A number of studies over the past 20 years have shown the beneficial effects of low glycaemic index (GI) foods in relation to development of chronic diseases such as type 2 diabetes (T2D)(Reference Jenkins, Kendall and McKeown-Eyssen1) and CVD(Reference Barclay, Petocz and McMillan-Price2). The United Nations FAO/WHO (1998) report recommends that the GI of foods be used in combination with information about food composition to guide food choices for better management and prevention of chronic diseases such as T2D and CVD(3).
India already has the highest number of people with T2D in the world(Reference Unwin, Whiting and Gan4). Controlling postprandial blood sugar is important for the prevention and control of T2D and its related complications(Reference Alberti, Zimmet and Shaw5, 6). There is a large body of evidence to suggest that if a reduction in postprandial glycaemia is to be part of the strategy for prevention and management of diabetes and CVD, the GI (or quality) is as relevant as the quantity of carbohydrate(Reference Jenkins, Kendall and McKeown-Eyssen1, Reference Brand-Miller, Hayne and Petocz7). Foods low in GI may reduce the insulin demand(Reference Rizkalla, Taghrid and Laromiguiere8), improve blood glucose control(Reference Rizkalla, Taghrid and Laromiguiere8), reduce blood lipid concentrations(Reference Kelly, Frost and Whittaker9) and body weight(Reference Ebbeling, Leidig and Sinclair10–Reference Slabber, Barnard and Kuyl12) and thereby could help prevent diabetes-related cardiovascular events(Reference Salmerón, Ascherio and Rimm13–Reference Liu, Willett and Stampfer15).
Carbohydrate foods (cereal-based), particularly rice and wheat (60–65 %), provide the bulk of the energies in the Asian Indian diet(16). Rice consumption is higher in southern(Reference Radhika, VanDam and Sudha17) and eastern parts of the country, while wheat consumption is higher in northern India(Reference Chatterjee, Rae and Ray18). Almost half (46·9 %) of the daily energies among South Indian population is derived from refined grains (mean intake 333 g/d) of which white rice is a major contributor (mean intake 253·4 g/d)(Reference Radhika, VanDam and Sudha17). Understanding the GI of such staples is necessary for the proper selection of foods and may be of particular benefit to Indians, who are more insulin resistant(Reference Mohan, Sharp and Cloke19, Reference Sharp, Mohan and Levy20). It is only quite recently that whole grain-based foods of traditional Indian diets have been reintroduced in the Indian market, but the GI of most products has not been tested. There is hence a need to determine the GI of local food products using standardised methodology(3, Reference Henry, Lightowler and Newens21). In the present study, we compare the GI of rotis (unleavened flatbread) made of whole wheat flour and with a newly developed atta mix containing bengal gram, psyllium husk and debittered fenugreek flour in healthy non-diabetic subjects.
Materials and methods
Subjects
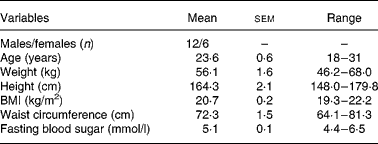
Non-diabetic healthy volunteers aged between 18 and 45 years were recruited from the diabetes centre, which included staff/students or their relatives. Subjects were excluded if BMI was >22·9 kg/m2. According to the WHO Asian Pacific guidelines, overweight in Asians is defined as BMI>22·9 kg/m2 and obesity as ≥ 25 kg/m2(22). Subject characteristics do not appear to have a significant effect on mean GI values, and therefore for routine testing, healthy human volunteers are recommended(Reference Brouns, Bjorck and Frayn23). Subjects were also excluded if fasting blood glucose value>5·6 mmol/l(24), if they were on any special diet, had a family history of diabetes, were suffering form any illness or food allergies or were on any medication. A total of thirty subjects volunteered to participate in the GI testing, of whom five were dropped, one due to change in BMI and four due to sickness. Three subjects were excluded because they had impaired glucose tolerance and an additional four subjects were excluded as they were found to be outliers (individual GI values greater or lesser than 2 sd of the mean GI values). Thus, a total of eighteen subjects (twelve men and six women) were included in the analysis.
Anthropometric measurements including height, weight and waist circumference were taken in the fasting state using standardised techniques as described elsewhere(Reference Henry, Lightowler and Newens21). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were also approved by the institution's Ethical Committee of the Madras Diabetes Research Foundation. Subjects were given full details of the study protocol and the opportunity to ask questions. All subjects gave written informed consent before participation.
Experimental protocol
The protocol used to measure GI was adapted from that described by Wolever et al. (Reference Wolever, Jenkins and Jenkins25) and Brouns et al. (Reference Brouns, Bjorck and Frayn23) and is in line with procedure recommended by the FAO/WHO(3). The procedure has been standardised with an international laboratory that took part in an Inter-laboratory study(Reference Wolever, Brand-Miller and Abernethy26) and has been published elsewhere(Reference Henry, Lightowler and Newens21). In the present study, the number of subjects has been increased to detect the small differences in GI and to ensure greater precision(Reference Brouns, Bjorck and Frayn23).
On the day before the test, subjects were asked not to smoke, consume alcohol, to undertake any vigorous physical activity and to consume their usual meal of similar size and composition, which was verified by a 24 h dietary recall and a brief questionnaire on behavioural status. If there was any deviation, the appointments were rescheduled. Subjects visited the GI testing centre in the morning after a 10–12 h overnight fast, which was standardised to within ± 15 min of the chosen interval on all five occasions.
Test food
The portion size of the test foods (whole wheat flour and atta mix) was calculated using the available carbohydrate estimated as per Association of Official Analytical Chemists (AOAC) method(Reference Englyst, Cummings, Furda and Brine27). Available carbohydrate content of the wheat flour was determined after gelatinising the flour using direct measurement, although there is a possibility that some available carbohydrate may be lost during the cooking process. Branded commercial whole wheat flour (Pillsbury, General Mills India Pvt. Ltd., Mumbai, India) was purchased from the super market, and was used to prepare the whole wheat flour roti. Atta mix is a proprietary (patented), functional food, made of roasted bengal gram flour (legumes), psyllium/ispaghula husk powder (the husk of the seeds of Plantago ovata) and debittered fenugreek (methi) powder. This mix was used in the ratio (4·4:1) of 459 g atta mix added to 2 kg whole wheat flour, as recommend by the manufacturer (Marico Ltd. K.C. Marg, Mumbai, India), and the cooking procedure was standardised by a nutritionist. Roti was prepared from dough that was rolled out to approximately 15 cm in diameter, cooked fairly well on both sides on hot griddle and tossed on direct flame to puff. Description and macronutrient composition and fibre content of the test foods are shown in Tables 1 and 2. Sensory attributes of roti were rated using a 15 cm structured graphical hedonic scale(Reference Holt, Brand Miller and Petocz28).
Table 1 Description of the test foods
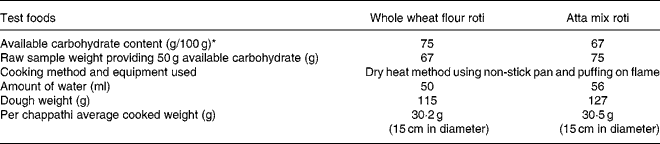
Available carbohydrate value on dry weight basis.
* Glycemic Index Testing Laboratory, Madras Diabetes Research Foundation, India.
Table 2 Macronutrient and dietary fibre content of the test foods*

* Test foods are whole wheat flow (commercial), atta mix.
† Source: from the Food and Nutrition label: ingredient: whole wheat atta, no additives or preservatives used.
‡ Source: from Food and Nutrition label: ingredient: roasted bengal gram flour, psyllium husk powder and debittered fenugreek powder. Whole wheat atta (commercial) and atta mix was mixed in the ratio of 4·4:1.
§ Available carbohydrate by direct method.
Reference food
Fifty-five grams of dextrose (glucose monohydrate) dissolved in 200 ml water were used as the reference food (Glucon-D® glucose powder, Heinz India (P) Ltd., Mumbai, India). The reference food was consumed during the first, middle and last test sessions, while the two types of roti were consumed in random order in between the reference food sessions(Reference Brouns, Bjorck and Frayn23), with at least 3 d gap between measurements to minimise carry-over effects. Subjects were given 200 ml water along with the test food and an extra 200 ml was given during the subsequent 2 h.
Blood glucose measurement
Fasting blood samples were obtained by collecting finger-prick capillary blood samples, at − 5 min and 0 min. The baseline value was taken as the mean of these two values. The subjects then consumed the reference/test food immediately after this. The first bite in the mouth is set as time 0 and the first blood sample is taken exactly 15 min afterwards. Further blood samples were obtained at 30, 45, 60, 90 and 120 min after the start of the test meal. Capillary blood sample was used in order to improve sensitivity and to remove the potential for variations in measured GI due to fluctuations in factors such as ambient temperature(3, Reference Wolever, Brand-Miller and Abernethy26, Reference Wolever, Vorster and Bjorck29). Blood glucose was measured using an automatic lancet device (Accu-Chek® Sensor, Roche Diagnostics GmbH, Mannheim, Germany), which was calibrated daily using the control solution and the Hemocue Glucose 201+ analyzer (Hemocue® Ltd, Angelholm, Sweden). The reliability of the glucometer was tested against Hemocue® analyzer among twenty-four volunteers by measuring the incremental area under the curve (IAUC). The CV was 2·5 % and the correlation coefficient of IAUC values was r 0·989, P < 0·001.
Calculation of the glycaemic index
The incremental area under the blood glucose response curves (IAUC) to test and reference foods were calculated geometrically using the trapezoid rule (FAO/WHO)(3), ignoring the area beneath the baseline. For each subject, a GI value for each test food was calculated by expressing each subject's IAUC after the test food as a percentage of the same subject's mean reference IAUC.
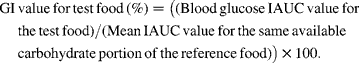
Individual GI values for any subject that were greater or less than 2 sd of the group mean GI were considered to be outliers and were excluded from the analysis.
Statistical analysis
Statistical analyses were performed with SAS software (version 9.1; SAS institute, Cary, NC, USA). Data are shown as means with their standard errors unless otherwise stated. Before statistical analysis, the normality of the data was tested using the Shapiro–Wilks statistics. The significance of difference between test foods was tested by paired t test. Levels of inter- and intra-individual variation of the three reference (glucose) tests were assessed by determining CV %. Using analysis of covariance, the effects of age, sex and BMI on GI were analysed for the two test foods. Statistical significance was set at P < 0·05.
Results
The demographic and clinical characteristics of the study subjects are presented in Table 3. The results of the sensory evaluation of the atta mix roti showed no significant difference in terms of appearance (10 (sem 0·4) v. 11 (sem 0·6)), texture (10 (sem 0·5) v. 11 (sem 0·6)), flavour (11 (sem 0·4) v. 10 (sem 0·4)), taste (10 (sem 0·5) v. 11 (sem 0·6)) and overall acceptability (11 (sem 0·4) v. 10 (sem 0·5)) in comparison with whole wheat flour roti.
Table 3 Demographic and clinical characteristics of the subjects studied (n 18)
(Mean values with their standard errors and ranges)
The mean fasting blood glucose was similar before each test meal, 4·95 (sem 0·08) mmol/l for whole wheat flour roti, 5·05 (sem 0·07) mmol/l for atta mix roti and 4·97 (sem 0·06) mmol/l (P = 0·605) for the glucose. The blood glucose response to atta mix roti was significantly lower at 30 (P = 0·025), 45 (P = 0·005), 60 (P = 0·009) and 90 min (P = 0·016) compared to the whole wheat flour roti, and the mean blood glucose response curve of the two test foods are shown in Fig. 1.
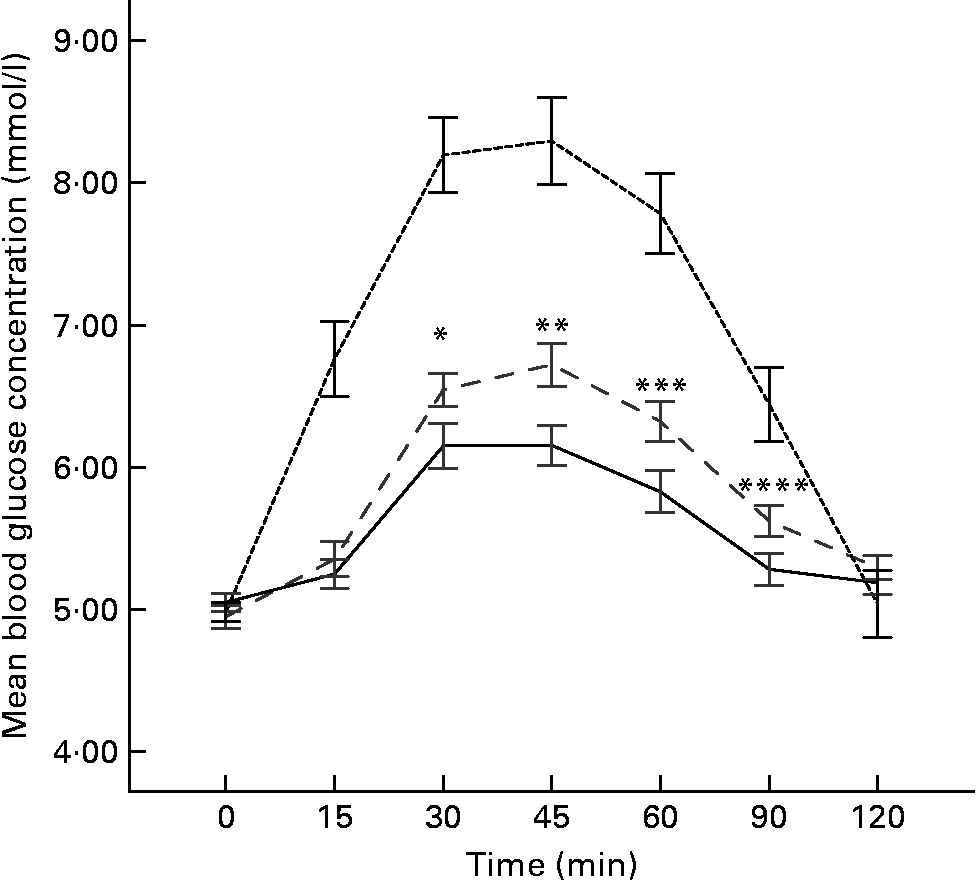
Fig. 1 Graphical representation showing mean blood glucose concentrations between reference (glucose), whole wheat flour roti and atta mix roti (n 18). Mean values were significantly different between atta mix roti and whole wheat flour roti: * P = 0·025; ** P = 0·005; *** P = 0·009; **** P = 0·016. - -, Glucose; – –, whole wheat flour roti; —, atta mix roti.
Table 4 shows the mean IAUC and GI of the test foods. The mean IAUC of atta mix roti (66·2 (sem 6·94) mmol/l) was significantly lower than the whole wheat flour roti (109·6 (sem 10·6) mmol/l, P < 0·001). The mean GI of atta mix roti showed significantly lower (27·3 (sem 2·2)) value than the whole wheat flour roti (45·1 (sem 3·5), P < 0·001), which represents a 39·5 % decreases in GI value. The mean intra-individual CV glucose tested thrice was 23·2 (sem 2·4) %. The individual values for reference CV were negatively related to the mean IAUC values (r 2 0·247, P = 0·036), but were not related to sex, age, BMI or effect of test foods.
Table 4 Incremental area under the curve (IAUC) and glycaemic index (GI) of the test foods (n 18)
(Mean values with their standard errors)

* P value ( < 0·001) refers to differences in IAUC and GI values between atta mix roti and whole wheat flour roti.
Discussion
The study reports on GI of Indian rotis prepared using whole wheat flour and with atta mix. The present study results demonstrate that both the whole wheat flour and atta mix rotis show lower GI. However, the atta mix rotis had 39·5 % lower GI units as compared to whole wheat flour roti. This would be of great relevance in the context of southeast Asia, which is currently the epicentre of the diabetes epidemic(Reference Unwin, Whiting and Gan4, Reference Mohan, Deepa and Deepa30, Reference Ramachandran, Mary and Yamuna31) and where the diets that usually consist of high carbohydrate-based foods (cereal staples) leading to high glycaemic load (GL)(Reference Radhika, VanDam and Sudha17). We have recently shown that GL is an independent risk factor for T2D(Reference Mohan, Radhika and Sathya32) and low HDL concentration, a component of metabolic syndrome(Reference Radhika, Ganesan and Sathya33). Use of atta mix rotis could help to reduce the dietary GL of the diet consumed in this part of the world.
The present results cannot be directly compared with the previously published values(Reference Chaturvedi, Sarojini and Nirmala34, Reference Urooj and Puttaraj35) as studies on wheat chappathi were done on mixed meals rather than on a single food. However, a study conducted by Chaturvedi et al. (Reference Chaturvedi, Sarojini and Nirmala34) showed medium GI (66 %) for a chappathi served with bottle gourd and tomato curry. A study by Urooj & Puttaraj(Reference Urooj and Puttaraj35) showed two extreme GI ranges (44–81 %) for chappathi, wheat flour, thin, with green gram dhal. These differences could be due to wheat with varied gluten content, the method of processing and preparation such as type of fat, dry roasting (amount of resistant starch formed during dry toasting)(Reference Thorne, Thompson and Jenkins36), presence of antinutrients and addition of salt(Reference Brand, Nicholson and Thorburn37), all which have been shown to influence the GI of roti. However, none of the earlier studies have evaluated different types of rotis, with respect to their GI.
The whole wheat flour roti tested in the present study also showed low GI, as it contains the whole grain components (wheat bran and germ) that are well known for its lower glycaemic response(Reference Holm, Hadander and Bjorck38). Studies have also found that the α-amylase inhibitors present in wheat, which can withstand cooking temperature, are effective in reducing blood glucose response(Reference Urooj and Puttaraj39). All these factors perhaps lowered the GI of whole wheat flour chappathi.
The therapeutic effects of bengal gram (legume)(Reference Dilawari, Kamath and Batta40), psyllium/ispaghula husk(Reference Ziai, Larijani and Akhoondzadeh41, Reference Sierra, García and Fernández42) and debittered fenugreek powder(Reference Madar, Abel and Samish43–Reference Sharma and Raghuram45) on lowering postprandial glucose levels are probably due to high viscous soluble fibre, the galactomannans (polysaccharides) that are not hydrolysed by the digestive enzymes. In contrast to insoluble fibre, soluble fibre results in high viscous intestinal contents with gelling properties that could delay gastric emptying and also intestinal absorption(Reference Jenkins, Jenkins and Wolever46). The soluble fibre content of the atta mix was not provided in the nutrition label and to measure the same is beyond the scope of the present study. However, published nutritive value of Indian foods(Reference Gopalan, Sastri and Balasubramanian47) showed appreciable amount of soluble fibre content in bengal gram dhal (2·6 g/100 g edible portion) and in fenugreek seeds (20·0 g/100 g edible portion). When galactomannans from bengal gram, psyllium and fenugreek seeds are well mixed with the carbohydrate portion of the food, it may change the physical availability of carbohydrate to hydrolytic enzymes thus converting the carbohydrates to a slow release form, thereby lowering the plasma glucose levels(Reference Dilawari, Kamath and Batta40, Reference Hallfrish and Behall48, Reference Brennan, Blake and Ellis49). In the present study, we note that the addition of atta mix to wheat flour neither changed the sensory characteristics nor the intrinsic food matrix of the whole wheat flour and yet showed significant drop in the GI units by 39·5 %. The outliers were removed for the calculations of IAUC and this could have ‘artificially’ lowered the coefficients of variation for AUC and GI values. However, this did not misclassify the GI values.
In the present study, age, sex, BMI or individual GI values were not related to intra-subject variation as measured by the CV of the reference food. This is consistent with a previously published study from our centre(Reference Henry, Lightowler and Newens21) and other reported values(Reference Wolever, Brand-Miller and Abernethy26, Reference Wolever, Vorster and Bjorck29), which suggests that GI is a property of food and not of the subject in whom it is measured.
Our previously published works(Reference Mohan, Radhika and Sathya32, Reference Radhika, Ganesan and Sathya33) and studies from the West(Reference Salmerón, Ascherio and Rimm13, Reference Salmeron, Manson and Stampfer14) had shown that the diets with a high glycaemic load and decreased intake of whole grains and fibre were positively associated with the risk of T2D. In the present study, GL of whole wheat flour roti (22·6/test feed serving) was higher than the atta mix roti (GL: 13·7/test feed serving). Thus, this could potentially benefit South Asians, as rotis consumed as the staple food, especially in the northern states of India. However, prospective studies using low GL diets are needed to see whether diabetes can be prevented using this approach.
The present study is the first of its kind to test the GI of whole wheat flour and atta mix using validated standardised GI protocol(Reference Henry, Lightowler and Newens21). Our subjects also showed low intra-individual (within-subject) variability for repeated tests of the reference food (23 %), which is substantially lower than that recorded by others(Reference Vega-Lopez, Ausman and Griffith50). Future studies should consider the measurement of insulin in addition to glycaemic response, as some low GI foods produce excessive insulin levels.
In conclusion, both whole wheat roti and atta mix roti show low GI food values. Hence, both could be incorporated into the Indian diets to replace existing high GI food choices such as refined grains. However, selecting the atta mix could further reduce the overall dietary glycaemic load which could be beneficial in a population, which is highly susceptible to T2D and insulin resistance(Reference Mohan and Deepa51).
Acknowledgements
We thank the volunteers participated in the GI testing study. Our special thanks to Ms P. Muthumariyammal and Ms Tamil Selvi for assisting in the manuscript. This is the first publication from our GI testing centre. The present study was supported by the Marico Limited, Mumbai, India, and we wish to thank Mr Anand and Mrs Ankita for their support.
V. M. is the guarantor. V. M., V. S. and G. R. planned and designed the study. G. R. and C. S. conducted the study. G. R. and A. G. contributed to the data analysis. G. R. wrote the first draft of the manuscript. V. M. and V. S. rewrote the subsequent drafts. V. M., V. S., G. R. and C. J. K. H. contributed the interpretation of the data and all contributors participated in the revision and final draft of the manuscript. They approved the final version and will take public responsibility for the content of the present paper. There are no conflicts of interest any with other organisation.







