Earlier, the beneficial effects of antimicrobial growth promoters (AGP) in production animals were attributed to their antibiotic character( Reference Dibner and Richards 1 ). This is unlikely for a variety of reasons, predominantly due to the sub-therapeutic concentrations used, the absence of a relationship between antibiotic activity and antibiotic spectrum and the use as AGP, and the fact that they work similarly in different species with widely divergent microbiota composition. It has therefore been proposed that AGP such as oxytetracycline (OTC) work rather by direct inhibition of the (intestinal) inflammatory response( Reference Niewold 2 ). Another argument is that AGP still appeared to work in the presence of widespread antibiotic resistance( Reference Kalmokoff, Waddington and Thomas 3 ). It also explains why non-antibiotic anti-inflammatory compounds such as acetylsalicylic acid have been described to have a similar effect (e.g. Xu et al. ( Reference Xu, Kornegay and Sweet 4 )). In recent years, several studies have supported the concept of direct inhibition of inflammation by AGP in pigs and poultry( Reference Buret 5 – Reference Lillehoj and Lee 8 ). Consequently, alternatives to AGP should be anti-inflammatory rather than antibiotic. Among the proposed anti-inflammatory alternatives to antibiotics are phytobiotics such as Macleaya cordata (syn. Bocconia cordata Willd.) extract (MCE). It contains isoquinoline alkaloids, such as sanguinarine and chelerythrine, with anti-inflammatory activities( Reference Simanek, Vespalec, Sedo and Schneider 9 ). The aim of the present study was to confirm the anti-inflammatory properties of MCE in an in vitro standard model of inflammation, and correlate it with in vivo growth promotion in broilers using OTC as a positive control. Conditions at experimental farms are often too optimal to detect any effect of growth promoters because of the absence of any challenge. In order to provide a challenge, in the present experiment, no coccidiostats were used in any of the experimental diets.
Materials and methods
Evaluation of the in vitro anti-inflammatory activity of Macleaya cordata extract
MCE was Sangrovit water soluble obtained from Phytobiotics Futterzusatzstoffe GmbH. Sangrovit water soluble has been registered in the European Union as a complementary feed for all animal species, and has entered the registration process in the USA. In the European Union registration trials, Sangrovit water soluble, with inclusion levels up to 1000 mg/l, did not show any increased mortality in broilers (data not shown). The MCE batch used in vitro contained 14 229 mg/kg of sanguinarine and 4400 mg/kg of chelerythrine, as established using the method of Kosina et al. ( Reference Kosina, Walterová and Ulrichová 10 ). The anti-inflammatory effect of MCE was compared with that of ultra-pure oxytetracycline dihydrate (reference no. O4636; Sigma-Aldrich).
The anti-inflammatory activity of MCE and OTC was tested using the RAW 264.7 assay, essentially as described by Wu et al. ( Reference Wu, Chen and Chen 11 ). Briefly, the monocytic murine cell line RAW 264.7 (American Type Culture Collection; ATCC) was grown in cell-culture flasks in Dulbecco's modified Eagle's medium containing 4·5 g/l of glucose, l-glutamine and sodium bicarbonate, without pyruvate, with 10 % fetal bovine serum (Biochrom AG, International Medical) until confluence, and then scraped off, resuspended in the same medium but without phenol red, and seeded into a ninety-six-well plate (100 μl/well) with a cell density of 1 × 106 cells/ml (Nunc-sterile; Sigma-Aldrich). The cells were incubated for 24 h at 37°C. Then, 50 μl/well of serially diluted OTC and sanguinarine-containing extract (MCE) were added and incubated for 4 h at 37°C. Subsequently, 50 μl of medium or 50 μl of medium containing lipopolysaccharide (LPS; 50 ng LPS/ml) were added, giving a final concentration of 12·5 ng LPS/ml, and incubated for 24 h at 37°C. The LPS used was from E. coli 055:B5 (Sigma-Aldrich). Then, 100 μl of medium were taken and pipetted into another ninety-six-well plate (Nunc-sterile; Sigma-Aldrich). NO production was measured with Griess reagent using a serial dilution of NaNO2 as a standard. Samples were run in duplicate. Preparations were titrated in a ninety-six-well plate containing 105 cells/well. The inflammatory response was measured by the production of NO. The half-maximal inhibitory concentration (IC50) was calculated using the sigmoidal dose–response (variable slope) method, using GraphPad Prism 5 for Windows (GraphPad Software, Inc.).
Evaluation of in vivo growth promotion and anti-inflammatory activity
The experimental animal protocol was approved by the local ethics committee for animal experiments at KU Leuven. The experiment is a completely randomised design of treatments with OTC and MCE at three different concentrations. In the in vivo experiment, oxytetracycline hydrochloride (112 reference no. O5875, ≥ 95 % HPLC, crystalline; Sigma-Aldrich) was used for economic reasons. The MCE batch used in vivo contained 11 078 mg/kg of sanguinarine and 4320 mg/kg of chelerythrine as established using the method of Kosina et al. ( Reference Kosina, Walterová and Ulrichová 10 ), meaning that it contained approximately 1·5 % of active compounds. Growth parameters were determined during the experiment, and body composition was determined at the end of the study. The anti-inflammatory effect of the dietary treatments was measured periodically by level of the acute-phase protein α1-acid glycoprotein (α1-AG) in plasma, and the local anti-inflammatory effect was determined by the jejunal expression of IL-1β and IL-10, as well as that of inducible NO synthase (iNOS).
A total of 900 male broiler chickens (Ross 308), aged 1 d old, of similar mean body weight (BW; 46·5 (sem 0·01) g), vaccinated for Newcastle disease, were obtained from a commercial hatchery (Belgabroed, Merksplas, Belgium). The chicks were housed in pens of 0·8 m2 and raised on wood shavings at the Zootechnical Centre of KU Leuven. Temperature was controlled with heaters under the initial condition of 35°C at the chick level, followed by a reduction of 0·5°C each day until 22°C was reached at the 4th week of age. The chicks were exposed to the following lighting schedule: day 1 to day 4, 23·30 h; day 4 to day 35, 18 h. The experiment was carried out in sixty pens with fifteen broilers per pen, and twelve pens per treatment. For each pen, one broiler (sentinel) was randomly chosen on day 1 and marked by colour for blood sampling on days 21 and 35, and for the determination of body composition and jejunal gene expression on day 35. Commercial starter and grower diets were offered to the broilers from 1 to 14 d of age and from 15 to 35 d of age, respectively (Table 1); both diets were without coccidiostats. Feed and water were provided ad libitum throughout the experiment. On day 14, vaccines against Gumboro disease and Newcastle disease were added to the drinking-water. From day 1 onwards, the chicks were fed with the following five treatments: three different concentrations of MCE (25, 50 and 100 mg/l) in drinking-water; OTC (200 mg/kg) in feed; the control. The intake of OTC was calculated based on the consumption of feed. The estimated intake of MCE could not be calculated based on the measured water consumption as the spillage was too high. Therefore, the calculation was based on the assumption that water intake was twice the feed intake (FI)( Reference Leeson and Summers 12 , Reference Lott, Dozier and Simmons 13 ).
Table 1 Composition and calculated contents of the experimental diets

* Premix supplied the following amounts of minerals and vitamins per kg diet: Co (carbonate), 1·02 mg; Cu (copper sulphate), 10·04 mg; Fe (sulphate), 48·57 mg; I (calcium iodate), 1·06 mg; Mn (oxide), 53·35 mg; Se (sodium selenite), 0·30 mg; Zn (oxide), 61·21 mg; Cl, 2·03 g; K, 9·08 g; Na+KCl, 240·79 mEq; Na, 1·82 g; vitamin A, 4050 μg; vitamin D3, 62·5 μg; vitamin E, 50·47 mg; 25-hydroxyvitamin D3, 0·04 mg; butylated hydroxytoluene, 19·99 mg.
Growth parameters and body composition
BW, FI and gain:feed (G:F) ratio were determined on days 14, 21 and 35. FI was corrected for mortality, which was recorded on a daily basis. Weights of the intestine, abdominal fat, liver and the remainder of the body were recorded at the end of the experiment from sixty marked chicks euthanised by decapitation.
Plasma α1-acid glycoprotein measurements
Heparin blood samples were taken from the wing vein of sentinels on days 21 and 35, and plasma was separated by centrifugation at 750 g for 15 min and stored at − 20°C until further use. Plasma levels of α1-AG were investigated using a commercial radial immunodiffusion kit. Chicken α1-AG plates, incorporating specific anti-chicken α1-AG serum of rabbit origin in agarose gel, were used (Saikin Kagaku Institute Company Limited) as described by Takahashi et al. ( Reference Takahashi, Kaji and Akiba 14 ). A standard curve of 0, 125, 250, 500 and 1000 μg/ml of α1-AG was made, 5 μl of plasma or standard were added to a well, and the plates were incubated overnight in a moisture chamber at 25°C. The α1-AG concentration of each test sample was calculated from the diameter of the precipitin ring in the samples and from the standard curve.
Intestinal expression of the inflammatory cytokines IL-1β and IL-10, and inducible nitric oxide synthase
A 10 cm section of the mid-jejunum was taken from the sixty marked chicks at the end of the experiment on day 35. The chymus was removed by washing with ice-cold PBS, and the mucosa was scraped off with a glass slide. Scrapings were snap-frozen in liquid N2 and then kept at − 80°C until analysis. Total RNA was isolated using TRIzol reagent (Invitrogen) and further purified by RNeasy mini-columns (RNeasy Mini Kit; Qiagen Benelux), according to the manufacturer's protocols. The isolated RNA was tested for purity and quantity by measuring the optical density at 260 nm with a spectrophotometer (NanoDrop ND-1000; Isogen Life Science). The RNA was converted to complementary DNA (cDNA) using random primers (Promega Benelux), dNTP mix (VWR) and RT (AMV-RTase; Promega) with buffer and Recombinant RNasin Ribonuclease Inhibitors (Promega) for transcription using a standard protocol( Reference Sambrook and Russell 15 ). The resulting cDNA was stored at − 20°C until quantitative real-time PCR analysis.
Primers (Table 5) were designed with software DNAman 5.0 (Lynnon BioSoft), designed to span exon–exon junctions, and obtained from Invitrogen. For data normalisation, five genes were evaluated as housekeeping genes: ubiquitin (UB); β-actin (ACTB); glyceraldehyde-3-phosphate dehydrogenase (GAPDH); β2-microglobulin (B2M); succinate dehydrogenase complex, subunit A (SDHA). To determine the most appropriate housekeeping genes, a relative standard curve was used as the quantitative RT-PCR method to measure the relative mRNA abundance of potential reference genes( Reference Larionov, Krause and Miller 16 ). Relative mRNA values from these five housekeeping genes were analysed with specific software (geNorm, version 3.4). UB and ACTB showed the lowest average M value, and were identified as the two most stable reference genes, as described by Vandesompele et al. ( Reference Vandesompele, De Preter and Pattyn 17 ), and used to normalise mRNA measurements.
Quantitative real-time PCR was performed for iNOS, IL-1β, IL-10, ACTB and UB using an ABI Prism 7700 sequence (Applied Biosystems) detection system. A calibration curve was built with serial dilutions of pooled cDNA to quantify each gene expression( Reference Yuan, Reed and Chen 18 ). Each PCR included 10 ng of template cDNA (sample or pool), primers, SYBR Green PCR Master Mix (Applied Biosystems) and RNase-Free Water (Qiagen) up to 20 μl. Each assay included cDNA samples in triplicate, together with a blank (reactions lacking cDNA) and the calibration curve. The amplification programme included initial denaturation at 95°C for 10 min followed by forty cycles of denaturation at 95°C for 15 s and 60°C for 1 min, and a dissociation curve at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. Melting curves were generated at the end of the amplification to verify the presence of a single product. A standard curve including two reference genes was generated using serial dilutions of a pooled RNA. PCR efficiency of 90–110 % (3·2 < slope>3·8) together with a correlation coefficient of >0·98 were accepted.
Statistical analysis
Statistical analysis was performed with SAS for Windows (version 9·4; SAS Institute, Inc.). The pen was considered the experimental unit for the measurements of BW, FI and G:F ratio. For α1-AG, body composition and gene expression measurements (performed in sentinels), individual chicks were considered the experimental unit. All data were checked for outliers and normality of the residuals. Normality of the sample distribution was assessed with the Kolmogorov–Smirnov test, and data that were not normally distributed were log-transformed. The weight of the intestine, abdominal fat and liver, and gene expression were analysed using the general linear model. Additionally, contrasts were specified for dose–response modelling with linear, quadratic and cubic polynomial contrasts, correcting for unequal spacing for MCE (0, 25, 50 and 100 mg/l), and the fourth contrast was used for comparing OTC with the control. Data subject to transformation for α1-AG, BW, FI and G:F ratio that were recorded at different time points were subjected to the linear mixed model( Reference Gilmour, Cullis and Gogel 19 ), with treatment, time (repeated option) and the treatment × time interaction as fixed effects. The linear mixed model was as follows:
where μ is the overall mean; A i is the effect of treatment; B j is the fixed effect of time; AB ij is the interaction of time per treatment; and e ij is the random residual error. In addition, the dose–response of MCE (0, 25, 50 and 100 mg/l) was modelled with three polynomial contrasts (linear, quadratic and cubic), correcting for unequal spacing, and the fourth contrast was used for comparing OTC with the control at different time points. No cubic relationships were found.
Results
Evaluation of in vitro anti-inflammatory activity
OTC inhibited LPS-induced NO production with an IC50 value of 88 (95 % CI 88, 99) mg/l, while MCE inhibited at an IC50 value of 132 (95 % CI 94, 170) mg/l. The inhibition of NO production was not the consequence of cell death because it was established earlier using the tetrazolium salt method that cell viability was not affected in any of the treatments (data not shown).
Evaluation of in vivo growth promotion and anti-inflammatory activity
No cubic relationships were found to be significant (data not shown). The effects of the five dietary treatments on BW, FI and G:F ratio are summarised in Table 2 . OTC significantly enhanced the BW of broilers at all days (P< 0·01) compared with the control. MCE enhanced BW significantly in a linear manner at 21 and 35 d (P= 0·012, and P= 0·0002, respectively). Concerning the average feed consumption rate over 0–3 and 0–5 weeks, OTC-fed chicks consumed significantly more feed than the control group, whereas MCE had no effect on FI. OTC-fed chicks showed a significantly higher G:F ratio than the control group at all the three periods, whereas for chicks fed with MCE, a quadratic effect (P= 0·035) was observed in the first 2 weeks and a strong linear effect (P= 0·005) over the 5-week period. There were significant interactions (P< 0·01) observed between time and treatment for BW, FI and G:F ratio. Mortality was not significantly (P= 0·08) associated with treatment, but was mostly associated with the typical symptoms of coccidiosis. The intake of OTC and MCE calculated based on the consumption of feed and water, respectively, was over 7 weeks for MCE (25, 50 and 100 mg/l) and OTC (87, 155, 310 and 321 mg/kg BW).
Table 2 Effect of the experimental diets on the performance indices of broilers (Mean values with their standard errors)
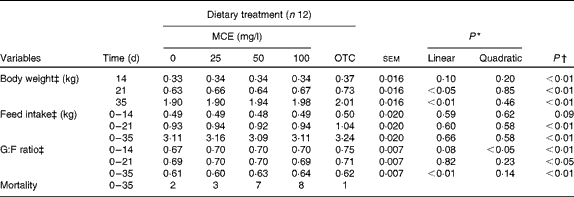
MCE, Macleaya cordata extract; OTC, oxytetracycline; G:F, gain:feed.
* P values were obtained from polynomial tests (only for MCE).
† P values were obtained by comparison of OTC v. 0 mg MCE/l (control).
‡ There was a significant effect for the time × treatment interaction (P< 0·01).
For the determination of other parameters, the sixty marked chicks were used. For BW, the latter chicks were not significantly different from the treatment group (data not shown).
The weights of the liver, intestine and body without the organs were not different by dietary treatments compared with the control. No differences were found in the weight of abdominal fat in chicks fed with OTC, whereas a significant (P= 0·011) linear lower percentage of abdominal fat was observed in chicks fed with increasing concentration of MCE (Table 3). No significant differences in the plasma concentrations of α1-AG were found between the treatments, although the values were numerically lower compared with the control (Table 4).
Table 3 Effect of the experimental diets on the relative organ weights of broilers at 35th day (Mean values with their standard errors)

MCE, Macleaya cordata extract; OTC, oxytetracycline.
* P values were obtained from polynomial tests (only for MCE).
† P values were obtained by comparison of OTC v. 0 mg MCE/l (control).
Table 4 Effect of the experimental diets on the plasma acute-phase protein concentration of broilers (Mean values with their standard errors)

MCE, Macleaya cordata extract; OTC, oxytetracycline.
* P values were obtained from polynomial tests (only for MCE).
† There was no significant effect for the time × treatment interaction (P>0·05).
‡ P values were obtained by comparison of OTC v. 0 mg MCE/l (control).
The expression of the five possible reference genes and three target genes (Table 5) was established using quantitative RT-PCR). The M values found for the five tested reference genes were 1·13 for GAPDH, 0·84 for B2M, 0·76 for SDHA, and 0·72 for both ACTB and UB. The latter two genes were used for normalisation of the expression of target genes as they had the lowest average M (stability) value. The expression of the inflammatory cytokine IL-10 was not significantly affected by the dietary treatments. For IL-1β, a quadratic effect of MCE was observed (P= 0·026). For iNOS, a significantly lower expression was observed in chicks treated with OTC (P= 0·003), and a significant linear decrease in jejunal expression was found in MCE-treated chicks (P= 0·003) (Table 6).
Table 5 Primer information for quantitative RT-PCR assays

F, forward; R, reverse.
Table 6 Effect of the experimental diets on the mRNA expression levels of selected jejunal genes in arbitrary units (Mean values with their standard errors)
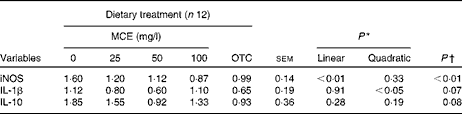
MCE, Macleaya cordata extract; OTC, oxytetracycline; iNOS, inducible NO synthase.
* P values were obtained from polynomial tests (only for MCE).
† P values were obtained by comparison of OTC v. 0 mg MCE/l (control).
Discussion
Inflammation resulting from either feed or disease is inversely related to growth and health( Reference Klasing, Laurin and Peng 20 ). Broilers without any inflammation can reach 100 % of their genetic growth potential. Inflammation causes a reduction in growth (through inappetence and muscle catabolism), the size of which depends on the magnitude of the stimulus. This explains why the anti-inflammatory properties of AGP are much more effective during the greatest challenge. Effective growth promoters must be inhibitors of inflammatory responses, including metabolic inflammation( Reference Niewold 2 , Reference Niewold, Garnsworthy and Wiseman 21 ). Established growth promoters such as tetracyclines have in vivo and in vitro anti-inflammatory properties( Reference Esterly, Koransky and Furey 22 – Reference Kelly, Sutton and Weathered 28 ). In fact, tetracyclines are very effective inhibitors of NO biosynthesis with an IC50 value of 72 mg/l for doxycycline in RAW cells( Reference Amin, Attur and Thakker 24 ), which is very similar to the IC50 value of 88 mg/l found in the present study for OTC. This means that tetracyclines are much more effective than established anti-inflammatory drugs such as aspirin and indomethacin with an IC50 value of 540 and 720 mg/l, respectively( Reference Terra, Valls and Vitrac 29 ). In broilers, 50 mg OTC/kg in feed had an immunosuppressant effect mainly on macrophage function( Reference Al-Ankari and Homeida 30 ). Most recently, it has been shown in a mouse model that chlortetracycline had a down-regulatory effect on intestinal inflammatory cytokines while reducing weight loss( Reference Costa, Uwiera and Kastelic 31 ).
Several in vitro and in vivo studies have identified the anti-inflammatory effects of sanguinarine and MCE( Reference Simanek, Vespalec, Sedo and Schneider 9 , Reference Lenfeld, Kroutil and Marsalek 32 – Reference Chaturvedi, Kumar and Darnay 34 ). These alkaloids exhibit anti-inflammatory effects on carrageenan-induced paw oedema in rats( Reference Lenfeld, Kroutil and Marsalek 32 ). Chaturvedi et al. ( Reference Chaturvedi, Kumar and Darnay 34 ) reported that sanguinarine is an inhibitor of NF-κB activation in human myeloid ML-1 cells. This provides evidence for a mechanism by which sanguinarine exerts its anti-inflammatory effects. The extract used in vitro contained 1·8 % of active compounds, which means that the IC50 value for the latter is about 3 mg/l, which agrees with the IC50 value of pure sanguinarine for human polymorphonucleocytes( Reference Agarwal, Reynolds and Pou 33 ). Here, we show that MCE has in vitro anti-inflammatory activity very similar to OTC.
On the basis of this finding, it was anticipated that MCE would behave similarly in vivo as well. A prerequisite for in vivo activity is that the proximal intestinal uptake should be low, in order to maintain effective concentrations in the distal small intestine. This is, for instance, the case with certain polyphenols that have been proposed as useful additives in inflammatory bowel disease in humans( Reference Biasi, Astegiano and Maina 35 ). In contrast, SCFA such as butyrate are absorbed almost totally in the stomach and proximal small intestine, and the same is true for essential oils( Reference Michiels, Missotten and Van Hoorick 36 ), which may explain the often conflicting results published( Reference Le Gall, Gallois and Sève 37 ). To compensate for absorption, the in-feed dose should be increased or stomach-resistant encapsulation is required, both of which are costly. The limited available data (from rats) show that 58 % of OTC( Reference Agwuh and MacGowan 38 ) and 1–2 % of sanguinarine( Reference Kosina, Walterová and Ulrichová 10 ) are absorbed in the stomach. This still leaves a sizeable proportion of both MCE and OTC to reach the small intestine, and exert anti-inflammatory activity.
Based on in vitro anti-inflammatory properties, it was expected that both OTC and MCE would improve BW, FI and G:F ratio, reduce abdominal fat and reduce inflammatory parameters in the plasma and intestine. In-feed supplementation of 200 mg OTC/kg resulted in significantly enhanced BW, FI and G:F ratio compared with the control (MCE 0 mg/l). This latter finding is in agreement with the report of Dong et al. ( Reference Dong, Gao and Su 39 ) using chlortetracycline, except that the latter found the effect on G:F ratio to be limited to the first 2 weeks. Inflammation depresses growth by decreasing appetite and enhancing muscle catabolism, and in a recent study, it has been estimated that decreased appetite is the largest contribution (70 %) to inflammation-induced growth depression in chickens (Klasing, personal communication). The increment in FI in the OTC group indicates that it is, indeed, also active as an anti-inflammatory agent in vivo, because the increased appetite cannot easily be explained from the antibiotic theory on AGP. Significant effects of MCE were mainly found on performance indices such as BW and G:F ratio. The effects on performance as with the other parameters were mostly significant in a linear fashion, although a modest quadratic effect was found for the G:F ratio in the first 14 d only, and for the jejunal expression of IL-1β, both without a concurrent linear effect. Because the biological significance of this effect is unclear, it is not further discussed. Furthermore, no cubic relationships were found.
Similar to OTC-fed chicks, the chicks fed with MCE showed a significant linear increase in BW compared with the control group on days 21 and 35. In contrast to OTC-treated chicks, the FI of chicks treated with MCE was not different, but there was a strong linear effect on their G:F ratio over the 5-week period. The present results for BW and G:F ratio are similar to the findings reported by Vieira et al. ( Reference Vieira, Oyarzabal and Freitas 40 ) in broilers. Concerning body composition, liver and intestinal weights were not significantly affected by the dietary treatments compared with the control. These results are consistent with those reported by Hernandez et al. ( Reference Hernandez, Madrid and Garcia 41 ) who did not find any differences between the control treatment and diets containing an antibiotic or mixtures of plant extracts in relation to the organ weight of broilers. In the present study, we found a significantly linear effect of MCE on the percentage of abdominal fat compared with the control. The lower percentage of abdominal fat is consistent with the suppression of the inflammatory response in which corticosteroids govern the repartition of fat deposits, and studies( Reference Sapolsky, Romero and Munck 42 , Reference Davison, Rowell and Rea 43 ) have shown that abdominal fat in broilers was increased 6-fold by the two highest (dietary) corticosterone concentrations (20 and 40 mg/kg). Conversely, anti-inflammatory compounds are expected to lower the percentage of abdominal fat, which holds true for MCE but not for OTC. Here, we found that plasma acute-phase protein levels were not significantly reduced compared with the control; however, they were numerically lower in the treated groups v. the control group, which is suggestive of an anti-inflammatory effect. Most of the aforementioned results suggest that both OTC and MCE work through an anti-inflammatory mechanism. Although both are similar, there are also some differences and seeming inconsistencies.
For MCE, the results are consistent with what we expected from an anti-inflammatory compound except for FI, as opposed to OTC. This suggests that MCE lacks the expected effect on appetite that OTC clearly had. The improved G:F ratio in chicks treated with MCE can be interpreted as a consequence of reduced loss of muscle, which is absent in chicks fed with OTC. The latter also does not show an effect on the percentage of abdominal fat, whereas MCE did. The differences between OTC and MCE cannot be easily explained. They may be due to hitherto unknown side effects, or to differences in the cellular level on which they act. One of the problems here is that despite the fact that the anti-inflammatory action of many antibiotics has been clearly established, nothing is known on the exact mechanism. The latter also holds true for the well known (non-antibiotic) anti-inflammatory compound acetylsalicylic acid. Acetylsalicylic acid is an inhibitor of cyclo-oxygenase-1 (COX1) expressed in most cell types, but is not regulated after inflammatory stimulation as opposed to COX2( Reference Botting 44 ). Using the murine macrophage cell line RAW 264.7 and stimulation by LPS, it has been demonstrated that the anti-inflammatory effect of acetylsalicylic acid could not be fully explained by the inhibition of COX1-mediated PG synthesis, but the NF-κB pathway has been found to be involved in this inhibition( Reference Kepka-Lenhart, Chen and Morris 45 ). In rat enterocytes, COX1 is also constitutive, and inhibition of LPS-induced COX2 expression was regulated by the acetylsalicylic acid analogue valerylsalicylic acid( Reference Longo, Damore and Mazuski 46 ). Interestingly, acetylsalicylic acid affects BW and FI, but not the G:F ratio( Reference Xu, Kornegay and Sweet 4 ), similar to OTC, suggesting a shared target. It also suggests that MCE partly operates at a different level. It is known that sanguinarine inhibits the expression of NF-κB, and that COX2 expression is induced by NF-κB, as well as the induction of iNOS, which leads to the production of NO that can be inhibited by both compounds as observed in the in vitro experiment of the present study. Furthermore, sanguinarine has been reported to inhibit the activation of mitogen-activated protein kinase in human peritoneal macrophages, which is mediated by NF-κB, and regulate NO production( Reference Niu, Fan and Li 47 ).
However, there are many steps involved between NF-κB induction and COX2 and NO production, and much is not known about the many signalling pathways involved in these processes( Reference Tsatsanis, Androulidaki and Venihaki 48 ). Furthermore, anti-inflammatory compounds do not always have the expected in vivo effect on inflammatory parameters such as that have been evidenced in human cancer cachexia( Reference Kumar, Kazi and Smith 49 ). It remains also possible that at least part of the mechanisms involved is predominantly at the local level. Hence, the effect of OTC and MCE on the expression level of selected small-intestinal genes was investigated. It was anticipated that OTC and MCE would down-regulate the pro-inflammatory cytokine IL-1β and iNOS, and up-regulate the anti-inflammatory cytokine IL-10. No significant effect of treatment was found on the two cytokines, although the levels of these cytokines were numerically lower than those of the control. Both cytokines are only two of dozens of different cytokines with overlapping functions that are active in a very complex and intricate network in (intestinal) inflammation, making it very hard to draw conclusions from the levels alone( Reference Kumar, Kazi and Smith 49 ). In any case, OTC and MCE did indeed down-regulate the expression of iNOS, showing that feeding both compounds had a definite anti-inflammatory effect on the mucosal level. Furthermore, for all the parameters measured, there was an apparent dose–response to MCE, which had a significant linear effect on BW on days 21 and 35, on the G:F ratio over 35 d, and on the percentage of abdominal fat and jejunal iNOS expression on day 35. This dose–response effect is very important because it makes it very unlikely that the effects observed are due to mere chance, but it rather points to a real biological phenomenon.
Much less is known about poultry gastrointestinal inflammatory responses, compared with mammalian species. However, available studies have demonstrated a general similarity with mammals in the presence and regulation of immunological transcripts and proteins in the small intestine of chickens( Reference van Hemert, Hoekman and Smits 50 – Reference Cox, Sumners and Kim 52 ). For example, dietary β-glucan did modulate the expression of jejunal iNOS in chickens as in mammals( Reference Cox, Sumners and Kim 52 ). On the basis of the latter finding, similar effects of MCE could be expected in other production species such as swine, but no such publications are available yet. In humans, phytogenic anti-inflammatory compounds such as MCE could possibly be helpful in the treatment of conditions such as inflammatory bowel disease.
Concerning growth promotion in production animals, it can be concluded that the current data on MCE and OTC are largely consistent with the anti-inflammatory theory, although the exact pathways and mechanisms involved remain to be elucidated. Therefore, effective alternatives to AGP can be selected successfully based on the latter finding.
Acknowledgements
The present study was financially supported by Phytobiotics Futterzusatzstoffe GmbH, Eltville, Germany.
The authors thank Kris de Backer, Yufeng Wang and Xiaoquan Guo for their help in sampling the broilers at the farm and for their skilled technical assistance.
The authors’ contributions are as follows: A. K. performed the animal experiment, the laboratory experiments, analysed the data, and wrote the first version of the manuscript under the supervision of L. S. and N. E.; T. A. N. formulated the research question, designed the study, and contributed to the writing of the final manuscript. All authors declare no conflict of interest.








