Man and other mammals cannot synthesise folate, and thus must obtain the vitamin from exogenous sources via intestinal absorption. Therefore, the intestine plays a central role in controlling and regulating body folate levels. Dietary folate is absorbed in the small intestine; however, folate can also be synthesised by the normal microflora of the large intestine and can be absorbed in that region of the gut(Reference Rong, Selhub, Goldin and Rosenberg1). Fe is an essential mineral, body levels of which are controlled by regulated intestinal absorption. Both micronutrients are added as supplements to foods and are combined in supplements to combat anaemia(Reference Picciano2). The recent finding that the intestinal transporter, proton-coupled folate transporter/haem carrier protein 1 (PCFT/HCP1) (SLC46A1) participates in the absorption of folate and may also transport an important dietary Fe source, haem, raises the possibility that there may be an interaction between these micronutrients at the intestinal level.
Regulation of intestinal uptake, tissue distribution and renal tubular reabsorption is essential for normal folate homeostasis and to ensure its adequate supply for normal human health. It is found to be essential for the biosynthesis of nucleic acid precursors (purine and pyrimidine) and metabolism of many other amino acids(Reference Selhub, Jacques and Bostom3). Folate deficiency can result from intestinal diseases(Reference Elsborg and Larsen4) that affect absorption, food factor interactions (for example, catechins and tea extracts)(Reference Alemdaroglu, Wolffram, Boissel, Closs, Spahn-Langguth and Langguth5), chronic alcohol consumption(Reference Hamid, Wani, Rana, Vaiphei, Mahmood and Kaur6) or genetic defects(Reference Geller, Kronn, Jayabose and Sandoval7). Thus, defects in intracellular folate homeostasis have been involved in embryonic abnormalities, cancer and CVD(Reference Barber, Bennett, Greer and Finnell8, Reference Finnell, Greer, Barber and Piedrahita9). In most of these clinical conditions, folate malabsorption results from the suppression of both of the processes of hydrolysis and intestinal uptake of folate(Reference Halsted10).
Several different mechanisms have been proposed to regulate intestinal epithelial folate uptake in mammals, including reduced folate carriers (RFC-1)(Reference Sirotnak and Tolner11) and glycosylphosphatidylinositol-linked folate receptors (FR), also known as folate-binding proteins (Folbp)(Reference Antony12). In the mouse, the SLC19A1 (RFC-1) transporter is more abundantly expressed in the epithelial cells of the villus tips than in the immature cells of the crypt and is localised at the apical membrane, suggesting a possible role in the absorption process(Reference Chiao, Roy, Tolner, Yang and Sirotnak13). After folates bind to the FR, the receptor–folate complex is believed to be internalised by an endocytotic mechanism, thereby delivering the folate cofactors into the cell's cytoplasm(Reference Selhub, Jacques and Bostom3, Reference Kamen and Smith14). Three FR isoforms have been identified and characterised in human cell lines. FR-α is present predominantly in epithelial cells(Reference Elwood15–Reference Shen, Ross, Wang and Ratnam17), FR-β is expressed at low to moderate levels in several different tissues(Reference Ratnam, Marquardt, Duhring and Freisheim18), whereas FR-γ/γ′ are specific for haematopoietic cells(Reference Shen, Ross, Wang and Ratnam17, Reference Shen, Wu, Ross, Miller and Ratnam19).
In contrast, although several haem transporters have been described in prokaryotes(Reference Bracken, Baer, bdur-Rashid, Helms and Stojiljkovic20, Reference Wandersman and Stojiljkovic21), the mechanism responsible for the uptake of haem in eukaryotic cells has been controversial. Studies showed that haem enters the small-intestinal absorptive cell as an intact Fe-porphyrin, and may be facilitated by a vesicular transport system(Reference Uzel and Conrad22), presumably binding first to the brush-border membrane of enterocytes, then undergoing internalisation into the cytoplasm, finally appearing within enclosed vesicles(Reference Wyllie and Kaufman23) and being broken down in the enterocyte by microsomal haem oxygenase(Reference Raffin, Woo, Roost, Price and Schmid24). In eukaryotic cells, the assessment of cellular haem uptake has been confounded by previous studies which suggested the existence of haem binding proteins in the mammalian enterocyte brush-border membrane(Reference Tenhunen, Grasbeck, Kouvonen and Lundberg25, Reference Grasbeck, Kouvonen, Lundberg and Tenhunen26), hepatocyte plasma membrane(Reference Worthington, Cohn, Miller, Luo and Berg27) and on the surface of haematopoietic cell lines(Reference Galbraith28, Reference Galbraith, Sassa and Kappas29).
Progress seemed to have been made recently, when a haem carrier protein (HCP1)(Reference Shayeghi, Latunde-Dada and Oakhill30) was isolated from the mouse duodenum and was shown to mediate the transport of haem and its analogues such as Zn protoporphyrin, although with a relatively low affinity. Subsequently, however, the same protein was identified as a proton-coupled folate transporter and named PCFT(Reference Qiu, Jansen, Sakaris, Min, Chattopadhyay, Tsai, Sandoval, Zhao, Akabas and Goldman31). PCFT/HCP1 has been shown to be altered in response to altered dietary folate levels in mice(Reference Qiu, Min, Jansen, Malhotra, Tsai, Cabelof, Matherly, Zhao, Akabas and Goldman32) and explains many kinetic properties of intestinal folate uptake(Reference Inoue, Nakai, Ueda, Kamigaso, Ohta, Hatakeyama, Hayashi, Otagiri and Yuasa33). PCFT can transport folate (as an oxidised form) and also reduced folates and methotrexate very efficiently in the presence of a proton gradient. Loss of the function of human PCFT/HCP1 due to genetic mutation leads to hereditary folate malabsorption, indicating that human PCFT/HCP1 plays a critical role in the absorption of dietary folate(Reference Qiu, Jansen, Sakaris, Min, Chattopadhyay, Tsai, Sandoval, Zhao, Akabas and Goldman31). Results in Xenopus laevis oocytes(Reference Shayeghi, Latunde-Dada and Oakhill30) suggested that PCFT/HCP1 (SLC46A1) is a relatively low affinity transporter of haem.
The present study aimed to investigate haem and folate transport characteristics of PCFT/HCP1 both in vivo in mice and in vitro and in cultured cells to elucidate further the substrate specificity and selectivity of PCFT/HCP1 and the possible interactions of haem and folate at the level of intestinal absorption.
Experimental methods
Reagent and chemicals
Chemicals and biochemicals were of Analar grade and either from BDH-Merck Ltd, (Poole, Dorset, UK) or Sigma Chemical Company Ltd (Poole, Dorset, UK). 59Fe (supplied as ferric chloride) was from PerkinElmer (specific activity 185 GBq/g; PerkinElmer, Beaconsfield, Bucks, UK). [59Fe]haem was prepared as described by Follett et al. (Reference Follett, Suzuki and Lonnerdal34), [55Fe] haem was purchased from RI Consultant LLC (Hudson, NH, USA; 1·79 mg, specific activity 10·36 MBq/mg (0·28 mCi/mg)), [3H]folate was obtained from Moravek Biochemicals (Brea, CA, USA) and HCP1 antibody was made by Sigma Genosys (Sigma-Aldrich, Cambridge, UK).
Animals
Male mice, CD1 strain, aged 6–8 weeks (Charles River, Margate, Kent, UK) were used throughout the study and housed in a light- and temperature-controlled room with ad libitum access to water and a standard pellet diet (RM1 diet; SDS Ltd, Witham, Essex, UK), which contains 0·7 mg folic acid/kg but has no haem content. Animal care and the regulation of scientific procedures met the criteria laid down by the United Kingdom Animals (Scientific Procedures) Act 1986. Chronic hypoxia was induced by placing mice in a hypobaric chamber at 0·5 atmospheres for 72 h(Reference Laftah, Raja, Latunde-Dada, Vergi, McKie, Simpson and Peters35); control mice were left at normal air pressure. Dietary Fe deficiency was induced by feeding CD1 male mice aged 4 weeks with a low-Fe diet (Formula TD 80 396; elemental Fe concentration 3–6 mg/kg; Harlan-Teklad, Madison, WI, USA) for 2 weeks.
Proton-coupled folate transporter/haem carrier protein 1 antibody
The antisera of mouse HCP1 was generated by Sigma Genosys (Poole, Dorset, UK) and the peptide of the C-terminal corresponding to amino acids 444–459 was KVNPHPEFQQFPQSP and was synthesised by N-term conjugation with 4-methoxybenzenesulfonyl and injected into rabbits(Reference Shayeghi, Latunde-Dada and Oakhill30).
Duodenal haem uptake
59Fe-labelled haem uptake and absorption were measured with tied-off duodenal segments in CD1 mice in vivo (Reference Laftah, Raja, Latunde-Dada, Vergi, McKie, Simpson and Peters35, Reference Simpson, Debnam, Laftah, Solanky, Beaumont, Bahram, Schumann and Srai36), in the presence or absence of an inhibiting antibody for PCFT/HCP1 or pre-immune serum. The activity of 59Fe present in the intestinal tissue is referred to as mucosal retention, whilst that in the carcass is referred to as mucosal transfer: the sum of the mucosal retention and mucosal transfer represents the total mucosal uptake(Reference Raja, Bjarnason, Simpson and Peters37).
In vitro uptake using everted duodenal segments
Mice were anaesthetised and the first 10 cm of the duodenum removed and cleared from any connective tissues and mesenteries. The segment was then washed with ice-cold 0·15 m-sodium chloride and then inverted longitudinally so as to expose the mucosal surface. Two tied-off segments were made from each segment by closing the two end parts with thread. After rinsing in 1 ml oxygenated physiological medium, the loops were incubated at 37°C in oxygenated physiological medium containing [3H]folate (1 μm) in the presence or absence of haem arginate (100 μm) with either pre-immune serum as a control or PCFT/HCP1 antisera at a dilution of 1:100 added to the incubation medium. After 10 min incubation, the uptake was terminated by rinsing the tissue in ice-cold Hanks' balanced salt solution (HBSS; Sigma) and then three times in 1 % bovine serum albumin for 1 min each. These rinses are optimal for maximum removal of any adherent folate without loss of intracellular radioactivity. After reblotting and weighing, the tissue was solublised, and radioactivity was assayed in the tissue and in 10 μl samples of the incubating medium using a twin channel β counter (LKB Wallac-1209; Wallac Oy, Turku, Finland). The results were expressed as pmol/mg wet tissue.
Cell culture and small interfering RNA transfections
Caco-2 cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). Cells were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10 % fetal calf serum (Sigma) and with penicillin (100 U/ml), streptomycin (100 μg/ml) and 1 % minimum essential amino acids. Cells were maintained at 37°C in a humidified incubator containing 95 % air–5 % CO2. Caco-2 cells were transiently transfected with PCFT/HCP1 small interfering RNA (siRNA) duplex (catalogue L-018653) or the siCONTROL non-targeting, non-homologous siRNA (catalogue D-001210-01) from Dharmacon, Inc. (Lafayette, CO, USA). Transfected cells were assayed after 72 h for PCFT/HCP1 mRNA expression by quantitative RT-PCR, [55Fe]haem uptake (2 μm) as described by Shayeghi et al. (Reference Shayeghi, Latunde-Dada and Oakhill30) and [3H]folate uptake (40 nmol) as in Qiu et al. (Reference Qiu, Jansen, Sakaris, Min, Chattopadhyay, Tsai, Sandoval, Zhao, Akabas and Goldman31). Briefly, cells were pre-incubated with HBSS (pH 7·0) at 37°C for 20 min before the uptake assay. After this, 0·25 ml [3H]folic acid in HBSS buffer (pH 5·5) was added to the cells and incubated at 37°C for 2 min. Cells were washed with ice-cold HBSS and lysed in 2 % sodium deoxycholate. A 20 μl sample was removed for protein analysis before adding the liquid scintillant and counted in a twin channel β counter (LKB Wallac-1209; Wallac Oy, Turku, Finland) for radioactivity.
Real-time reverse transcriptase polymerase chain reaction
Total RNA was extracted from liver samples and cells with TRIZOL reagent (Invitrogen, Life Technologies, Paisley, UK) according to the manufacturer's instructions as previously described(38). Quantitative RT-PCR was carried out using a two-step ABI Prism 7000HT Sequence Detection System. First-strand synthesis was performed using the ABI cDNA Synthesis Kit using 2 μg total RNA template according to the manufacturer's protocol. In the second step, transcripts of the various genes were amplified with the specific primer sequences (PCFT/HCP1, sense 5′-AGA GCT GGA CAA TGG ATC GGT-3′, antisense 5′-GCT GAT AGC CAT GAC TC-3′; 18S sense 5′-AAC TTT CGA TGG TAG TCG CCG-3′, antisense 5′-CCT TGG ATG TGG TAG CCG TTT-3′) using the ABI SYBR Green supermix protocol(Reference Takeuchi, Bjarnason, Laftah, Latunde-Dada, Simpson and McKie38). The efficacy of the amplification was confirmed by a melting curve analysis and gel electrophoresis to confirm the presence of a single product. Quantitative measurement of each gene was derived from a standard curve constructed from known concentrations of PCR product. The results were calculated by the ΔCt method that expresses the difference in threshold for the target gene relative to that of 18S.
Data analysis
Data were presented as means and standard deviations. The significance of simple two-group comparisons was obtained using Student's t test. Values were considered statistically different when P values were less than 0·05. One-, two- or three-way ANOVA was performed using SPSS (version 15 for Windows; SPSS Inc., Chicago, IL, USA). In studies where comparisons between three groups were necessary, Dunnett's post hoc test was used to identify significant differences with α set at 0·05.
Results
We previously reported that mucosal haem uptake was induced in hypoxic mice(Reference Shayeghi, Latunde-Dada and Oakhill30). The present study shows that there is induction in the mucosal transport of the radioiron from the lumen across the mucosa to the bloodstream (Fig. 1). The efficiency of the transfer (i.e. the percentage of radioiron take up that is transferred to the animal's body) was not affected by hypoxia (normal pressure, 84·4 %; hypoxia, 90·10 %). Addition of folic acid (1:1 molar ratio) had no overall effects on mucosal haem transport but the effect of folate in hypoxic mice was different from the effect in normal mice (Fig. 1), suggesting that folate inhibits uptake in hypoxia. In vivo uptake of 3H-labelled compounds is more difficult to measure accurately than uptake of γ-emitting isotopes such as 59Fe; therefore in vitro methods were used to measure the uptake of [3H]folate into everted segments of duodenum. Preliminary studies showed that [59Fe]haem uptake by everted duodenum segments showed increased uptake following hypoxic exposure of mice and was inhibited by pre-incubation of segments with anti-PCFT/HCP1 antibodies (data not shown). Folic acid uptake (as with haem uptake) was increased in duodenal segments from mice exposed to 3 d hypoxia (Fig. 2). Duodenum segments showed that the uptake of [3H]folate (1 μm) was strikingly reduced when the segment was previously incubated in physiological medium containing anti-PCFT/HCP1 antibodies (Fig. 2). Moreover, the uptake of folic acid was significantly (Table 1) reduced by the addition of haem (100 μm) to the medium (Fig. 2). Similar findings (i.e. significant main effects of hypoxia, anti-PCFT/HCP1 antibodies or 100 μm-haem) were found with 10 μm-[3H]folate (data not shown). When 100 μm-folate was used in the medium, only hypoxia significantly affected folate uptake (data not shown).

Fig. 1 In vivo mucosal uptake of [59Fe]haem (100 μm) in control and hypoxic mice. (□), Mucosal retention; (), mucosal transfer (the amount of Fe exported out of the duodenum); (), total mucosal uptake (mucosal retention + mucosal transfer). Results are means for four independent experiments in each group, with standard deviations represented by vertical bars. Two-way ANOVA revealed no overall significant effect of folate. Hypoxia significantly affected mucosal transfer (P < 0·001) and total mucosal uptake (P < 0·001). There was a significant interaction between the effect of folate and hypoxia for both mucosal transfer (P < 0·05) and total mucosal uptake (P < 0·05). Mucosal retention showed no significant effects for any factor.

Fig. 2 In vitro [3H]folate uptake by everted segments of duodenum from control (normal atmosphere) and 3 d hypoxic mice. Everted segments were incubated in oxygenated physiological medium for 10 min containing either pre-immunised serum or anti-proton-coupled folate transporter/haem carrier protein 1 (PCFT/HCP1) antibodies. Incubation medium contained 1 μm-folate and 100 μm-haem as indicated. Results are means for four mice in each experimental group, with standard deviations represented by vertical bars. PI, pre-immune serum; Ab, PCFT/HCP1 antibody; H, haem.
Table 1 Three-way ANOVA of the data in Fig. 2 summarising the significance of the factors and interactions on the uptake of folate

PCFT/HCP1 Ab, proton-coupled high-affinity folate transporter/intestinal haem carrier protein 1 antibody.
Caco-2 cells transiently transfected with siRNA PCFT/HCP1 duplex oligos resulted in a 69 % reduction in PCFT/HCP1 mRNA when compared with the control siRNA (control 29·2 (sd 10·3); siRNA 9·05 (sd 1·36), P < 0·03, as mean of three experiments respectively). Both haem and folic acid uptake were significantly (P < 0·05) reduced in cells transfected with PCFT/HCP1 siRNA (Fig. 3); however, the magnitude of reduction with folic acid uptake was greater (48 %) than that with haem uptake (22·5 %) (Fig. 3).
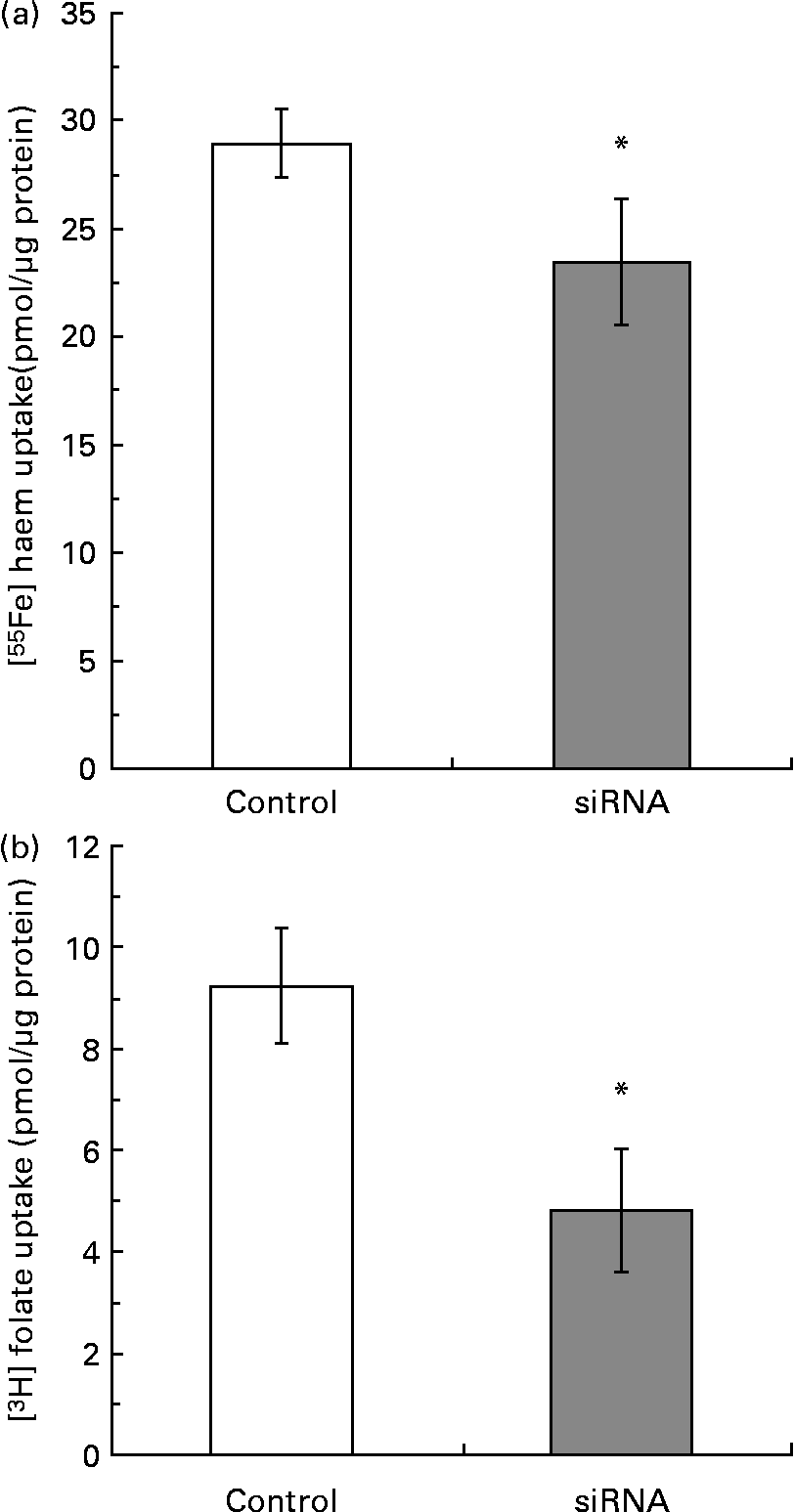
Fig. 3 [55Fe]haem (2 μm) (a) and [3H]folate (40 nm) (b) uptake in Caco-2 cells transfected with proton-coupled folate transporter/haem carrier protein 1 small interfering RNA (siRNA) or a control siRNA (Control). The data are the means of triplicate experiments, with standard deviations represented by vertical bars. * Mean value was significantly different from that for the control cells (P < 0·05).
Discussion
Our goal in the present study was to further investigate the specificity of PCFT/HCP1 toward folate and haem uptake by intestinal epithelial cells. We did so using various mammalian cell systems including confluent monolayers of human intestinal epithelial cells (Caco-2) as well as both in vivo and in vitro mouse duodenal models which our laboratory has previously established as suitable for the study of different aspects of the intestinal uptake of Fe(Reference Laftah, Ramesh, Simpson, Solanky, Bahram, Schumann, Debnam and Srai39).
It is well established that haem is an efficient source of Fe in the mammalian diet, and that duodenal epithelium is the major site of dietary haem Fe absorption in mammals. However, the identity of the putative haem transporters expressed by the proximal duodenal was unknown until recently when Shayeghi et al. (Reference Shayeghi, Latunde-Dada and Oakhill30) proposed HCP1 as a candidate protein to transport haem from the gut lumen into intestinal epithelial cells. The small intestine and in particular the duodenum also represent a major site of entry of essential folate compounds in mammals(Reference Balamurugan and Said40). Dietary folates, however, are usually polyglutamylated and must undergo conjugase digestion to monoglutamate forms before absorption by the enterocytes of adults(Reference Halsted10, Reference Chandler, Wang and Halsted41, Reference Wang, Chandler and Halsted42).
The present results showed the uptake of folate by mouse duodenum is decreased by pre-incubation with an antibody to PCFT/HCP1 and by the addition of a high (though physiologically plausible, based on intake of red meat with a haem content of approximately 0·5 mm(Reference Kalpalathika, Clark and Mahoney43) with a 5-fold dilution during digestion) concentration of haem to the medium. The effects suggest that a significant proportion of folate uptake by the duodenum is mediated via PCFT/HCP1. Under hypoxic conditions, the induction of folate uptake is only partially inhibited by an antibody or haem, suggesting that a distinct folate transporter is also present in hypoxic mice. In previous studies it had been shown that the reduced folate carrier is a carrier system also involved in folate absorption in the gut(Reference Balamurugan and Said40, Reference Subramanian, Chatterjee and Said44). However, the physiological role of the reduced folate carrier in folate absorption remains unclear since in humans it appears unable to compensate for the lack of PCFT/HCP1 at normal levels of dietary folate but may play a role when dietary folate levels are high during supplementation. Indeed, we found that folate uptake at higher concentrations (100 μm) was not inhibited by haem or anti-PCFT/HCP1 antibodies. The transport of folate mediated by PCFT/HCP1 was clearly shown to be pH dependent(Reference Nakai, Inoue, Abe, Hatakeyama, Ohta, Otagiri, Hayashi and Yuasa45, Reference Praetorius, Andreasen, Jensen, Ainsworth, Friis and Johansen46) and was greater at lower extracellular pH due to the greater inward proton gradient across the plasma membrane. In contrast, we found that haem uptake by PCFT/HCP1 was not pH dependent and had a Kmapp for haem of 125 μm(Reference Shayeghi, Latunde-Dada and Oakhill30). We would expect therefore that at lower pH values folate transport would be favoured, while at a pH nearer to 7·4, haem transport may be more favourable by PCFT/HCP1. Our data with mouse duodenum suggest that each substrate can inhibit the uptake of the other to some extent and the uptake of both can be inhibited by an antibody against PCFT/HCP1. The data also suggest that there are at least two uptake mechanisms for each substrate present in duodenum.
The intestinal transport of haem and folate may require other proteins involved in receptor-mediated endocytosis(Reference Rothberg47). In this process, folate or haem would bind to receptors mediated at the cell surface, which would then be internalised into endocytic vesicles. Within the cell, the acidity within endosomal vesicles could result in the dissociation of compounds from the receptor and a strong proton motive driving force that would favour folate transport from the vesicle via the PCFT/HCP1(Reference Paulos, Reddy, Leamon, Turk and Low48).
The results from Caco-2 cells confirmed that PCFT/HCP1 is an avid transporter of folate. We also have found that in Madin–Darby canine kidney (MDCK) cells transfected with a PCFT/HCP1–green fluorescent protein fusion protein, both haem and folate uptake were significantly increased compared with untransfected cells; however, the increase in [3H]folate uptake was quantitatively higher (28-fold increase) compared with haem uptake (1·8-fold increase) (AT Mckie, unpublished results). PCFT/HCP1 may, however, also play a physiological role in Fe nutrition and metabolism as a low-affinity transporter for haem. Another haem transporter, ABCG2, which also transports folate analogues(Reference Krishnamurthy, Ross, Nakanishi, Bailey-Dell, Zhou, Mercer, Sarkadi, Sorrentino and Schuetz49) and other members of the major facilitator superfamily, to which PCFT/HCP1 belongs, are characterised by promiscuity in substrates transported(Reference Burckhardt and Wolff50).
Coordination of intestinal Fe and folate uptake is desirable, as both nutrients are required for erythrocyte and Hb synthesis to combat anaemia; indeed, both nutrients are effective when given in combined supplements. Dietary folate intakes are small (200–400 μg/d)(Reference Ruston, Hoare, Henderson, Gregory, Bates, Prentice, Birch, Swan and Farron51) and are typically derived from vegetables, beans and fortified foods. Haem intakes are very variable, and depend on red meat intake. This suggests that a low-vegetable/bean, high-red meat diet would lead to a high haem:folate ratio in the intestinal lumen. In contrast a low-red meat, high-vegetable/bean diet should lead to a low haem:folate ratio in the intestine. Our data suggest that such diets may lead to competition between haem and folate for dietary absorption which may further exacerbate the risk of deficiency due to the unbalanced intake. This may be more important in the case of high-haem, low-folate-containing diets since other duodenal folate carriers appear unable to compensate for loss of PCFT/HCP1 whereas in a low haem diet, absorption of non-haem Fe could compensate for the loss of haem Fe absorption.
Acknowledgements
The present study was supported in part by grants from the UK BBSRC Agri Food Programme (Biotechnology and Biological Sciences Research Council) and by the Roche Foundation for Anemia Research (RoFAR). A. H. L. performed the animal studies, analysed the data and wrote the first draft of the paper. G. O. L.-D. performed the Caco cell studies, analysed the data and assisted with the final drafting of the paper. S. F. synthesised [59Fe]haem. R. C. H. assisted with the synthesis of [59Fe]haem. R. J. S. designed some studies, analysed the data and assisted with data interpretation and the final drafting of the paper. A. T. M. designed some studies, assisted with data interpretation and the final drafting of the paper and is the grant holder. There are no financial or contractual agreements that might cause conflicts of interest or be perceived to cause conflicts of interest. There is no financial agreement between the author(s) and any company whose product features prominently in the paper. The corresponding author and all of the authors read and approved the final submitted manuscript.






