Many naturally occurring compounds with antioxidative effects are now known to protect cellular components from oxidative damage and prevent diseases(Reference Gulcin1, Reference Gulcin2). A number of such compounds can activate the phase II detoxification enzymes, which can remove the toxic elements from our system. Exposure to such phytochemicals is therefore beneficial to human health. In addition, many natural compounds are now known to have a modulator role on physiological functions and biotransformation reactions involved in the detoxification process, thereby providing protection from cytotoxic, genotoxic and metabolic effects of environmental toxicants(Reference Saha and Das3). Numerous studies have demonstrated that a great number of medicinal and aromatic herbs, as well as fruits and leaves of some berry plants, biosynthesise phytochemicals possessing antioxidant property and may be used as a natural source of free radical scavenging compounds(Reference Yu, Zhou and Parry4, Reference Sacchetti, Maietti and Muzzoli5). Also, a great number of spices and aromatic herbs contain chemical compounds exhibiting antioxidant properties. These properties are attributed to various active phytochemicals including vitamins, carotenoids, terpenoids, alkaloids, flavonoids, lignans, simple phenols and phenolic acids, and so on(Reference Liu and Ng6).
Grapeseeds (GS) are a particularly rich source of complex polymers of flavonoids such as gallic acid, the monomeric flavan-3-ols catechin, epicatechin, gallocatechin, epigallocatechin, epicatechin 3-ogallate, dimeric-, trimeric and even more polymeric proanthocyanidins(Reference Segura, Marin and Delgado7–Reference Rezende, Graf and Guterres9). Furthermore, grape skins and seeds contain flavonoids (catechin, epicatechin, procyanidins and anthocyanins), phenolic acids (gallic and ellagic acids) and stilbenes(Reference Yilmaz and Toledo10). Earlier studies have demonstrated the potent free radical scavenger ability of GS procyanidins(Reference Da Silva, Darmon and Fernandez11). Animal studies have indicated that myocardial infarction rate reduces with dietary GS procyanidin extract supplementation(Reference Sato, Maulik and Ray12). Addition of 1 % GS procyanidin extract (w/w) to diet reduced atherosclerosis in the aorta without influencing the serum lipid profiles in rabbits(Reference Yamakoshi, Kataoka and Koga13). GS extract of Vitis vinifera L. has in vivo antioxidant property(Reference Sato, Bagchi and Tosaki14) and could be as important as vitamin E in preventing oxidative damage in tissues(Reference Tebib, Rouanet and Besancon15) by reducing the lipid oxidation(Reference Bouhamidi, Prevost and Nouvelot16) and/or inhibiting the production of free radicals(Reference Bagchi, Garg and Krohn17). Grapes containing either high or low flavanol contents showed anti-ulcer activity in rats(Reference Saito, Hosoyama and Ariga18). GS procyanidin extract and its phenolic acid, gallic acid, may play a role in inducing apoptosis or programmed cell death in the body(Reference Sakaguchi, Inoue and Ogihara19, Reference Joshi, Kuszynski and Bagchi20). Further, some authors have suggested that GS procyanidins have anti-tumoural property and can increase the anti-tumoural activity of the chemotherapeutic agent doxorubicin(Reference Zhang, Li and Wu21).
The reactive oxygen species are known to play a major role in either the initiation or the progression of carcinogenesis by inducing oxidative stress(Reference Gulcin22). Peroxides and superoxide anion (![]() ) produce cytotoxicity/genotoxicity in cellular system(Reference Gulcin, Tel and Kireci23, Reference Gulcin, Bursal and Sehitoglu24). Reactive oxygen species and nitrogen species are formed in the human body and endogenous antioxidant defences are not always sufficient to counteract them completely. A large number of studies support the hypothesis that oxidative damage to DNA, lipids and proteins may contribute to the development of CVD, cancer and neurodegenerative diseases(Reference Halliwell25, Reference Gulcin, Mshvildadze and Gepdiremen26). Diet-derived antioxidants may therefore be particularly important in protecting against chronic diseases(Reference Halliwell25, Reference Vendemiale, Grattagliano and Altomare27).
) produce cytotoxicity/genotoxicity in cellular system(Reference Gulcin, Tel and Kireci23, Reference Gulcin, Bursal and Sehitoglu24). Reactive oxygen species and nitrogen species are formed in the human body and endogenous antioxidant defences are not always sufficient to counteract them completely. A large number of studies support the hypothesis that oxidative damage to DNA, lipids and proteins may contribute to the development of CVD, cancer and neurodegenerative diseases(Reference Halliwell25, Reference Gulcin, Mshvildadze and Gepdiremen26). Diet-derived antioxidants may therefore be particularly important in protecting against chronic diseases(Reference Halliwell25, Reference Vendemiale, Grattagliano and Altomare27).
Turkey is, today, the fifth-largest producer of grapes and is becoming one of the important wine producers in the world(Reference Bozan, Tosun and Ozcan28). Although some studies(Reference Bozan, Tosun and Ozcan28, Reference Bakkalbasi, Yemis and Aslanova29–Reference Orak31) reported major flavanol contents and antioxidant activities, the literature lacks information on chemopreventive and antioxidant roles of GS varieties in Turkey. Therefore, the objective of this study was to determine hepatoprotective and antioxidant properties of Mazrona GS variety, which is widely grown in Mardin region located in south-eastern Turkey.
There is a growing interest of natural products in the human diet, both because of the possible negative effects of synthetic food additives on human health and the increased consumer awareness about this problem in recent years. As far as our literature survey could ascertain, no studies have been reported on hepatoprotective role and antioxidant capacity of GS supplementation. The objective of this study was to determine healthful potentials of GS against alcohol-induced oxidative stress by evaluating their in vivo hepatoprotective role and antioxidant capacity. Thus, in the present study, we have extensively studied the antioxidant activity of GS using in vivo models. For this aim, food containing 15 % powdered GS was given orally as treatment because the effect of the functional plant is well characterised in nutrition and GS is widely consumed in Turkey and worldwide. The serum enzymes were chosen because of their importance as index of hepatotoxin and hepatoprotective effects. The antioxidant activity of GS on some phase II detoxification antioxidant defence system (ADS) such as GSH, glutathione reductase, superoxide dismutase, glutathione S-transferase (GST) and glutathione peroxidase (GPx) and malondialdehyde (MDA) contents in the various tissues was evaluated during the experiment.
Materials and methods
Chemicals
Thiobarbituric acid, butylated hydroxytoluene, trichloroacetic acid, EDTA, GSH, metaphosphoric acid, 5,5′dithiobis-(2-nitrobenzoic acid), trihydroxymethyl aminomethane, 1-chloro-2,4-dinitrobenzene, GSSG, NADPH, KH2PO4 and NaCl of technical grade used in this study were supplied by Sigma Chemical (St Louis, MO, USA). Kits for the analysis of antioxidant enzymes were supplied by Randox Laboratories (Crumlin, County Antrim, UK).
Animals
Rats (Wistar albino) 4 months of age with an average weight 200–250 g were provided from the Experimental Animal Research Center, Yuzuncu Yil University (Van, Turkey), and were housed in four groups, each group containing six rats. The animals were housed at 20 ± 2°C in a daily light/dark cycle. All animals were fed a diet based on wheat and soyabean meal and water ad libitum in stainless cages, and received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institutes of Health. The ethics regulations were followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments. This study was approved by the ethic committee of the Yuzuncu Yıl University (no. B.30.2.YYÜ.0.05.06.00/300-12).
Preparation of foods
Briefly, GS were provided from a local winegrower in Mardin, a major grape-producing province of Turkey. The natural and organic GS of Mazrona variety used in the study were obtained from the Dargecit–Mardin region. This variety was chosen because of the remarkable total production of grapes in the region comes from Mazrona. GS were ground into powder and then the amount of powdered GS was adjusted 15 % of rat food.
Experimental design
The rats were randomly divided into four groups, each containing six rats.
Group I (control)
The rats received tap water and fed with standard pellet diet ad libitum.
Group II (alcohol)
The rats received 20 % ethanol water and fed with standard pellet diet ad libitum. Dose of ethanol was selected on the basis of a 20 % concentration, which caused oxidative stress administered orally(Reference Aykac, Uysal and Suha Yalcin32–Reference Yurt and Celik35).
Group II (alcohol)Group III (15 % grapeseeds)
The rats received tap water and fed with 15 % GS powder containing diet supplementation.
Group IV (15 % grapeseeds+20 % alcohol)
The rats received 20 % alcohol water and fed with 15 % GS powder containing diet supplementation.
Preparation of tissues supernatant and erythrocyte pellets
At the end of the 50-d experiments, the rats were anaesthetised by an injection of ketamine (5 mg/100 g body weight) intraperitoneally. The blood samples were obtained from a cardiac puncture, using syringe for the determination of serum marker enzyme levels and biochemical analysis. The serum samples were obtained by centrifuging blood samples at 4000 g for 15 min at 4 °C, and enzyme levels were measured from these serum samples. For biochemical analysis, blood samples were put immediately into disposable silicon glass tubes with EDTA as an anti-coagulant and were centrifuged at 4000 g for 15 min at 4°C and erythrocyte pellets were obtained. Then, the pellets were washed tree times with physiological saline (0·9 % NaCl).
The tissues of brain, kidney, spleen, heart and liver were dissected and put in Petri dishes. After washing the tissues with physiological saline (0·9 % NaCl), the samples were taken and kept at − 78°C during the analysis. The tissues were homogenised for 5 min in 50 mm ice-cold KH2PO4 solution (1:5 w/v) using stainless steel probe homogeniser (ultrasonic frequency 20 KHz; Jencons Scientific, Leighton Buzzard, Beds, UK) for 5 min and then centrifuged at 7000 g for 15 min. All processes were carried out at 4°C. Supernatants and erythrocyte pellets were used to determine ADS constituents and MDA contents(Reference Yurt and Celik35, Reference Celik, Temur and Isık36).
Biochemical analysis
The concentration of MDA in erythrocyte and tissues was determined using the method described by Jain et al. (Reference Jain, McVie and Duett37), based on thiobarbituric acid reactivity. The concentration of GSH in erythrocyte and tissues was measured using the method described by Beutler et al. (Reference Beutler, Dubon and Kelly38). GST was assayed by following the conjugation of GSH with 1-chloro-2,4-dinitrobenzene at 340 nm as described by Mannervik & Guthenberg(Reference Mannervik and Guthenberg39). Glutathione reductase activity was assayed as described by Carlberg & Mannervik(Reference Carlberg and Mannervik40) as the decrease in absorbance of NADPH at 340 nm. GPx activity was assayed as described by Paglia & Valentine(Reference Paglia and Valentine41) based on that of GPx catalyses and the oxidation of GSH by cumene hydroperoxide. Superoxide dismutase activity was measured at 505 nm by calculating inhibition percentage of formazan dye formation(Reference McCord and Fridovich42).
Measurement of enzyme levels
The activities of serum marker enzymes, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT) and lactate dehydrogenase (LDH), were measured by an auto analyser (BM/HITACHI–911; Boehringer Mannheim, Mannheim, Germany), using the kits.
Analysis of data
All data were expressed as mean and standard deviation. The statistical analyses were carried out using the Minitab 13 for Windows packet program. Means and standard deviations were calculated according to the standard methods for all parameters. One-way ANOVA was used to determine the differences between means of the experimental groups, accepting the significance level at P ≤ 0·05.
Results
Following the exposure of experimental groups, the effects of alcohol and the GS-supplemented diet on liver damage index and antioxidative role were evaluated as marker serum enzymes, ADS and MDA, content in blood and various tissues samples from control and treated rats. The results of experiment showed that the treatment of rats with alcohol and alcohol+GS-containing diet supplementation caused changes in the level of serum enzymes, MDA content and ADS constituents in comparison with control rats. As known, serum AST, ALT, GGT and LDH levels are susceptible to hepatotoxin and serve as markers of liver damage, which promotes the release of such aminotransferases from hepatocytes into the bloodstream. According to the results, levels of serum liver damage enzymes, such as AST, ALT, GGT and LDH, in alcohol-treated group were significantly increased compared with the control group whereas AST and LDH levels of GS-supplemented alcohol (alcohol+GS-treated group) resulted in marked decreases. There was no significant difference in ALT and GGT levels between control and alcohol+GS-treated groups (Table 1). With regard to MDA content and ADS constituents, the results of experiment showed that the treatment of rats with alcohol and alcohol+the functional food containing diet supplementation caused changes in MDA concentration and ADS in the erythrocyte, liver, brain, kidney, spleen and heart tissues in comparison to those of control rats. According to the results, the increased MDA content because of oxidative stress induced by alcohol in the all tissues was found to be decreased in the tissues in GS-treated groups. MDA content increased significantly in all the tissues in the alcohol-exposed group whereas it was decreased in the GS-treated group. On the other hand, while ethanol caused fluctuation in ADS constituent level as a result of oxidative stress condition in the rats, the healing effects of the GS against these fluctuations could not be determined (Table 2).
Table 1 Effect of grapeseed supplementation on liver damage marker enzymes in serum of rats
(Mean values and standard deviations)
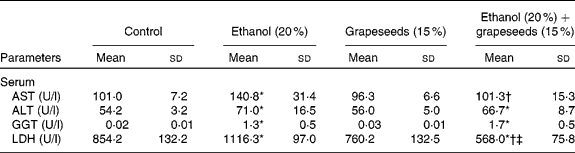
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; LDH, lactate dehydrogenase.
* Mean values were significantly different from control (P ≤ 0·05, one-way ANOVA).
† Mean values were significantly different from the ethanol group (P ≤ 0·05, one-way ANOVA).
‡ Mean values were significantly different from the grapeseeds group (P ≤ 0·05, one-way ANOVA).
Table 2 Effect of grapeseeds supplementation on antioxidant status and malondialdehyde (MDA) content in various tissues of rats
(Mean values and standard deviations)

GPx, glutathione peroxidase; GR, glutathione reductase; SOD, superoxide dismutase; GST, glutathione S-transferase.
* Mean values were significantly different from control (P ≤ 0·05, one-way ANOVA).
† Mean values were significantly different from the ethanol group (P ≤ 0·05, one-way ANOVA).
‡ Mean values were significantly different from the grapeseeds group (P ≤ 0·05, one-way ANOVA).
Discussion
Overexposures to oxidative stress caused by environmental pollutants are thought to increase the risk of cancer. Also, it is known that alcoholic liver disease is a major medical complication of alcohol intake. Oxidative stress plays an important role in the development of alcohol-related liver disease(Reference Gulcin43, Reference Coban, Beydemir and Gulcin44). Hence efforts are needed to provide effective protection from the damaging agents, and experimental studies have implicated the influence of a functional plant, GS, in this regard. The first aim of this study was to investigate whether GS supplementation could prevent hepatotoxicity of ethanol, decrease content of the MDA and efficacy of the ADS in rats.
The results of the present study show, for the first time, that the treatment of the rat with GS effectively protected the rat against alcohol-induced hepatotoxicity, as evidenced by decreased AST, ALT and LDH serum enzyme levels and MDA contents in all the tissues. In this study, experimental alcoholosis was induced in rats by feeding them with a diet containing 20 % ethanol in drinking water for 50 d. The known biochemical findings of the ethanol toxicity in rats were also observed in the present study. The hepatotoxic effects of alcohol have been well documented in various animal species. Reactive oxygen species and consequent peroxidative damage caused by alcohol are considered to be the main mechanisms leading to hepatotoxicity(Reference Aykac, Uysal and Suha Yalcin32–Reference Yurt and Celik35).
As shown in the Table 1, alcohol caused a significant elevation in the levels of AST, ALT, GGT and LDH in comparison to those of control rats whereas GS supplementation caused a significant decrease the serum marker enzymes in comparison to those of alcohol-treated rats. The reasons for such an effect of alcohol and the GS supplementation are certainly not understood at present. However, it is known that several soluble enzymes in blood serum, such as these enzymes, have been considered as indicators of the hepatic dysfunction and damage. Also, the increase in the activities of AST and ALT in plasma of rats treated with ethyl alcohol is mainly due to the leakage of these enzymes from the liver cytosol into the bloodstream(Reference Navarro, Montilla and Martin45). Further, ALT and AST levels are also of value indicating the existence of liver diseases, as this enzyme is present in large quantities in the liver. ALT increases in serum when cellular degeneration or destruction occurs in this organ(Reference Hassoun and Stohs46). Any interference in these enzymes leads to biochemical impairment and lesions of the tissue and cellular function(Reference Khan, Reddy and Mahboob47). Yousef et al. (Reference Yousef, Awad and Elhag48) reported that the changes in the activities of these enzymes in SnCl2-treated rats were regarded as the biochemical manifestation of the toxic action of inorganic tin. On the other hand, phosphatases and dehydrogenases are important and critical enzymes in biological processes too. They are responsible for detoxification, metabolism and biosynthesis of energetic macromolecules for different essential functions. The increase in plasma LDH activity may be due to the hepatocellular necrosis leading to leakage of the enzyme to the bloodstream(Reference Wang and Zhai49). Thus, when alcohol leads to the release of these enzymes into plasma as a result of autolytic breakdown or cellular necrosis, the GS supplement imparts protection against alcohol-induced oxidative injury that may result in development of liver damage.
As shown in Table 2, the present study shows that GS could have an antioxidative role in rats. This was obvious from our observation that, by the consequence of GS additional treatment in vivo, the concentration of MDA in the tissues differed from that of the alcohol-exposed group. According to the obtained results, while MDA concentrations appreciably increased in the erythrocytes, liver, brain, kidney, spleen and heart of rats treated with alcohol, the tissue MDA contents significantly decreased in the GS supplementation group compared with that of alcohol group. The reasons for such an effect of alcohol and the GS additions are not understood at present. But, the increased MDA content might have resulted from an increase of reactive oxygen species as a result of stress condition in the rats with ethyl alcohol intoxication. Studies have shown that alcohol consumption may result in increased oxidative stress with formation of lipid peroxides and free radicals(Reference Aykac, Uysal and Suha Yalcin32–Reference Yurt and Celik35, Reference Nordmann, Ribiere and Rouach50–Reference Gentry-Nielsen, Top and Snitily53). Alcohol-induced oxidative stress is linked to the metabolism of ethanol(Reference Zima, Fialova and Metsek54). On the other hand, it is known that the elevation of lipid peroxidation after the consumption of some xenobiotics and following superoxide overproduction, which produce dismutation singlet oxygen and H2O2, can be easily converted later into the reactive ∙OH. Both single oxygen and OH radicals have a high potential to initiate free radical chain reactions of lipid peroxidation. Further, it is known that ∙OH can initiate lipid peroxidation in tissues(Reference Halliwell25) and MDA is a major oxidation product of peroxidised PUFA and increased MDA content is an important indicator of lipid peroxidation(Reference Freeman and Crapo55).
Meanwhile, superoxide dismutase, glutathione reductase, GPx and GST activities and GSH levels fluctuated at appreciable level in the alcohol-treated rats. But the efficacy of the GS against these fluctuations could not be determined. The reasons for such an effect of functional plant's supplement are not understood at present. However, oxidative stress can affect the activities of protective enzymatic antioxidants in organisms exposed to alcohol. The increased GPx and GST activities may reflect an adaptive change against ethanol-induced lipid peroxide toxicity(Reference Aykac, Uysal and Suha Yalcin32). However, the increased activities of GST are known to serve as protective responses to eliminate xenobiotics(Reference Smith and Litwack56). Thus, the existence of an inducible antioxidant system may reflect an adaptation of organisms. Also, the reasons for such an effect of the GS addition may be due to polymers of flavonoids such as gallic acid, monomeric flavan-3-ols catechin, epicatechin, gallocatechin, epigallocatechin, epicatechin 3-ogallate, dimeric-, trimeric and even more polymeric proanthocyanidins(Reference Segura, Marin and Delgado7–Reference Yilmaz and Toledo10) in the GS, or that GS have strong antioxidant properties and may therefore protect cells and tissues against free oxygen radicals. So far, no study examining the preventive role of GS-supplemented food in vivo has been carried out on rat serum marker enzymes levels, ADS and MDA content in diet supplementation. Therefore, we had no chance to compare the present results with the previous ones. In addition, because of high variability in analysing serum enzymes–chemicals interaction in vitro and in vivo, and inconsistent factors like treatment time and manner, the setting of studies and species tissue differences etc., it is difficult to compare the present data to different studies regarding the chemopreventive properties. However, earlier studies have demonstrated the potent free radical scavenger ability of GS procyanidins(Reference Da Silva, Darmon and Fernandez11). Procyanidin- containing seed extracts of V. vinifera L. have in vivo antioxidant activity(Reference Sato, Bagchi and Tosaki14) and could be as important as vitamin E in preventing oxidative damage in tissues(Reference Tebib, Rouanet and Besancon15) by reducing lipid oxidation(Reference Bouhamidi, Prevost and Nouvelot16) and/or inhibiting the production of free radicals(Reference Bagchi, Garg and Krohn17). Despite treatment time and manner and the different setting of studies, the results of the afore-mentioned studies are in agreement with the present results.
In conclusion, the observations presented here led us to conclude that while administration of subchronic ethyl alcohol promotes MDA concentration fluctuations in the antioxidative systems and elevates liver damage serum marker enzymes, the GS supplement imparts protection against alcohol-induced liver injury and oxidative stress. The observations, along with changes, might also suggest that such a test will also be of value in chemopreventive studies, and also be of interest to understand the molecular basis of the refractoriness of the protective role of GS. Also, we wish to study alcohol-destructive effects and the healing effects of GS against alcohol before coming to any conclusion. Nevertheless, the results suggest that regular intake of the functional food may be useful for the prevention of chronic degenerative liver diseases.
Acknowledgements
None of the authors has a commercial interest, financial interest, and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies and/or medical devices or with commercial providers of medical services. The authors are grateful to the University of Yuzuncu Yil Grant Commission for providing financial assistance during the tenure of research (no. YYÜ-BAP-2010-FBE-YL033). I. C. was the main moderator of the study. A. D. performed the biochemical investigation and treatment in this study.




