Epidemiological and clinical studies have suggested health benefits of tomatoes and tomato-based food productsReference Blum, Monir, Wirsansky and Ben-Arzi1, Reference Agarwal and Rao2. Dietary intake of tomato and tomato products has been shown to be associated with a decreased risk of CVDReference Willcox, Catignani and Lazarus3, Reference Rao4 and of certain cancersReference Giovannucci5, including those of the digestive tractReference Franceschi, Bidoli, La Vecchia, Talamini, D'Avanzo and Negri6, prostateReference Giovannucci, Ascherio, Rimm, Stampfer, Colditz and Willett7, Reference Giovannucci, Rimm, Liu, Stampfer and Willett8 and pancreasReference Burney, Comstock and Morris9.
Tomato and tomato-based food products contain a large variety of micronutrients (pro-vitamin A, vitamin C, folate and K) and microconstituents, including polyphenols and non-pro-vitamin A carotenoidsReference Davies and Hobson10–Reference Campbell, Canene-Adams, Lindshield, Boileau, Clinton and Erdman12. Lycopene is the most representative carotenoid in ripe tomatoesReference Curl13 and it is responsible for the deep-red colour of tomatoes and tomato-based foodsReference Shi and Le Maguer14. It represents approximately 80 to 90 % of the pigments present. Most of this carotenoid is consumed from tomatoes and tomato products, such as juice, pasta and sauceReference Shi and Le Maguer14. Other carotenoids are also present in tomatoes, such as phytoene, phytofluene and, in minor amounts, α-carotene, β-carotene, lutein and cryptoxanthinReference Curl13.
In recent years there have been suggestions that lycopene may be responsible for the health benefits of tomato-based food products. In particular, an important study was conductedReference Giovannucci, Ascherio, Rimm, Stampfer, Colditz and Willett7 in which a significant number of men, supplemented with fresh tomatoes, tomato sauce and pizza, were followed from 1986 to 1992. This study clearly showed that the intake of lycopene but not that of other carotenoids, including α-carotene, β-carotene, lutein, and β-cryptoxanthinin, in tomatoes was associated with a lower risk for prostate cancers. Moreover, serum and tissue lycopene levels have been inversely related to the risk of prostate cancersReference Gann, Ma, Giovannucci, Willett, Sacks, Hennekens and Stampfer15.
Although several in vitro studies have been performed to elucidate possible mechanisms underlying the beneficial effects of lycopene on healthReference Heber and Lu16, experimental studies on carotenoid functions in cell-culture models are limited by the absence of an adequate method of solubilising lycopene, which could lead to misinterpretation of the physiological significance of the observed phenomena. In fact, the high hydrophobicity of this carotenoidReference Curl13 makes it very insoluble in aqueous systems and therefore poorly available for cell cultures. In most in vitro studies, lycopene was provided to cultured cells as a tetrahydrofuran solution. Although it has been reported that tetrahydrofuran can easily solubilise lycopeneReference Levy, Bosin, Feldman, Giat, Miinster, Danilenko and Sharoni17, it does not contribute to its stability in solutionReference Xu, Wang, Constantinou, Stacewicz-Sapuntzakis, Bowen and van Breemen18. Moreover, it can determine non-specific uptake of the carotenoid and it can cause problems of toxicity.
Therefore, the main aims of the present study were: (1) to define and utilise a physiological approach consisting of an in vitro tomato digestion, to deliver lycopene-containing tomatoes to colon cultured cells; (2) to analyse antitumoural effects of the aqueous fraction of tomato digestate. HT-29 and HCT-116 cells were used as the model system, since they are colon cancer cells, still exhibiting many morphological and biochemical similarities with intestinal cells. Colon carcinoma cell lines have been reported to accumulate carotenoidsReference Palozza, Serini, Maggiano, Angelini, Boninsegna, Di Nicuolo, Ranelletti and Calviello19.
Materials and methods
Preparation of tomato samples and in vitro simulated digestion
All manipulations with tomato samples were performed under subdued lighting and in amber glass bottles to minimise the destruction of carotenoids. Samples of 60 mg lyophilised ripe tomatoes (Lycopersicon esculentum Mill. cv Red Setter), corresponding to 1 g fresh tomato, were mixed with 1·8 ml saline (140 mm-NaCl, 5 mm-KCl, 150 μm-butylated hydroxytoluene in tetrahydrofuran) and hand-homogenised in a Teflon-glass Potter homogeniser (Thomas, Philadelphia, USA). In vitro simulated digestion was performed according to Garrett et al. Reference Garrett, Failla and Sarama20 with modifications. Briefly, after homogenisation, samples were acidified to pH 2·0 with 1 m-HCl before the addition of 50 μl pepsin, from porcine stomach mucosa (0·2 g pepsin in 5 ml of 0·1 m-HCl), and samples were incubated in a shaking water-bath for 60 min at 37°C. After gastric digestion, the pH was raised to 6·9 with 1 m-NaHCO3. Intestinal digestion was simulated by the addition of 200 μl pancreatin–bile solution from porcine pancreas (0·45 g porcine bile extract and 0·075 g pancreatin in 37·5 ml of 0·1 m-NaHCO3) and incubated in a shaking water-bath at 37°C for 120 min. The pH of the samples was then adjusted to 7·5.
The heat-treated tomatoes were obtained by 15 min incubation in a boiling water-bath before gastric digestion.
Samples were centrifuged at 12 000 rpm for 30 min at 4°C in a Sorvall SS-34 angle rotor (Du Point Instruments, Toronto, Canada), and supernatant fractions collected and stored at − 80°C.
The amount of lycopene in the different tomato preparations was expressed in μg/g fresh weight (60 mg lyophilised tomato correspond to 1 g fresh tomato).
Cell culture
HT-29 human colon adenocarcinoma cells (American Type Culture Collection, Rockville, MD, USA) were grown in modified Eagle's medium. HCT-116 colon carcinoma cells were cultured in McCoy's 5a. Cells were maintained in log phase by seeding them twice per week at the density of 3 × 105 cells/ml at 37°C under 5 % CO2–air atmosphere. The medium was supplemented with 10 % (v/v) fetal calf serum (Flow Laboratories, Irvine, Ayrshire, UK) and 2 mm-glutamine. The medium was not further replaced throughout the experiments. Experiments were routinely carried out on triplicate cultures. After the incubation, cells were harvested and quadruplicate haemocytometer counts were performed. The trypan blue dye exclusion method was used to evaluate the percentage of viable cells.
Cell growth-inhibition assay
Curves of cell growth inhibition were determined for both HT-29 and HCT-116 cells. Serial dilution of tomato digestate with culture medium was used (1:10, 1:20; 1:50). Cells were seeded in twenty-four-well plates with 3 × 104 cells/well and divided into control and treatment groups. Cells were maintained for 24 h before any treatment to facilitate their adhesion on the well. The control group consisted of cells treated with the same amount of digestion mixture, as indicated earlier, diluted with culture medium (1:10, 1:20; 1:50) and of cells without any treatment. Since no differences in terms of viability, cell cycle, caspase activity and cell-cycle-related proteins were found between the two groups, untreated cells are referred to as control cells. For each treatment, cells from four wells were used. At the time indicated (24 h), cells were removed from the wells, stained with trypan blue and counted under a microscope for viable and dead cells.
Cell cycle analysis
Cell cycle distribution was analysed by flow cytometry, as previously describedReference Crissman and Steinkamp21. Samples of 106 cells were harvested by centrifugation, washed in PBS and fixed with ice-cold 70 % ethanol. The cells were incubated at 4°C for 30 min and then centrifuged at 2500 g for 10 min. The pellet was resuspended in 0·5 ml PBS and 0·5 ml DNA-Prep stain (Coulter Reagents, Miami, FL, USA), containing RNAse (1 g/l) and propidium iodide (50 g/l). All samples were incubated for 30 min in the dark at 4°C. The DNA content of cells stained with propidium iodide was measured with a FACS instrument (EPICS XL-MCL Flow Cytometer; Coulter Electronics, FL, USA), by using Multicycle AV software.
Caspase-3 activity assay
The activity of caspase-3 was determined as indicatedReference Palozza, Serini, Torsello, Di Nicuolo, Maggiano, Ranelletti, Wolf and Calviello22. Briefly, after a 24 h treatment, cells (2 × 106) were lysed in 50 mm-tri(hydroxymethyl)-aminomethane-HCl buffer (pH 7·5) containing 0·5 mm-EDTA, 0·5 % IGEPAL® CA-630 (Sigma Aldrich, St. Louis, MO, USA) and 150 mm-NaCl, and cell lysate was incubated with 50 μm-fluorogenic substrate, coumarin (Alexis Biochemicals, San Diego, CA, USA), in a reaction buffer (10 mm-HEPES (pH 7·5) containing 50 mm-NaCl and 2·5 mm-dithiothreitol) for 120 min at 37°C. The release of coumarin was measured with excitation at 380 nm and emission at 460 nm using a fluorescence spectrophotometer.
Western blot analysis of cyclin D1, p53, p21WAF-1/CIP-1, p27, Bax, Bcl-2 and Bcl-xL expression
Cells (10 × 106) were harvested, washed once with ice-cold PBS and gently lysed for 30 min in ice-cold lysis buffer (1 mm-MgCl2, 350 mm-NaCl, 20 mm-HEPES, 0·5 mm-EDTA, 0·1 mm-ethylene glycol-bis(β-aminoethylether)-N, N, N′, N′-tetra-acetic acid, 1 mm-dithiothreitol, 1 mm-Na4P2O7, 1 mm-phenylmethylsulfonyl fluoride, 1 mm-aprotinin, 1·5 mm-leupeptin, 1 mm-Na3VO4, 20 % glycerol and 1 % Nonidet P40). Cell lysates were centrifuged for 10 min at 4°C (10 000 g) to obtain the supernatant fractions, which were used for Western blot analysis. The anti-cyclin D1 (clone 72-13G, catalogue no. SC-450), anti-p21WAF-1/CIP-1 (clone F-5, catalogue no. 6246), anti-p27 (clone N-20, catalogue no. SC-527), anti-Bax (clone P-19, catalogue no. SC-526) and anti-Bcl-xL S/l (clone L-19, catalogue no. SC-1041) monoclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-p53 (clone DO-1, catalogue no. SC-126) and the anti-Bcl-2 (clone Bcl-2/100/D5) monoclonal antibodies were purchased from YLEM (Rome, Italy). The blots were washed with PBS and exposed to horseradish peroxidase-labelled secondary antibodies (Amersham Pharmacia Biotech, Arlington Heights, IL, USA) for 45 min at room temperature. The immunocomplexes were visualised by the enhanced chemiluminescence detection system (ECLTM Western blotting Analysis System; Amersham, Chalfont, Bucks, UK) and quantified by densitometric scanning.
Extraction and analysis of lycopene
Lycopene was extracted from fresh (2 g) and lyophilised (120 mg reconstituted in 2 ml saline solution) tomato digestates and from cells (10 × 106) as describedReference Palozza, Sheriff, Serini, Boninsegna, Maggiano, Ranelletti, Calviello and Cittadini23, Reference Liu, Glahn and Liu24. Cell pellets were resuspendend in 6 m-guanidine hydrochloride in 20 mm-potassium phosphate (pH 2·3) and then extracted twice with hexane (0·5 mg butylated hydroxytoluene/l). Hexane layers were combined and evaporated to dryness under a stream of N2. The residue was redissolved in 200 μl methanol–tetrahydrofuran (90:10, v/v, 0·5 mg butylated hydroxytoluene/l) and 20 μl was injected into an HPLC system. The carotenoid was analysed by HPLC, as describedReference Marletta, Lucarini, Ruggeri and Carnovale25. Chromatographic analyses were performed by the HPLC system provided with a Waters 600 pump, a C18 Inertsil ODS-80 Å reversed-phase column (5 μm, 250 × 4·6 mm; GL Sciences Inc., Torrance, CA, USA) and a photodiode array detector (Waters 996). The mobile phase was constituted by a mixture of CH3CN–CH2Cl2–CH3OH (70/20/10, by vol.).
Statistical analysis
Three separate cultures per treatment were utilised for analysis in each experiment. Values are presented as mean values with their standard errors. One-way ANOVA was adopted to assess any differences among the treatments or the concentrations. When significant values were found (P < 0·05), post hoc comparisons of means were made using Fisher's test. Differences were analysed using Minitab Software (Minitab, Inc., State College, PA, USA).
Results
Total lycopene content in fresh tomatoes, in lyophilised tomatoes and in digestate is shown in Table 1. During the dehydration process, lycopene content remained essentially constant, the amount of this carotenoid in fresh tomatoes being quite similar to that found in dehydrated ones. The amount of lycopene transferred to the aqueous fraction during the digestion procedure was 8·2 (sem 0·6) μg/g fresh weight, which corresponds to roughly one-tenth of the amount of lycopene contained in the fresh tomatoes. Lycopene was not detected in the aqueous fraction when no digestive enzymes and bile extract were added to the homogenate (data not shown). Boiling the lyophilised tomatoes for 15 min before digestion increased the micellarisation of lycopene, the carotenoid content in such preparations being 10·3 (sem 0·9) μg/g fresh weight.
Table 1 Lycopene content in fresh, lyophilised and digested tomatoes (Mean values with their standard errors of three different determinations)

a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05) (Fisher's test).
* 60 mg Lyophilised tomato correspond to 1 g fresh tomato.
As shown in Fig. 1, cells incorporated and/or associated lycopene in a linear manner. A time-dependent increase in lycopene content was observed in HT-29 cells treated with 100 ml digestate/l for 24 h. The cellular carotenoid amount increased in a dose-dependent manner, being 0·10 (sem 0·01), 0·30 (sem 0·03) and 0·60 (sem 0·06) ng/106 cells after the addition of 20, 50 and 100 ml digestate/l, respectively, for 24 h. The digestion mixture itself, for example, proteolytic and lypolitic enzymes without tomato, diluted with culture medium and administered to the cells at the same concentration (20–100 ml/l) was not cytotoxic, as measured by the trypan blue exclusion method and caspase-3 activation.

Fig. 1 Lycopene incorporation and/or association in HT-29 cells treated with tomato digestate (100 ml/l) for 24 h. Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
Tomato digestate inhibited the growth of HT-29 cells in a dose-dependent manner, as measured by direct cell counting (Fig. 2). This inhibition is shown at 24 h of incubation, but it was also found at 12 h (data not shown). Interestingly, the growth-inhibitory effects of tomato digestate were enhanced by boiling the tomatoes for 15 min before digestion. After the addition of tomato digestate at the concentration of 100 ml/l to culture medium for 24 h, cell growth was inhibited by 36 (sem 1·8) % and 51 (sem 2·8) % using raw and heated tomato digestate, respectively.

Fig. 2 Effects of varying tomato digestate concentrations on the growth of HT-29 cells treated for 24 h. (□), Control; (■), tomato digestate (20 ml/l); (![]() ), tomato digestate (50 ml/l); (
), tomato digestate (50 ml/l); (![]() ), tomato digestate (100 ml/l). Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
), tomato digestate (100 ml/l). Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
To elucidate the mechanisms involved in the growth-inhibitory effects of tomatoes, we first examined whether the reduction in cell number by tomato digestate was associated with changes in cell cycle progression. As shown in Table 2, treatment with tomato digestate for 24 h resulted in a significant dose-dependent inhibition of cell cycle progression, manifested by the accumulation of cells in the G0/G1 phase and by a concomitant decrease in the percentage of cells in the S phase and in the G2/M phase. Interestingly, the analysis of DNA histograms revealed the appearance of a pre-G1 peak (subdiploid DNA content), which is characteristic of apoptotic cell death, in HT-29 cells treated with tomato digestate. This value increased in a dose-dependent manner.
Table 2 Effect of lyophilised tomato digestate on cell cycle distribution in HT-29 colon adenocarcinoma cells (Mean values with their standard errors of three different determinations)
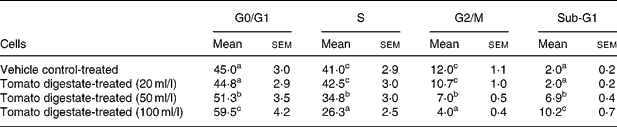
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05) (Fisher's test).
Apoptosis induction by tomato digestate was further studied by analysing the activation of caspase-3, one of the most important cell death executioners for apoptosis (Fig. 3). According to the results obtained by the analysis of the cell cycle, we found that a 24 h treatment with tomato digestate resulted in a strong increase in 7-amido-4-methylcoumarin fluorescence, indicative of the activation of caspase-3 in HT-29 cells.

Fig. 3 Effects of varying tomato digestate concentrations on caspase-3 activation in HT-29 cells treated for 24 h. (□), Control; (■), tomato digestate (20 ml/l); (![]() ), tomato digestate (50 ml/l); (
), tomato digestate (50 ml/l); (![]() ), tomato digestate (100 ml/l). Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
), tomato digestate (100 ml/l). Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
We also investigated possible mechanisms by which tomato digestate would interfere with cell cycle progression and apoptosis. Therefore, we evaluated the cellular content of cyclin D1, which is a regulatory protein during the G0/G1 phase of the cell cycle (Fig. 4 (A)). HT-29 treated with tomato digestate for 24 h showed a strong decrease in cyclin D1 expression compared with control cells. The effect was dose-dependent. This finding was consistent with the blockage of cell cycle progression in the G0/G1 phase. In contrast, the expression of p53 (Fig. 4 (B)), p21 and p27 (Fig. 4 (C)), three other proteins regulating the cell cycle, was not modified by a 24 h tomato digestate treatment.

Fig. 4 Representative Western blot analyses of cyclin D1 (A), p53 (B) and p21 and p27 (C) expression in HT-29 cells treated with varying tomato digestate concentrations for 24 h. C, control; T20, 20 ml tomato digestate/l; T50, 50 ml tomato digestate/l; T100, 100 ml tomato digestate/l. Values are expressed as protein:actin ratios.
To explore the effects of tomato digestate on apoptosis-regulating proteins, we examined the expression of Bcl-2 and Bcl-xL, which suppress programmed cell death, and that of Bax, which promotes it, in HT-29 cells treated for 24 h (Fig. 5). Treatment with tomato digestate significantly reduced the expression of both Bcl-2 and Bcl-xL in a dose-dependent manner. In contrast, no significant changes in the expression of Bax were found in HT-29 cells following a 24 h treatment.

Fig. 5 Representative Western blot analyses of Bax, Bcl-2 and Bcl-xL expression in HT-29 cells treated with varying tomato digestate concentrations for 24 h. C, control; T20, 20 ml tomato digestate/l; T50, 50 ml tomato digestate/l; T100, 100 ml tomato digestate/l. Values are expressed as protein:actin ratios.
Similar effects of tomato digestate on cell growth (Fig. 6 (A)), apoptosis (Fig. 6 (B)) and protein expression (Fig. 6 (C)), including cyclin D1 and Bcl-2, were observed in HCT-116 cells, another human adenocarcinoma cell line (Fig. 6).

Fig. 6 Effects of tomato digestate on the growth (A), caspase-3 activation (B) and cyclin D1 and Bcl-2 expression (C) in HCT-116 cells treated with tomato digestate (100 ml/l; ■) for 24 h. (□), Control cells. Data are mean values of three different experiments, with standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05) (Fisher's test).
Discussion
In the present study, we digested tomatoes in vitro with a procedure that mimics the physiological process in intestinal cells and we have investigated the mechanisms involved in the growth-inhibitory effects of tomato products in colon cancer cells. In particular, we administered ripe tomatoes rich in lycopene to cultured cells after the in vitro digestion treatment. We then characterised lycopene accumulation into the treated cells and studied the antitumoural effects of the aqueous fraction digested, by focusing on some molecular pathways involved in cell proliferation and apoptosis. The present data show that tomato acts as a potent anticarcinogenic agent not only in prostate cancer cells as it has been reported in the literature, but also in colon cancer cells, by inhibiting cell cycle progression through a decrease in cyclin D1 expression and by inducing apoptosis through the modulation of Bcl-2 and Bcl-xL proteins.
The in vitro digestion method has been used effectively for assessing the bioaccessibility and bioavailability of FeReference Etcheverry, Wallingford, Miller and Glahn26, amino acidsReference Cave27, cholesterolReference Fouad, Farrell, Marshall and van de Voort28, vitamin B6Reference Ekanayake and Nelson29 and, recently, carotenoids from baby-food mealsReference Garrett, Failla and Sarama20. It has been reported that the micellarisation of lutein exceeded that of α-carotene, β-carotene and lycopeneReference Garrett, Failla and Sarama20, Reference Reboul, Borel, Mikail, Abou, Charbonnier, Caris-Veyrat, Goupy, Portugal, Lairon and Amiot30. The poor ability of lycopene to form micelles with respect to that of lutein is probably due to the lower hydrophilicity of hydrocarbon carotenoids than oxycarotenoids as well as to their different subcellular locations and molecular interactions in plant foods. The location of carotenoids within the oil droplet which is formed during digestion is affected by its polarity and probably represents an important factor that influences the transfer to mixed micellesReference Borel, Grolier, Armand, Partier, Lafont, Lairon and Azais-Braesco31. Moreover, hydrocarbon carotenoids, such as lycopene, are buried in the core of the oil droplet, whereas oxycarotenoids, such as lutein, reside near the surfaceReference Borel, Grolier, Armand, Partier, Lafont, Lairon and Azais-Braesco31.
Although the micellarisation of lycopene has been reported to be extremely poor in baby-food meals after in vitro digestionReference Garrett, Failla and Sarama20, it should be noted that tomatoes possess a greater amount of lycopene than other foodsReference Heber and Lu16. Our finding that heating the samples allowed the release of higher amounts of lycopene from tomatoes is in agreement with the observation that the availability of lycopene during in vitro digestion is higher in tomato paste and in tomatoes cooked in the presence of oil. The combination of bile salts and pancreatic enzymes is essential for the efficient micellarisation of lycopene from tomatoesReference Garrett, Failla and Sarama20.
The present data are in agreement with epidemiological evidence of a possible protective effect of lycopene against cancerReference Giovannucci, Ascherio, Rimm, Stampfer, Colditz and Willett7, and with several clinical, animal, and tissue and cell-culture studies that have demonstrated through other approaches its anticarcinogenic potentialReference Levy, Bosin, Feldman, Giat, Miinster, Danilenko and Sharoni17, Reference Kim, Rao and Rao32.
The different isomeric structures of lycopene and in general of carotenoids have been suggested to be important factors that influence their bioavailability and absorptionReference Omoni and Aluko33. Since also the composition and the structure of the food matrix have a strong impact on bioavailability of these molecules, in the present study we have chosen to utilise in vitro simulated digestion in order to release from tomatoes the carotenoid pool, focusing in particular on lycopene.
In the present paper, we reported that HT-29 cells were capable of accumulating lycopene from mixed micelles in a dose-dependent manner. Such an observation is not surprising in view of previous reports showing that another human colonic carcinoma cell line, Caco-2, accumulates carotenoids present in the aqueous or micellar fraction after in vitro digestion of the mealReference Garrett, Failla and Sarama20. The present data demonstrate that ripe tomato digestates, very rich in lycopene, may act as potent growth-inhibitory agents in vitro, confirming the observations of antitumoural effects of tomato products in vivo Reference Giovannucci5, Reference La Vecchia34. Growth-inhibitory effects of purified lycopene have been reported in different tumour cell linesReference Bhuvaneswari and Nagini35, including prostateReference Hwang and Bowen36, mammaryReference Ben-Dor, Nahum, Danilenko, Giat, Stahl, Martin, Emmerich, Noy, Levy and Sharoni37, Reference Hirsch, Atzmon, Danilenko, Levy and Sharoni38 and endometrialReference Nahum, Hirsch, Danilenko, Watts, Prall, Levy and Sharoni39 cancer cells and promyelocytic leukaemia cellsReference Amir, Karas, Giat, Danilenko, Levy, Yermiahu, Levy and Sharoni40. However, it is important to underline that tomato digestates obtained by our experimental approach contain a complex mix of compounds besides lycopene, including other carotenoids such as phytoene and phytofluene, carotenoid metabolites and oxidative products, which better mimics the in vivo situation and that can be responsible for the growth-inhibitory effects observed.
The inhibition of HT-29 cell growth by tomato digestate was associated with a slowing of cell cycle progression at the G0/G1 phase. Such an effect seems to involve a down regulation of cyclin D1, which has been implicated in the control of this phase of the cell cycle. It is well known that cyclin D1 is an oncogene and it is over-expressed in several cancer cell linesReference Diehl41. It is interesting to note that lycopene alone has been reported to inhibit tumour cell growth by an arrest in cell cycle progression and a concomitant decrease in cyclin D1 expression. In fact, G0/G1 arrest was observed in lycopene-treated HL-60 cellsReference Amir, Karas, Giat, Danilenko, Levy, Yermiahu, Levy and Sharoni40 and in RAT-1 immortalised fibroblastsReference Palozza, Sheriff, Serini, Boninsegna, Maggiano, Ranelletti, Calviello and Cittadini23. In MCF-7Reference Ben-Dor, Nahum, Danilenko, Giat, Stahl, Martin, Emmerich, Noy, Levy and Sharoni37 and T47D breast cancer cells, as well as in ECC-1 endometrial cancer cellsReference Hirsch, Atzmon, Danilenko, Levy and Sharoni38, lycopene delayed G1-S transition by down regulating cyclin D1 and D3 protein expression, suggesting that the regulation of cell cycle progression by lycopene involves a modulation of cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors. The present data on the modulation of cyclin D1 by tomato digestate confirm such findings and strongly support the hypothesis that lycopene could be directly implicated in the anticarcinogenic effects of tomatoes and tomato products.
Tomato digestate was able to induce apoptosis in colon cancer cells. There are few reports on apoptosis induction by lycopene and tomato products in cancer cells. Some authorsReference Hall42, Reference Kotake-Nara, Kushiro, Zhang, Sugawara, Miyashita and Nagao43 did not find apoptosis induction by lycopene in different human prostate cancer cells even at very high lycopene concentrations. Lycopene was also apparently unable to induce apoptosis in HL-60 cellsReference Amir, Karas, Giat, Danilenko, Levy, Yermiahu, Levy and Sharoni40 as well as in MCF-7 mammary cancer cellsReference Karas, Amir, Fishman, Danilenko, Segal, Nahum, Koifmann, Giat, Levy and Sharoni44. On the other hand, recent studies demonstrate that apoptosis can be detected by using lycopene in LNCaP human prostate cancer cellsReference Hwang and Bowen36, Reference Hantz, Young and Martin45. Oxidative metabolites of lycopene have been reported to act as potent apoptosis inducersReference Nara, Hayashi, Kotake, Miyashita and Nagao46. In addition, tomato sauce increased the percentage of apoptotic cells in prostate carcinoma and, to a lower extent, also in prostatic hyperplasiaReference Bowen, Chen, Stacewicz-Sapuntzakis, Duncan, Sharifi, Ghosh, Kim, Christov Tzelkov and van Breemen47, suggesting that the type of cells, the amount of lycopene administered, as well as tomato processing, may deeply influence the ability of the carotenoid to act as a pro-apoptotic agent.
Moreover, in the present study we have demonstrated that tomato digestate was able to decrease the expression of Bcl-2 and Bcl-xL, two proteins belonging to the Bcl-2 family of proteins, both acting as inhibitors of programmed cell death. This effect was dose-dependent and strictly related to apoptosis induction. In contrast, the pro-apoptotic protein Bax was not affected by the treatment with tomato digestate.
In conclusion, the present data suggest that this in vitro tomato digestion procedure represents a useful and physiological approach to deliver tomato – and therefore lycopene and its related molecules – to cultured cells and to study molecular mechanisms underlying the anticarcinogenic properties of this vegetable. In particular, we demonstrated that tomato digestate is able to inhibit the growth of colon cancer cells by modulating the expression of regulators of the cell cycle and apoptosis.
Acknowledgements
The present study was supported by MIPAF, ‘OGM in Agriculture’, LYCOCARD, European Integrated Project no. 016213 and MIUR. We thank Dr Francesco Cellini (Metapontum Agrobios, Metaponto (MT), Italy) for providing lyophilised tomatoes.










