant crypt foci (ACF)(3–Reference Kowalczyk, Orlowski and Siermontowski5). ACF can be identified microscopically on the surface of the entire colonic mucosa, being considered a biomarker for evaluating the progression of CRC(Reference Perse and Cerar6).
Colorectal carcinogenesis can be influenced by genetic factors and modifiable factors such as food intake, in which a greater consumption of processed and ultra-processed foods and a reduction in fresh foods are observed(1,3) . In this context, sources of bioactive compounds with antioxidant, anti-inflammatory, anti-carcinogenic properties, among others, are of great scientific interest, as they are related to reducing the risk of developing chronic diseases such as cancer. Among the sources of bioactive compounds, the Hibiscus sabdariffa L. (H. sabdariffa) of the Malvaceae family stands out, commonly known as hibiscus, roselia, sorrel or sour okra, and is widely cultivated in Africa, Southeast Asia and some countries in America. It is consumed in the form of tea, jellies, fermented products, among others, due to its considerable concentration of phenolic compounds and anthocyanins(Reference Sáyago-Ayerdi, Arranz and Serrano7–Reference Maciel, Carmo and Azevedo9).
Plant-derived phenolic compounds include simple phenols, phenolic acids, tannins, flavonoids, among others(Reference Sequetto, Oliveira and Soares10). Anthocyanins belong to the group of flavonoids and are the pigments responsible for the coloration of H. sabdariffa. These compounds play anti-inflammatory, anti-proliferative and hepatoprotective functions, in addition to a powerful antioxidant capacity, acting in the neutralisation of free radicals, reducing the risk of damage to the DNA molecule and the development of diseases or injuries associated with oxidative stress(Reference Huang, Chang and Kao11–Reference Popović, Kocić and Katić14).
Given the information above, phenolic compounds and total anthocyanins from dehydrated H. sabdariffa calyces (DHSC) can be used to reduce oxidative stress and the inflammatory state, and consequently to suppress the carcinogenesis process(Reference Lin, Chan and Sheu15–Reference Juhari, Bredie and Toldam-Andersen18). Therefore, the aim of the study was to evaluate the effects of supplementation of 5 % and 10 % of DHSC during the development of pre-neoplastic lesions induced by 1,2-dimethylhydrazine (DMH) in BALB/c mice.
Methodology
Sample acquisition and preparation
Ten kg of H. sabdariffa calyces were originally obtained from a producer in Coimbra – Minas Gerais (latitude: −20,830,127 and longitude: −42,801,351) and purchased from a local market in Viçosa – Minas Gerais. H. sabdariffa calyces were then pulverised in a knife mill and stored at −20°C.
Dehydrated H. sabdariffa calyces characterisation
Centesimal composition and dietary fibre
The determination of the proximate composition of the DHSC followed the methodologies proposed by the Association of Official Analytical Chemists (AOAC)(19). Proteins were determined by the Kjeldahl method. Lipids were quantified by extraction with ethyl ether in Soxhlet. Ashes were determined (fixed mineral residue) by a gravimetric process. The total fibre content was quantified by the gravimetric–enzymatic method. Finally, carbohydrates were calculated by difference (100 – (moisture + protein + lipids + fibre + ash)).
Total phenolic compounds, total anthocyanins and antioxidant activity
The extraction of total phenolic compounds was performed according to Borrás-Linares et al. (Reference Borrás-Linares, Fernández-Gutiérrez and Segura-Carretero8), in which 20 g of DHSC was macerated in 100 ml of extraction solution (70 % ethanol acidified with 0·1 % HCl) at a ratio of 1:10 (p/v). The extraction took place for 24 h, at refrigeration temperature (8 ± 2ºC). Finally, the extract was filtered and concentrated in a rotary evaporator at 40°C.
The determination of total phenolic compounds was performed according to the Folin–Ciocateu colorimetric method, and the results were expressed in mg of Gallic Acid Equivalents/100 g of sample(Reference Singleton and Rossi20). Total anthocyanins were quantified by the differential pH method, and the results were expressed in mg of cyanidin-3-glucoside/100 g of sample(Reference Giusti and Wrolstad21).
The scavenging capacity of the 2,2-diphenyl-1-picrylhydrazyl radical was determined using the stable 2,2-diphenyl-1-picrylhydrazyl radical, as previously described(Reference Borrás-Linares, Fernández-Gutiérrez and Segura-Carretero8,Reference Pukalskas, Beek and Venskutonis22) . The antioxidant activity of DHSC was defined using a trolox standard curve, and the result expressed as µmol equivalent of trolox/g of DHSC.
Experimental design
Forty-five male BALB/c mice, 7 weeks old, initial weight between 20 and 40 g, purchased from the Animal Facility of the Biological Sciences Center of the Universidade Federal de Viçosa were used. Animal protocol was approved by the Ethics Committee on Animal Experimentation from the Universidade Federal de Viçosa, Brazil, under the process number 10/2017. BALB/c mice were used because they develop neoplasms more easily; in addition, male mice were defined due to the hormonal variability of females. The sample calculation was performed according to Mera, Thompson and Prasad(Reference Mera, Thompson and Prasad23) who consider the effect of treatment between the experimental groups on the outcome variables of interest. The animals were housed in collective cages, with controlled temperature (22 ± 2°C), 12-h photoperiod and ad libitum access to water and feed. After 2 weeks of acclimatisation, the animals were randomised according to body weight and distributed in ascending order in the cages in three experimental groups: control group – CON (diet AIN-93M, American Institute of Nutrition for Maintenance, n 15), group 5DHSC (diet AIN – 93M + 5 % DHSC, n 15) and the 10DHSC group (diet AIN-93M + 10 % DHSC, n 15) (Table 1). The 5 % and 10 % DHSC supplementation was defined in order to provide about 100–200 mg of anthocyanins. Such amounts can be incorporated into the human diet. The difference in the average weights between the animals was not greater than 5 % and the cages were properly identified. The experimental period was 12 weeks, and during the first 8 weeks all animals received an intraperitoneal injection in a single dose/week of the drug DMH (20 mg/kg of body weight) for induction of pre-neoplastic lesions (Fig. 1).
Table 1. Composition of experimental diets AIM-93M* (g 100 g−1)

DHSC, dehydrated H. sabdariffa calyces.
* AIN-93M (American Institute of Nutrition for Maintenance).
† Control group (AIM-93M).
‡ 5DHSC group (AIM-93M supplemented with 5 % dietary DHSC).
§ 10DHSC group (AIM-93M supplemented with 10 % dietary DHSC).
At the end of the experimental period, the mice were fasted for 12 h, then anaesthetised in the Experimental Nutrition Laboratory with 3 % isoflurane (Cristália®, Brazil), followed by total exsanguination through the cervical retro-orbital sinus. The entire colon was dissected, washed with PBS buffer to remove luminal content, cut along the mesenteric margin and then fixed in Karnovsky’s solution for 24 h for ACF analysis. Faecal samples were collected 1 week before killing and used for SCFA analysis. For immunophenotyping, after dissection, the colon was washed with cold PBS buffer (pH 7·2), cut into small pieces and incubated in DMEM (Dulbecco modification of Minimum Essential Media) (Sigma-Aldrich™) for 90 min at 37°C. Another part of the colon was fixed in Carson’s formalin solution for 24 h for histological analyses. Livers were excised, weighed, immediately frozen in liquid N2 and stored at −80°C until liver enzyme activity was determined.
Antioxidant liver enzymes and serum markers
The activity of liver antioxidant enzymes was determined in liver homogenate. Catalase (CAT) concentration was determined according to Aebi(Reference Aebi24), and superoxide dismutase (SOD) concentration was determined based on the ability of this enzyme to reduce pyrogallol auto-oxidation(Reference Dieterich, Bieligk and Beulich25). All readings were performed in a spectrophotometer (Thermo Scientific®, model Multiskan GO), and data were expressed in units (U)/mg protein. The determination of the concentration of proteins resident in the liver tissue used in the CAT and SOD analyses was carried out according to Lowry et al. (Reference Lowry, Rosebrough and Farr26). Readings were performed in a spectrophotometer (Thermo Scientific®, model Multiskan GO) at a wavelength of 700 nm.
Colonic aberrant crypt foci score
The colon was measured and divided into three equidistant segments, identified as proximal, medial and distal in relation to the caecum. To count the ACF, the colon segments were stained with 0·1 % methylene blue solution for 2 min. Counting was performed with the aid of an optical microscope (Olympus America Inc., Model CBA) at 100× magnification by two trained double-blind observers. The categorisation of ACF was performed based on the number of aberrant crypts per focus, defined as foci with more than three (ACF > 3) or less than three aberrant crypts (ACF ≤ 3)(Reference Bird27).
Quantification of faecal SCFA
SCFA extraction and quantification followed the Smiricky-Tjardeset et al.(Reference Smiricky-Tjardes, Grieshop and Flickinger28) and Marcon et al.(Reference Marcon, Moraes and Cruz29). Acetic and butyric acids were detected and quantified by an UV detector (model SPD-20A VP) at 210 nm. Results were expressed as µmol SCFA/g of faeces.
Histological analyses in the colon
The colon fragments were fixed in Carson’s formalin and subsequently dehydrated in increasing concentrations of ethanol and cleared with xylene and embedded in paraffin (Sigma Aldrich®). About 5-μm-thick cross sections were obtained on a rotating microtome (Olympus America Inc., Model CUT 4055). The slides were stained with haematoxylin and eosin to verify the presence of inflammatory infiltrates. For analysis of goblet cells, slides were stained with Alcian Blue (AB, pH 2·5) and periodic acid Schiff. All slides underwent a pre- and post-staining step and were then analysed under an optical microscope (40×) (Leica Microsystems®, Inc.).
The counting of inflammatory infiltrates and the determination of goblet cells were performed with the aid of image analysis software (Image Pro Plus 4·5, Media Cybernetcs Inc.).
Determination of leucocytes in the colon mucosa by immunophenotyping
Leucocytes were quantified and characterised in the colon mucosa as previously described by Belkaid, Jouin e Milon(Reference Belkaid, Jouin and Milon30) and Marcon et al. (Reference Marcon, Moraes and Cruz29). The colon was removed and washed in ice-cold PBS, cut into small fragments and incubated in cell culture medium, DMEM, pH 7·2 (Sigma-Aldrich™) for 90 min at 37°C. Thereafter, the suspension was centrifuged three times at 42 g for 5 min, and the supernatant was removed. Then it was centrifuged again at 543 g for 10 min. Afterwards, the remaining sediment was resuspended with PBS buffer (100 µl, pH 7·2). Cell viability was assessed using Trypan blue dye and, after staining, the cells were counted in a Neubauer chamber. The leucocytes obtained were incubated with the following antibodies, according to the manufacturer’s instructions: anti-CD4 (PeCy5), anti-CD25 FITC-conjugate, anti-CD196 (anti-CRC6) PE-conjugate, anti-CD49b (anti-PanNK) APC-conjugated, and PECy7-conjugated anti-CD8 (Biolegend, San Diego). Leucocytes (1 × 104 events) were acquired (FACSVerse™ and BD FACSuite software; BD Biosciences PharMingen) according to size (direct scatter) and granularity (side scatter). One or two stains were used to identify CD4 T lymphocytes (CD4+), CD8 T lymphocytes (CD8+), regulatory T cells (CD4 + CD25+), Th17 lymphocytes (CD4 + CD196+) and natural killer (NK) cells (CD49b +). Results are expressed as means and standard deviations of the percentage of each subpopulation stained with the specific antibody within the blocked cells.
Statistical analysis
Results are presented as means and standard deviations. The means of the three groups (CON, 5DHSC and 10DHSC) were compared with each other by means of ANOVA complemented with Tukey’s test to verify the difference between the CON group and the groups supplemented with H. sabdariffa. A significance level of 5 % (P < 0·05) was considered. Data were analysed using GraphPad Prism 5 software, version 5.01.
Results and discussion
Centesimal characterisation, dietary fibre and phenolic compounds of dehydrated H. sabdariffa calyces
The chemical composition of the H. sabdariffa calyces in the present work showed a predominance of total dietary fibre, with the highest concentration of insoluble fibre, followed by proteins, ash, total phenolic compounds, lipids, carbohydrates and anthocyanins (Table 2). Differences in the chemical composition of H. sabdariffa calyces between different studies are common and attributed to factors such as different cultivars, planting conditions, storage, climate type, soil type, harvest time, among other factors(Reference Borrás-Linares, Fernández-Gutiérrez and Segura-Carretero8,Reference Maciel, Carmo and Azevedo9,Reference Sáyago-Ayerdi, Velázquez-López and Montalvo-González31) .
Table 2. Characterisation of the centesimal composition, dietary fibre and phenolic compounds of DHSC (Mean values and standard deviations)

DHSC, dehydrated H. sabdariffa calyces; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
Data are expressed as mean ± sd of the analyses in triplicate.
In contrast to our results, Jabuer et al. (Reference Jabeur, Pereira and Barros32), after to analyse the proximate composition of DHSC, observed higher concentrations of carbohydrates, followed by ash, proteins and lipids. Regarding dietary fibre, in the present study, the concentration of soluble and insoluble fibres in the DHSC was higher than that reported by Kalla et al.(Reference Kalla, Jong and Kayem33), who found a total dietary fibre concentration of 6·15 g/100 g, with 5·86 g/100 g of insoluble fibre and 0·29 g/100 g of soluble fibre. However, higher concentrations of dietary fibre were reported by Sáyago-Ayerdi et al.(Reference Sáyago-Ayerdi, Velázquez-López and Montalvo-González31), with a total dietary fibre concentration ranging from 36 to 39 g/100 g, in which soluble fibre ranged from 7·06 to 8·32 g/100 g and insoluble fibre from 13·0 to 17·08 g/100 g among different cultivars of H. sabdariffa calyces of whole flower stems analysed in the study.
As for bioactive compounds, the concentration of total phenolic compounds in the present work was lower than that found by Maciel et al.(Reference Maciel, Carmo and Azevedo9), with a total concentration of phenolic compounds of 1·71 g in 100 g, but the value of anthocyanins was like our study, with a concentration of 0·24 g in 100 g. The antioxidant activity of DHSC in the present study was similar to that reported by Borrás-Linares et al.(Reference Borrás-Linares, Fernández-Gutiérrez and Segura-Carretero8), with a concentration ranging from 27·4 to 112 µmol of trolox/g of dehydrated calyx. The therapeutic potential of anthocyanidins was already demonstrated by Hemmati et al.(Reference Hemmati, Foroozan and Houshmand34) and Casao et al.(Reference Casao, Pinheiro and Sarandy35), which relate to the action of this compound in inflammatory diseases due to its antioxidant and anti-inflammatory properties. It indicates that its therapeutic action is probably related to the high level of phenolic compounds associated with anthocyanins.
Body weight and food intake of mice
Body weight did not differ between the groups (Fig. 2(a)). However, considering the total food consumption per week (Fig. 2(b)), all groups showed a reduction in diet intake during the second, third and fourth experimental weeks. The CON and the 5DHSC groups presented lower food intake compared with the 10DHSC group between the second and fourth week. Interestingly, such decrement in food consumption matches the beginning of DMH protocol. After the fifth week, no difference in food consumption was observed.
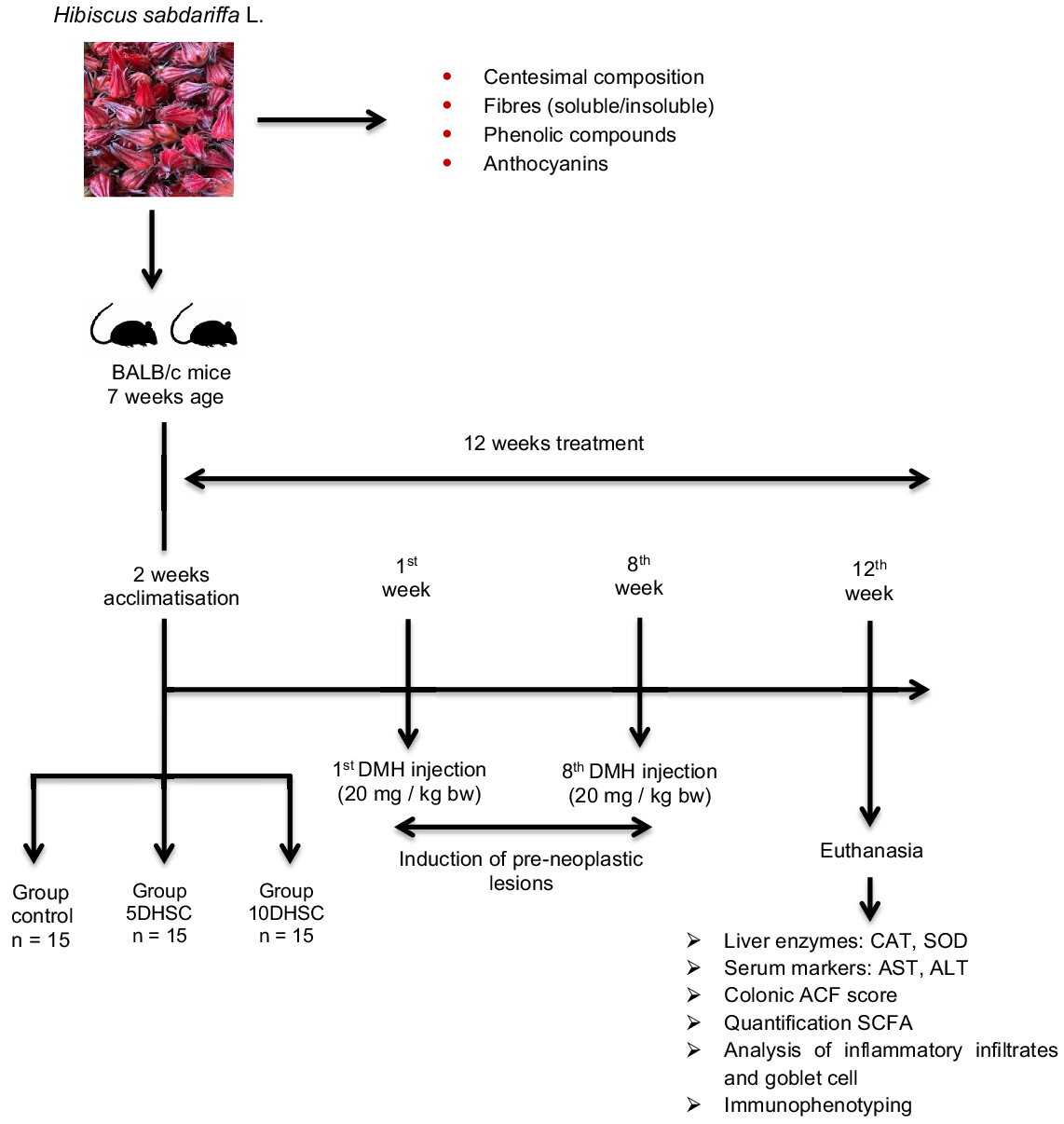
Fig. 1. Flow diagram of the study steps. DMH, 1,2-dimethylhydrazine; CAT, catalase; SOD, superoxide dismutase; ACF, aberrant crypt foci.

Fig. 2. Effect of DHSC supplementation on body weight (a) and food intake (b) of mice induced to pre-neoplastic lesions with DMH. DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; CON, control.
According to the average DHSC consumption of the animals, the 5DHSC group consumed 250 mg of DHSC/d which provided 23·05 mg of total dietary fibre, with 4·4 mg of soluble fibre and 18·65 of insoluble fibre, in addition to 2·73 mg of total phenolic compounds and 0·55 mg of total anthocyanins. In contrast, the 10DHSC group was supplemented with twice the amount of DHSC in the diet, and this group consumed 530 mg of DHSC/d containing 48·87 mg of total dietary fibre, with 9·33 mg of soluble fibre and 39·54 mg of insoluble fibre, in addition to 5·77 mg of total phenolic compounds and 1·17 mg of total anthocyanins. After, it was possible to calculate the quantity of DHSC possibly ingested by humans. This amount was determined through the method of normalisation of the body surface area considering the mice’s diet consumption. According to the calculations of Reagan-Shaw, Nihal and Ahmad(Reference Reagan-Shaw, Nihal and Ahmad36), considering the average weight of an adult to be 70 kg, the equivalent ingestion of DHSC for a human would be approximately 36 g/d (5DHSC) and 75 g/d (10DHSC) incorporated in preparations such as cakes, jams, icecreams and yogurts, for example(Reference Borrás-Linares, Fernández-Gutiérrez and Segura-Carretero8,Reference Maciel, Carmo and Azevedo9) .
Liver enzyme activity and serum markers in serum
No significant differences in SOD enzyme activity were observed between the experimental groups (Table 3). However, there was an increase in CAT enzyme activity in the group supplemented with 10 % DHSC. The drug DMH is a powerful carcinogen that induces oxidative stress, in addition to hepatotoxicity through its hepatic metabolism(Reference Ribeiro, Silva and Campanholo37,Reference Shebbo, Joumaa and Kawach38) . Therefore, phenolic compounds and anthocyanins, due to their antioxidant properties, can help reduce the risk of oxidative damage, in addition to contributing to hepatoprotective effects by increasing the activity of the antioxidant enzymes, such as CAT and SOD, as shown in the literature(Reference Kao, Hsu and Wang39–Reference Sunkara, Shackelford and Walker42).
Table 3. Effects of DHSC supplementation on liver enzyme activity and liver serum markers in BALB/c mice (Mean values and standard deviations)

DHSC, dehydrated H. sabdariffa calyces; CON, control; CAT, catalase; SOD, superoxide dismutase; 5DHSC, 5 % DHSC; 10DHSC, 10 % DHSC,
CAT, SOD (n 10).
Different letters mean statistical difference according to ANOVA complemented with Tukey’s test (P < 0·05).
These enzymes are extremely important in the fight against the generation of free radicals within the cell. When the tissue DMH exposure occurs, the generation of reactive oxygen species usually increases, which changes the lipids, protein and DNA of the cells, causing tissue stress and decreased cellular function(Reference Hemmati, Foroozan and Houshmand34,Reference Guo and DiPietro43) . Therefore, the formation of these radical species is controlled by the antioxidant enzymes, and it is desirable that the increase of these enzymes is to protect the tissue from the activity of these radicals. We believed that the increase in the antioxidant system stimulated by 10 % DHSC was important to reduce oxidative damage and, consequently, to reduce the risk of liver disease. The mechanism of action of antioxidant enzymes begins with the action of the SOD enzyme, which converts the superoxide anion into hydrogen peroxide; then, by the action of the CAT enzyme, this peroxide is transformed into water, reducing oxidative damage(Reference Ribeiro, Silva and Campanholo37,Reference Nandi, Yan and Jana44) .
Effect of dehydrated H. sabdariffa calyces on the development of aberrant crypt foci in the colon
In the present study, it was observed that, in the proximal and medial segments of the colon, there was no difference in the number of ACF between the groups (Table 4). However, in the distal portion of the colon, the groups supplemented with 5 % or 10 % of DHSC demonstrated a reduction of 34 % and 48 %, respectively, in ACF compared with the CON group. Still, considering the entire portion of the tissue (proximal, medial and distal), there is a reduction in ACF in the 5 % and 10 % DHSC groups compared with the CON group. Similarly, studies in the literature have shown that phenolic compounds are able to attenuate the formation of ACF in animals induced to CRC, reducing the risk of progression of the carcinogenesis process(Reference Kao, Hsu and Wang39,Reference Popović, Đukić and Katić45–Reference Al-Henhena, Khalifa and Ying47) . ACF can be observed in Fig. S1 in the online Supplementary material.
Table 4. Effect of DHSC supplementation (5 % and 10 %) on ACF formation in mice with DMH-induced pre-neoplastic lesions(Mean values and standard deviations)

DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; ACF, aberrant crypt foci; CON, control.
* Total number of ACF in all colon segments (proximal, medial and distal).
Different letters in each line mean statistical difference according to ANOVA complemented with Tukey’s test.
It has been reported that DMH exposure causes deep modifications in tissue associated with inflammation and oxidative stress and consequently promotes intestinal crypt proliferation and increases ammonia concentration, which can damage healthy cells. The lesions induced by this drug, presence of epithelial origin, and histological, morphological and anatomic characteristics are considered highly reproducible for CRC studies. Furthermore, they are highly specific, leading to the initiation and promotion of carcinogenesis in a dose-dependent manner(Reference Perse and Cerar6,Reference Venkatachalam, Vinayagam and Anand48) .
In our study, we believe that the reduction in ACF after DHSC exposure occurred because the DHSC interferes in the inflammatory and oxidative process and promotes a regression in the morphological alteration imposed by the carcinogenic process. Corroborating with our study, Chewonarin et al.(Reference Chewonarin, Kinouchi and Kataoka49) showed that chemopreventive effects are more effective in the early stages, in which the H. sabdariffa calyces extract reduced the formation of ACF in the early stage of carcinogenesis, but in the post-initiation stage the group treated with H. sabdariffa showed a formation of ACF similar to the untreated group, especially the ACF that had four or more crypts/focus. Phenolic compounds, in general, contribute to attenuating the progression of colorectal carcinogenesis by acting as natural chemopreventives, promoting inhibitory effects on the colorectal carcinogenesis process(Reference Costea, Hudiţă and Ciolac50–Reference Wang, Cui and Pan52).
It is noteworthy that ACF with more than three aberrant crypts/focus (ACF > 3) are more likely to progress to the tumor during colorectal carcinogenesis(Reference Wang, Cui and Pan52). In the present study, no ACF > 3 crypts/focus were found. It is suggested that this result is associated with the antioxidant properties of phenolic compounds and anthocyanins present in DHSC, since the increase in antioxidant defences helps to reduce the risk of developing pre-neoplastic lesions.
Effects of dehydrated H. sabdariffa calyces supplementation on butyrate production
SCFA (acetic, butyric and propionic acids) are the main metabolites produced by the fermentation of dietary fibres through the bacteria that make up the intestinal microbiota(Reference Takahashi, Yamada and Ohkubo53). It was observed that the concentration of acetic and butyric acid varied between the experimental groups (Fig. 3(a) and (b)). The diet supplemented with 5 % or 10 % DHSC did not significantly influence the concentration of faecal acetic acid (Fig. 3(a)); however, butyric acid was higher in the 10DHSC group compared with the CON group (Fig. 3(b)). Among the experimental weeks, a reduction in butyric acid is noted in the group supplemented with 10 % DHSC from the 1st to the 12th week. However, it should be noted that all fatty acids showed a reduction in their concentrations from the beginning to the end of the experimental period, but this reduction, even if significant, was smaller in the 10DHSC group compared with the CON and 5DHSC groups.

Fig. 3. Faecal concentration of acetic and butyric acid (µmols SCFA/g feces) in mice induced to pre-neoplastic lesions with DMH and supplemented with DHSC. SCFA were quantified on weeks 1, 5 and 12 of the experiment. Data are expressed as mean ± sd (n 5). *Means statistical difference according to ANOVA complemented with Tukey’s test (P < 0·05). DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; CON, control.
According to Liu et al.(Reference Liu, Wang and Jing54), animals treated with myristyl extract rich in anthocyanins showed a high representation of Lachnospiraceae and Ruminococcaceae in the microbiota, in which the presence of members of these families is considered an important producer of butyric acid. This result was consistent with the increase in butyric acid concentration in the supplemented group. These changes in the structure of the intestinal microbiome of animals treated with anthocyanins contribute to increased SCFA production, especially butyric acid.
The ingestion of dietary fibre and its fermentation by the intestinal microbiota increases SCFA production. Fibre intake significantly alters the microbiota population, increasing SCFA-producing bacteria and their metabolite levels, and may play a protective role in colon cancer(Reference Bishehsari, Engen and Preite55). Therefore, it is suggested in the present study that such an increase in butyric acid concentration in the 10DHSC group can be attributed to the higher consumption of DHSC and, consequently, fibre, phenolic compounds and anthocyanins. Acetic acid is absorbed in the colonic epithelium, transported and metabolised in the liver where it can be used by some ways such as lipogenesis, ketogenesis, production of cholesterol, glutamine and glutamate. Another part of the acetic acid reaches the circulation and is captured and oxidised by the muscle for energy production(Reference Bultman56). Butyric acid, however, is absorbed in the colonic epithelium, so its bioavailability is mainly restricted to the colon. It is later metabolised to produce ATP, providing 70 % of energy to colonocytes, in addition to playing other roles in maintaining colon homoeostasis and epithelial integrity(Reference Bultman56–Reference Bultman59).
Butyric acid can exert immunomodulatory effects, such as the inhibition of histone deacetylases; by inhibiting the activity of HDAC, butyric acid induces the expression of the p21Waf1/Cip1 gene, which can interrupt cell proliferation(Reference Hamer, Jonkers and Venema57,Reference Bultman59) . The anti-inflammatory mechanisms of butyric acid involve the suppression of activation of the NF-κβ. The inhibition of NF-κβ by butyric acid can result in the reduction of myoperoxicities, COX-2, adhesion molecules and pro-inflammatory cytokines. In addition, butyric acid can inhibit interferon-&x1D67; production and regulate PPAR&x1D67; signalling. Activation of this receptor on colonic epithelial cells can inhibit the production of inflammatory cytokines, promoting anti-inflammatory effects(Reference Bultman56).
Intake of fibre and/or polyphenols has been associated with reduced inflammation and inflammatory diseases due to the production of metabolites from polyphenols (valerolactones, aromatics, among others) and fibre (SCFA)(Reference Kuo60,Reference Gasaly, Hermoso and Gotteland61) . Therefore, the increased production of butyric acid in the 10DHSC group in the present study may be associated with a reduction of ACF and the presence of inflammatory infiltrates in this group.
Histological analyses of the distal colon
The groups treated with 5 % and 10 % DHSC in the diet showed a significant reduction in the percentage of inflammatory infiltrates compared with the animals in the CON group (Fig. 4).

Fig. 4. Percentage (%) of inflammatory infiltrates (a) in the control group (b), 5DHSC (c) and 10DHSC (d) in the distal colon portion of BALB/c mice induced to pre-neoplastic lesions with DMH and supplemented with DHSC. Arrows indicate inflammatory infiltrates. Data are expressed as mean ± SD (n 7). Different letters mean statistical difference according to ANOVA complemented with Tukey’s test (P < 0·05). DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; CON, control.
Possibly, the phenolic compounds present in the DHSC showed antioxidant and anti-inflammatory properties during the development of pre-neoplastic lesions. In our study, the inflammation observed in CON group is probably related to DMH exposure, since it has already been known that this drug causes oxidative stress through the methylation of epithelial cell biomolecules resulting in an inflammatory state that contributes to the progression of these lesions(Reference Hamiza, Rehman and Tahir62). To better understand the inflammatory process that occurred, it is known that, after the appearance of a tissue injury, the recruitment of inflammatory cells to the injury site begins. The infiltration and enhanced activation of inflammatory cells in the injured tissue can initiate and promote the progression of the carcinogenesis process, since these inflammatory cells can produce inflammatory cytokines, several of which play crucial roles during this process(Reference Li, Kundu and Seow63–Reference Bahrami, Amerizadeh and ShahidSales65). Furthermore, it is noteworthy that with the progression of the inflammatory process, the intestinal mucosa is exposed to oxidative processes, which contributes to a greater risk of transformation of normal cells into malignant ones(Reference Guina, Biasi and Calfapietra66). We believed that the reduction in inflammatory infiltrates after DHSC exposure occurred due to the presence of phenolic compounds and anthocyanins may involve some mechanisms such as the neutralisation of free radicals, regulation of pro-inflammatory compounds and inflammation-associated cells(Reference Szymanowska and Baraniak67). Thus, the antioxidants present in the DHSC may be able to alleviate the damage to the colon caused by free radicals, with less infiltration of inflammatory cells in the tissue of the treated animals.
In relation to the goblet cells, based on area and diameter values (Fig. 5(a) and (b)), the group supplemented with 10 % DHSC had more hypertrophied goblet cells compared with the CON group. In addition, the CON group demonstrated a reduction in the volume density of goblet cells per tissue area, that is, a depletion of these cells in relation to the 10DHSC group (Fig. 5(c)). The mechanisms that could explain this hypertrophy is that after the development of tissue inflammation, the immune system induces goblet cells to extensive mucus release to remove intruders, making them larger due to increased mucus production(Reference Johansson and Hansson68,Reference Kaur, Saxena and Debnath69) .

Fig. 5. Area (µm), diameter (µm) and percentage (%) volumetric density of goblet cells present in the colonic mucosa of BALB/C mice induced to pre-neoplastic lesions with DMH and supplemented with DHSC. Staining was performed with Alcian Blue pH 2·5 (control – D, 5DHSC – E, 10DHSC – F) and periodic acid Schiff (control – G, 5DHSC – H, 10DHSC – I). Arrows indicate goblet cells. Data are expressed as mean ± sd (n 10). *Means statistical difference according to ANOVA complemented with Tukey’s test (P < 0·05). DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; CON, control.
Bioactive compounds can help maintain the integrity of goblet cells in the colon as reported by Almagrami et al.(Reference Almagrami, Alshawsh and Saif-Ali70), in which the authors observed that animals in the CON group induced to CRC with azoxymethane showed a significant reduction in the size of goblet cells and a depletion of mucin. On the other hand, the group of animals treated with Acanthus ilicifolius Linn in the diet (250 mg/kg of body weight) showed a slight reduction in these cells. Acanthus ilicifolius Linn is a prickly herb that is a source of flavonoids, terpenes and alkaloids. Furthermore, Rehman et al. (Reference Rehman and Rather71) demonstrated that prophylactic treatment for 14 d with the flavanone myricetin (25 and 50 mg/kg of body weight) during cisplatin-induced colon toxicity was able to maintain the integrity of the goblet cells in addition to preventing the mucin depletion. Goblet cells perform necessary functions to combat oxidative damage, inflammatory disorders and intestinal infections, in addition to being part of the innate immune system of the mucosa to defend the host against possible pathogens(Reference Zuo, Cao and Xue72).
Determination of the presence of leucocytes in the colonic mucosa
No significant differences were observed in the percentage of CD4, CD8, Th17 and Treg cells between groups (Fig. 6(a), (c), (d) and (e)). However, the group that was supplemented with 10 % DHSC showed an increase in NK cell infiltration into the colonic mucosa (Fig. 6(b)).

Fig. 6. Leucocytes quantified in the colon mucosa of BALB/c mice induced to pre-neoplastic lesions with DMH and supplemented with DHSC. Data are expressed as mean ± standard deviation (n 6). Different letters between bars mean statistical difference according to ANOVA complemented with Tukey’s test (P < 0·05). DHSC, dehydrated H. sabdariffa calyces; DMH, 1,2-dimethylhydrazine; CON, control; NK, natural killer.
NK cells are cytotoxic lymphocytes that play a role in immune defence. These cells through class I MHC can distinguish between normal and altered cells and can lyse malignant cells, acting against the formation and development of tumours(Reference Stojanovic, Fiegler and Brunner-Weinzierl73,Reference Gidlund, Örn and Pattengale74) .
Unlike normal cells, pre-neoplastic cells express specific receptors on the cell surface. These receptors are recognised by NK cells and then the immune response is initiated through the release of granules containing perforin, a protein that breaks the cell membrane and induces cell apoptosis(Reference Maria, Barnes and Weist75). Cell death mediated by NK cells may be more effective in the early stage of differentiation of carcinogenic cells(Reference Nagarsheth, Wicha and Zou76). Thus, the greater infiltration of NK cells in the colonic mucosa of animals in the 10DHSC group may be associated with the reduction in ACF that was observed in this same group of animals.
Conclusion
Supplementation with 5 % and 10 % of DHSC promoted a significant reduction in ACF, and this result is consistent with the reduction in the presence of inflammatory infiltrates in these groups. Supplementation with 10 % DHSC was more effective, as in addition to these changes in ACF and inflammatory infiltrates, there was an increase in hepatic CAT enzyme activity, butyrate production and a greater infiltration of NK cells in the colon. Therefore, it is suggested that the consumption of DHSC attenuates cellular responses in the early stage of the development of pre-neoplastic lesions. Intervention in the initial phase is relevant as it can help reduce the risk of progression of the carcinogenesis process. Furthermore, oxidative stress can be attenuated due to the potential antioxidant capacity of phenolic compounds and anthocyanins present in the DHSC included in the diet. The reduction of the oxidative process is another important factor to reduce the risk of developing pre-neoplastic lesions.
Acknowledgements
This study was supported by the Coordination for the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), the Fundação de Amparo à Pesquisa of the State of Minas Gerais (FAPEMIG) and the Universidade Federal de Viçosa (UFV). All authors contributed significantly to the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522001222













