l-Glutamate (Glu) is a non-essential amino acid with versatile functions in animal physiology and metabolism( Reference Rezaei, Knabe and Tekwe 1 ). Glu has an important role in the amino acid metabolism through its conversion to α-ketoglutarate or other amino acids, including alanine, aspartate, ornithine and proline in intestine( Reference Reeds, Burrin and Stoll 2 , Reference Blachier, Boutry and Bos 3 ). Thus, Glu can serve both locally inside enterocytes and through the production of other amino acids in an interorgan metabolic perspective( Reference Reeds, Burrin and Stoll 2 ). In addition, Glu is a key transamination partner and is required for the synthesis of GSH, which is an important component in the defence against oxidative stress( Reference Johnson, Kaufmann and Luo 4 ). Our previous studies demonstrated that dietary Glu supplementation increased intestinal anti-superoxide anion (ASA), anti-hydroxy radical (AHR), glutathione reductase (GR), catalase (CAT) and total superoxide dismutase (T-SOD) activities, and GSH content in grass carp Ctenopharyngodon idella ( Reference Zhao, Hu and Zhou 5 ), improved antioxidant capacity and regulated antioxidant-related signalling molecule expression of fish enterocytes( Reference Jiang, Shi and Zhou 6 ). Sivakumar et al. ( Reference Sivakumar, Babu and Srinivasulu Shyamaladevi 7 ) reported that Glu alleviated isoproterenol-induced oxidative stress in rats by increasing antioxidant enzyme activities and GSH content from exogenous Glu. These results suggested that Glu could offer protection during intestinal oxidative stress.
Cu is known for its essentiality for living organisms, including fish, which is required for maintaining cellular function and acting as a cofactor for a number of key metabolic enzymes( Reference Puig and Thiele 8 , Reference Tang, Feng and Jiang 9 ). Nevertheless, Cu is one of the most important pollution-causing metals, because it is commonly released into the environment through industrial wastes and used in the form of copper sulphate as algaecide, fungicide, bactericide and herbicide. In fish culture systems, Cu is regularly used in the form of copper sulphate (CuSO4) to control algal blooms and aquatic macrophyte infestations( Reference Jiang, Wu and Kuang 16 ). Recently, aquatic organisms may suffer from exposure to Cu concentrations that might be ten to fifty times higher than the required concentrations( Reference Hall, Scott and Killen 10 ). The danger of Cu is aggravated by their almost indefinite persistence in water because they cannot be destroyed biologically but are only transformed from one state to another( Reference James, Sampath and Jothilakshmi 11 ). Also, high concentrations of waterborne Cu can be toxic, because perturbations in Cu homoeostasis result in oxidative stress and increased free-radical production( Reference Eyckmans, Celis and Horemans 12 – Reference Sandrini, Bianchini and Trindade 14 ). The intestine is very sensitive to a wide range of stressors( Reference Olsen, Sundell and Mayhew 15 ). The recent reports show that the intestine is a major target for waterborne Cu toxicity in both freshwater and seawater fish( Reference Jiang, Wu and Kuang 16 – Reference Al-Bairuty, Shaw and Handy 18 ). Although Cu uptake is via gills, Cu exposure highly elevates fish intestinal Cu load( Reference Ransberry, Morash and Blewett 17 ). In grass carp, accumulation of Cu in intestine is higher than that in gill( Reference Liu, Ni and Chen 19 ). Our previous study demonstrated that Cu exposure could induce oxidative stress in intestine and the enterocytes of juvenile Jian carp Cyprinus carpio var. Jian( Reference Jiang, Wu and Kuang 16 ). However, few studies examined how to efficiently protect the intestine against Cu-induced oxidative damage. Given the increasing release of Cu into the environment and its potentially harmful effects on fish, it is important to expand our knowledge of how to protect fish against Cu toxicity. Some nutrients could prevent Cu-induced oxidative damage and change antioxidant capacity in fish enterocytes( Reference Jiang, Wu and Kuang 16 ).
Piscine antioxidant capacity can be assessed by the content of non-enzymatic compounds (e.g. GSH) and activities of antioxidant enzymes including superoxide dismutase (SOD), CAT and glutathione peroxidase (GPx), glutathione S-transferase (GST) and GR( Reference Martinez-Alvarez, Morales and Sanz 20 ). These antioxidant compounds and enzymes have key roles in eliminating the reactive oxygen species (ROS) in fish( Reference Wu, Jiang and Liu 21 – Reference Chen, Zhou and Feng 23 ). ROS are generated during normal cellular function, but high doses and/or inadequate removal of ROS results in oxidative stress that may cause severe metabolic malfunctions and impair cell health status( Reference Kohen and Nyska 24 ). NF-E2-related nuclear factor 2 (Nrf2) is an important transcription factor that can bind to the antioxidant responsive element (ARE) and induce transcription of antioxidant enzyme genes( Reference Muthusamy, Kannan and Sadhaasivam 25 ). Kelch-like ECH-associated protein 1 (Keap1) was identified as an Nrf2-binding protein, which depresses Nrf2 translocation to the nucleus( Reference Ma 26 ). Our previous report showed that antioxidant enzyme activities were regulated by the Nrf2 and Keap1 signalling molecules in fish( Reference Jiang, Liu and Hu 27 ). Chen et al.( Reference Chen, Zou and Li 28 ) reported that the up-regulation of Nrf2 expression could elevate the antioxidant gene (including SOD, CAT, GPx, GR and GST) expression levels in the mouse liver. However, to date, no study has addressed the effect of Glu on Nrf2 signalling pathway in fish. Our recent study showed that Glu could regulate Nrf2 and Keap1a gene expression, maybe mediating the signal transduction involved in increased gene expressions of antioxidant enzymes in fish enterocytes( Reference Jiang, Shi and Zhou 6 ).
The present study was designed to investigate whether Glu could attenuate Cu-induced cellular oxidative damage, mediating through Nrf2 signalling pathways regulating mRNA expressions of antioxidant enzymes genes and synthesis of GSH in fish intestine.
Methods
In vivo experiments
Animal collection and acclimation conditions
Animal Care advisory Committee of Sichuan Agricultural University specifically approved this study. Yong grass carp were obtained from Tong Wei fisheries and acclimated for 4 weeks. Dissolved O2 was not <6·0 mg/l. Water temperature and pH were 24 (sem 3)°C and 7·5 (sem 0·5), respectively.
Protective effect of glutamate in copper-induced oxidative stress in the intestine
The formulations of the basal and experimental diets (Table 1) were similar as in our previous study( Reference Zhao, Hu and Zhou 5 ). In brief, it contained 280 g of crude protein/kg diet. The basal diet was Glu unsupplemented control (Ctrl). Glu was added to the basal diet to provide 8 g Glu/kg diet, which was the required Glu concentration for optimal growth established by our previous study( Reference Zhao, Hu and Zhou 5 ). Procedures for diet preparation and storage were the same as those described by Shiau & Su( Reference Shiau and Su 29 ). A total of 180 fish with an average initial weight of 247 (sem 7·5) g from the acclimatisation tank were randomly assigned into two groups of three replicates each. The groups were fed either the Ctrl diet or the Glu diet for 56 d. The experimental conditions were the same as in our previous study( Reference Zhao, Hu and Zhou 5 ).
Table 1 Feed formulation and chemical composition of diets

* Vitamin premix (g/kg): retinyl acetate (150g/kg), 0·80 g; cholecalciferol (12·5g/kg), 0·48 g; dl-α-tocopheryl acetate (500 g/kg), 2000 g; menadione (230 g/kg), 0·22 g; thiamine hydrochloride (980 g/kg), 0·12 g; riboflavin (800 g/kg), 0·99 g; pyridoxine hydrochloride (980 g/kg), 0·62 g; cyanocobalamin (10 g/kg), 0·10 g; niacin (990 g/kg), 2·58 g; d-biotin (20 g/kg), 5·00 g; meso-inositol (990 g/kg), 52·33 g; folic acid (960 g/kg), 0·52 g; ascorbyl acetate (930 g/kg), 7·16 g; calcium-d-pantothenate (900 g/kg), 2·78 g. All ingredients were diluted with maize starch to 1 kg.
† Mineral premix (g/kg): FeSO4.H2O, 25·00 g; CuSO4.5H2O, 0·60 g; ZnSO4.H2O, 4·35 g; MnSO4.H2O, 2·04 g; KI, 1·10 g; NaSeO3, 2·50 g; MgSO4.H2O, 230·67 g. All ingredients were diluted with CaCO3 to 1 kg.
At the end of the feeding trial, the fish in each tank were weighed and collected for Cu exposure. Fish with a similar body weight from both the Ctrl and Glu groups were exposed to 0·7 mg Cu/l water for 96 h, which has been proved to induce oxidative stress in grass carp according to our previous study( Reference Wang, Feng and Jiang 30 ). In addition, the Ctrl/Ctrl treatment (fish from the Ctrl) was performed by exposing the fish from the Glu unsupplemented group to Cu-free water. Therefore, there were three different pre-treatment/exposure groups, Ctrl/Ctrl, Ctrl/Cu and Glu/Cu, with three replicates per group and twelve fish per replicate (thirty-six fish for each group) (Fig. 1). During the Cu exposure period in replicates, the experimental conditions were the same as those in the growth trial, but no food was provided and the water was not renewed( Reference Jiang, Wu and Kuang 16 ). At the end of the challenge trial, all of the living fish from each tank were anaesthetised in a benzocaine bath according to Basic et al.( Reference Basic, Schjolden and Krogdahl 31 ). The intestines of fish were quickly removed, frozen in liquid N2 and stored at −80°C for further analysis. Because the fish were fasted for 96 h during Cu exposure, it was not necessary to empty the intestinal lumen( Reference Enamorado, Martins and Flores 32 ).
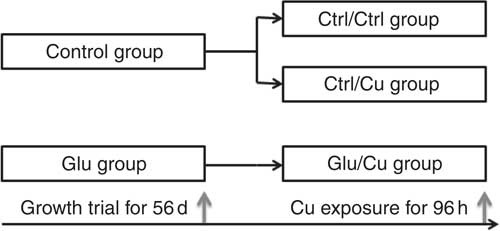
Fig. 1 Overview of experiment design in vivo.
In vitro experiments
Chemicals
Copper sulphate pentahydrate (CuSO4.5H2O), Glu, insulin, collagenase, dispase, d-sorbitol, Triton X-100, transferrin, benzyl penicillin and streptomycin sulphate were purchased from Sigma. Dulbecco’s Modified Eagle’s Medium (DMEM), Hank’s balanced salt solution (HBSS) and fetal bovine serum (FBS) were purchased from Hyclone. Glu-free DMEM was ordered from Beijing Tsing Skywing Bio Tech Co. Ltd. 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was purchased from Promega Corporation.
Primary enterocyte culture
The isolation and culture of primary enterocytes from grass carp C. idella intestine were performed according to the methods of Jiang et al.( Reference Jiang, Zheng and Zhou 22 ) with minor modifications. In brief, healthy grass carp with an average weight of 48·5 (sem 1·2) g were food deprived for 24 h before the experiment and killed by decapitation. The intestines were rapidly separated from the carcass, opened and rinsed with HBSS containing antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Enterocytes were isolated by collagenase and dispase digestion. Next, cells were suspended in DMEM (containing 2 % d-sorbitol, S-DMEM) and washed with S-DMEM five times to remove any undigested material and single cells according to Booth & O’Shea( Reference Booth and O’Shea 33 ) with slight modifications. Isolated enterocytes were seeded in twenty-four-well culture plates (Falcon) at the density of 2×103 cells per well that had been previously coated with collagen I (Sigma), as previously described by us( Reference Jiang 34 ). The cells were cultured in DMEM supplemented with 5 % FBS, 0·02 mg transferrin/ml, 0·01 mg insulin/ml and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 26 (sem 0·5)°C under a Biochemical Incubator (Shanghai Boxun Industry & Commerce Co. Ltd) in an air atmosphere. The cells were allowed to attach to plates for 72 h.
Prevention of copper-induced oxidative stress by glutamate in fish enterocytes
To investigate the effect of Glu on Cu-induced oxidative stress in fish enterocytes, cells were pre-treated with different concentrations of Glu (0–12 mmol/l) for 72 h. Next, cells were exposed to 6 mg Cu/l Glu-free medium for 24 h in a 27°C incubator. The Cu exposure concentration was chosen because previous experiments showed that 6 mg/l Cu of medium could induce oxidative stress in carp enterocytes( Reference Jiang, Wu and Kuang 16 ). Thus, there were eight groups (pre-treatment/Cu exposure): Ctrl/Ctrl, Ctrl/Cu, 2mmol/l Glu/Cu, 4mmol/l Glu/Cu, 6mmol/l Glu/Cu, 8mmol/l Glu/Cu, 10mmol/l Glu/Cu and 12mmol/l Glu/Cu. At the end of the exposure, the MTS assay was performed. Cytotoxicity was determined by measuring lactate dehydrogenase (LDH) activity and malondialdehyde (MDA) in the culture media supernatants. Cell lysates were collected to detect protein carbonyl (PC) and GSH contents, alkaline phosphatase (AKP), ASA, AHR, SOD, CAT, GPx, GST and GR activities, and CAT, GPx, GST, GR, Nrf2 and Keap1a mRNA expression.
Analysis and measurement
Cell viability and differentiation assays
Cell viability in vivo experiments were quantified using the CellTiter 96® AQueous One Solution cell proliferation assay kit (Promega). In brief, at the end of the experiment, 40 µl of MTS working solution was added to each well. After incubation for 2 h, the amount of formazan was determined by measuring the optical density (OD) at 490 nm on a plate reader (Wellscan MK3; Labsystems). AKP activity was assayed according to Krogdahl et al. ( Reference Krogdahl, Bakke Mckellep and Baeverfjord 35 ).
Lactate dehydrogenase, malondialdehyde, protein carbonyl and antioxidant parameter analysis
Cu-induced cytotoxicity was quantified by measuring the amounts of LDH released into the culture medium from injured cell in vitro experiments( Reference Ahn, Hong and Park 36 , Reference Tang, Wu and Wu 37 ). The amount of LDH released was measured using the method of Mulier et al.( Reference Mulier, Rahman and Watchorn 38 ). MDA content was analysed as described by Zhang et al.( Reference Zhang, Zhu and Cai 39 ) using the thiobarbituric acid reaction. The content of PC was determined according to the method described by Armenteros et al.( Reference Armenteros, Heinonen and Ollilainen 40 ) using 2, 4-dinitrophenylhydrazine reagent. The activities of SOD, CAT and GPx were determined by the method described by Chen et al.( Reference Chen, Zou and Li 28 ). GST and GR activities were measured by the method described by Pandey et al.( Reference Pandey, Parvez and Ansari 41 ) with minor modification. GSH contents were determined by using a method described by Chen et al.( Reference Chen, Zhou and Feng 23 ) with a minor modification. The method is based on the formation of yellow colour when dithio nitrobenzoic acid reacts with compounds containing sulfhydryl groups. The amount of GSH was expressed as nmol of GSH per mg protein. ASA (anti-superoxide anion) and AHR (anti-hydroxy radical) activities were assayed according to the method described by Jiang et al.( Reference Jiang, Feng and Liu 42 ).
ASA was determined using the Superoxide Anion Free Radical Detection Kit (Nanjing Jiancheng Bioengineer Institute). Superoxide anion (O2∙ −) were generated by the action of xanthine and xanthine oxidase. When the electron acceptor added, a coloration reaction is developed using the nitro blue tetrazolium. The coloration degree is directly proportional to the quantity of superoxide anion in the reaction. If the sample has anti-superoxide anion activity, the superoxide anion in the reaction will decrease, so the coloration will be weak; if the sample can promote the production of superoxide anion, the coloration will be strong. The superoxide anion can be measured by colorimetry, and then ASA of the homogenate was calculated by the following formula: ASA (U/mg protein) = (ODcontrol−ODsample)/(ODstandard−ODblank)×standard V C concentration (mmol/l)/protein contain (mg). One unit is 1 mg of homogenate scavenged superoxide anion free radical, which equals 1 mg vitamin C scavenging in 40 min at 37°C.
AHR was determined using the Hydroxyl Radical Detection Kit (Nanjing Jiancheng Bioengineer Institute). It was on the basis of Fenton reaction (Fe2++H2O2→Fe3++OH−+∙OH). According to the principle, hydroxyl radicals are generated by Fenton reaction. When the electron acceptor added, a coloration reaction is developed using the nitro blue tetrazolium. The coloration degree is directly proportional to the quantity of hydroxyl radicals in the reaction. If the sample has anti-hydroxy radical activity, the hydroxyl radicals in the reaction will decrease, so the coloration will be weak; if the sample can promote the production of hydroxyl radicals, the coloration will be strong. The hydroxyl radicals can be measured by colorimetry, and then AHR of the homogenate was calculated by the following formula: AHR (U/mg protein)=(ODControl−ODSample)/(ODstandard−ODblank)×standard H2O2 concentration (mmol/l)/protein contain (mg). One unit is 1 mg homogenate decreased 1 mmol/l H2O2 in 1 min at 37°C. The protein contents were measured using the method of Bradford with bovine serum albumin standards( Reference Bradford 43 ).
Real-time quantitative PCR
Total RNA was isolated using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. The RNA purity of each sample was determined by calculating the 260:280 ratio. The RNA integrity was assessed by inspection of the 28S and 18S ribosomal RNA bands in a 1 % agarose gel. Subsequently, the 2 μl total RNA was used to synthesise complementary DNA (cDNA) using the PrimeScript® RT reagent Kit with gDNA Eraser (Takara Biotechnology Co. Ltd). Real-time quantitative PCR analysis of CAT, GPx, GST, GR and house-keeping gene (β-actin) was performed in a CFX96 Real-Time PCR Detection System (Bio-Rad). The gene-specific primers used in this study were listed in Table 2. The PCR mixture consisted of l μl of the first-strand cDNA sample, 0·5 μl each of forward and reverse primers from 10 μm stocks, 3 μl of RNase-free dH2O and 5 μl of 2×Ssofast EvaGreen Supermix (Bio-Rad). Cycling conditions were 98°C for 10 s, followed forty cycles of 98°C for 5 s, annealing at a different temperature (Table 2) for each gene for 10 s and 72°C for 15 s. Target gene mRNA levels were normalised to the mRNA levels of the reference gene β-actin. The amount of the target gene was based on the threshold cycle number (CT), and the CT for each sample was determined using the CFX ManagerTM software. All of the primer amplification efficiencies were approximately 100 %. The gene expression results were analysed using the
![]() $${\rm 2}^{{\minus}} ^{{^{{\Delta \Delta CT}} }} $$
method according to Jiang et al.(
Reference Jiang, Shi and Zhou
44
).
$${\rm 2}^{{\minus}} ^{{^{{\Delta \Delta CT}} }} $$
method according to Jiang et al.(
Reference Jiang, Shi and Zhou
44
).
Table 2 The primers and annealing temperatures used in real-time quantitative PCR

CAT, catalase; GR, glutathione reductase; GPx, glutathione peroxidase; GST, glutathione S-transferase; Nrf2, NF-E2-related nuclear factor 2; Keap1, Kelch-like ECH-associated protein 1.
Statistical analysis
A t test was used for comparisons between two groups in the growth trial. The other data were analysed by one-way ANOVA using SPSS 13.0 (SPSS Inc.). Duncan’s multiple-range test was used to determine significant differences. Data are presented as means with their standard errors. P<0·05 was considered to be statistically significant.
Results
Glutamate prevented copper-induced oxidative damage of the intestine in vivo
Dietary supplementation with Glu significantly increased the growth of grass carp when compared with the Ctrl group – final weight: 614 (sem 12·2) v. 560 (sem 5·6) g (P<0·05). The effects of Glu on MDA, PC, SOD, CAT, GPx, GST, GR, ASA, AHR and GSH contents in intestine of grass carp under Cu exposure are displayed in Table 3. Compared with the Ctrl/Ctrl group, Ctrl/Cu exposure caused a significant increase in MDA and PC content in the intestine (P<0·05). However, dietary Glu pre-supplementation (Glu/Cu) significantly decreased MDA and PC formation (P<0·05). The activity of CAT in the intestine of grass carp was significantly increased by Cu exposure (Ctrl/Cu) (P<0·05), whereas dietary Glu pre-supplementation (Glu/Cu) significantly prevented the increase in CAT activity (P<0·05). In contrast, Ctrl/Cu exposure significantly decreased ASA, AHR, SOD, GPx, GST and GR activities in the intestine (P<0·05). Glu pre-supplementation (Glu/Cu) significantly prevented the adverse effects of Cu on these enzyme activities (P<0·05). The intestinal GSH content was the lowest in the Ctrl/Cu treatment, followed by the Glu/Cu treatment, and the GSH content was the highest in the Ctrl/Ctrl group (P<0·05).
Table 3 Malondialdehyde (MDA), protein carbonyl (PC) and GSH contents (nmol/mg protein), anti-superoxide anion (ASA), anti-hydroxy radical (AHR), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR) activities (U/mg protein) in the intestine of grass carp-fed diets containing different glutamate levels for 56 d, followed by exposure to 0·7 mg/l Cu for 96 h (Mean values with their standard errors, n 6)

a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·05).
Glutamate reduced copper-induced oxidative damage of enterocytes in vitro
The present study has investigated the protective effects of Glu pre-treatment on Cu-induced enterocyte oxidative stress. The Cu exposure (Ctrl/Cu) significantly increased LDH release, MDA content in the medium and PC content in enterocytes as compared with the unexposed Ctrl group (Ctrl/Ctrl) (P<0·05) (Table 4). However, pre-treatment with 6–12 mmol/l before exposure to Cu significantly reduced LDH release, MDA generation and PC formation induced by Cu (P<0·05) (Table 4). Exposure to Cu was shown to cause a significant decrease in MTS OD values and AKP activity compared with that of the Ctrl (P<0·05) (Table 4). As expected, pre-treatment with Glu partially prevented the decrease in cell viability and AKP activity induced by Cu (P<0·05) (Table 4). ASA and AHR activities of enterocytes are shown in Fig. 2. Cu exposure alone significantly decreased ASA and AHR activities in enterocytes. When cells were pre-treated with increasing doses of Glu, before Cu stress, ASA and AHR activities were increased in a dose-dependent manner (P<0·05).
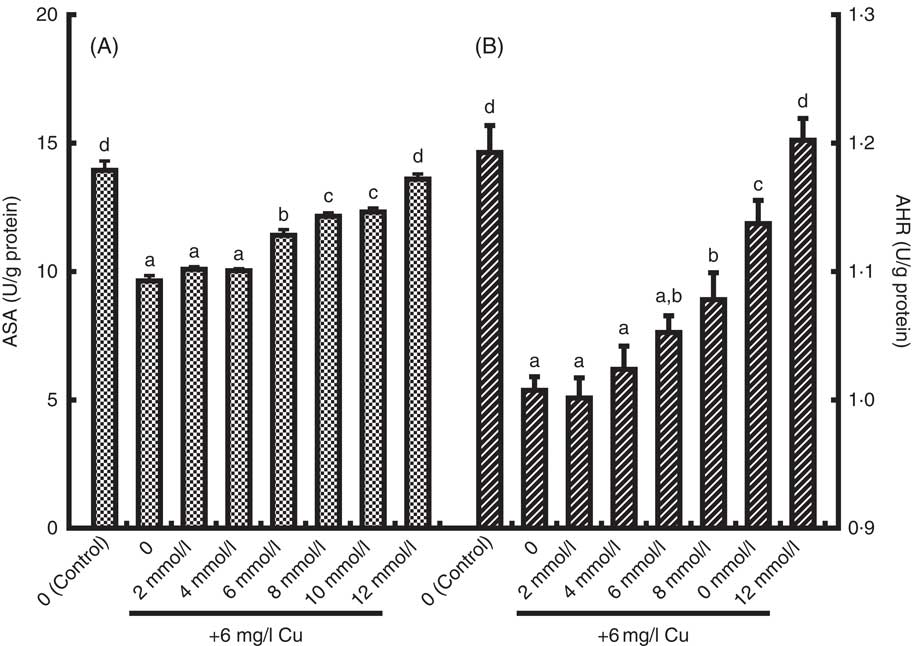
Fig. 2 The anti-superoxide anion (ASA, U/g protein) (A) and anti-hydroxyl radical (AHR, U/g protein), (B) activities in enterocytes cultured with medium containing graded levels of Glu for 72 h, followed by exposure to 6 mg/l Cu for 24 h. Values are means of six replicates, with their standard errors. a,b,c,d Mean values with unlike letters were significantly different (P<0·05).
Table 4 Effect of different concentrations of glutamate on lactate dehydrogenase (LDH) activity and malondialdehyde (MDA) content in media, 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium optical density (MTS OD), alkaline phosphatase (AKP) activities and protein carbonyl (PC) content in copper-exposed grass carp enterocytesFootnote * (Mean values with their standard errors, n 6)

a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* The cells were pre-treated with different concentrations of Glu for 72 h, followed by exposure to 6 mg/l Cu for 24 h.
The effects of Glu on antioxidant parameters in enterocytes under Cu exposure are displayed in Table 5. The T-SOD, GST and GR activities and GSH content were significantly decreased in cells exposed to Cu as compared with the unexposed Ctrl treatment (P<0·05). However, pre-treatment with Glu partially prevented a marked reduction in T-SOD, GST and GR activities and GSH content induced by Cu (P<0·05) (Table 5). In contrast, Cu exposure alone significantly increased the CAT and GPx activities. However, pre-treatment with Glu partially prevented the increase in CAT activity. Interestingly, cells pre-treated with Glu further increased high GPx activity (Table 5).
Table 5 Effect of different concentrations (U/mg protein) of glutamate on total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR) activities and GSH content (nmol/mg protein) in copper-exposed grass carp enterocytesFootnote * (Mean values with their standard errors, n 6)

a,b,c,d,e Mean values within a column with unlike superscript letters were significantly different (P<0·05).
* The cells were pre-treated with different concentrations (0, 2, 4, 6, 8, 10, 12 mmol/l) of Glu for 72 h, followed by exposure to 6 mg/l of Cu for 24 h.
Effects of glutamate on antioxidant-related and NF-E2-related nuclear factor 2 signalling molecule gene expression in the intestine in vivo
As shown in Fig. 3, the Ctrl/Cu treatment increased the relative mRNA expression levels of CAT in the intestine of grass carp compared with the Ctrl/Ctrl group (P<0·05). Dietary Glu treatment (Glu/Cu) significantly prevented the up-regulation of CAT mRNA expression (P<0·05). The Ctrl/Cu treatment caused a significant decrease in GR, GPx and GST mRNA expression in the intestine when compared with the Ctrl/Ctrl (P<0·05). Pre-treatment with Glu prevented down-regulation of the GR, GPx and GST mRNA expression (P<0·05). The effects of Glu on Nrf2 and Keap1a mRNA expression in the intestine of fish following Cu exposure are presented in Fig. 3. Fish exposed to Cu showed a decrease in Nrf2 mRNA expression of intestine as compared with the Ctrl/Ctrl group (P<0·05), and Glu markedly inhibited down-regulation of Cu-induced Nrf2 mRNA expression (P<0·05). Exposure to Cu significantly increased Keap1a transcript abundances in intestine of grass carp compared with the untreated control (P<0·05). Pre-treatment with Glu decreased Keap1a mRNA expression (P<0·05).

Fig. 3 Relative gene expression (shows to be relative to Ctrl/Ctrl (![]() ), which was set at 1·0, in arbitrary units) of catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), NF-E2-related nuclear factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) in the intestine of young grass carp (Ctenopharyngodon idella) fed diets containing different Glu levels for 56 d, followed by exposure to 0·7 mg/l Cu for 96 h. Values are means of six replicates, with their standard errors. a,b,c Mean values with unlike letters were significantly different (P<0·05).
), which was set at 1·0, in arbitrary units) of catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), NF-E2-related nuclear factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) in the intestine of young grass carp (Ctenopharyngodon idella) fed diets containing different Glu levels for 56 d, followed by exposure to 0·7 mg/l Cu for 96 h. Values are means of six replicates, with their standard errors. a,b,c Mean values with unlike letters were significantly different (P<0·05). ![]() , Ctrl/Cu;
, Ctrl/Cu; ![]() , Glu/Cu.
, Glu/Cu.
Effects of glutamate on antioxidant enzyme genes and NF-E2-related nuclear factor 2 signalling molecule in vitro
Relative gene expressions of CAT, GPx, GST, GR, Nfr2 and Keap1a in enterocytes were presented in Fig. 4. Cu exposure alone significantly increased CAT and GPx mRNA expression (P<0·05). Pre-treatment with Glu resulted in a significant increase of levels of GPx mRNA as compared with the Cu stress treatment. However, GST and GR mRNA expression were significantly decreased by Cu stress alone, and Glu markedly inhibited Cu-induced down-regulation of GST and GR mRNA expression (P<0·05). The relative expression level of Nrf2 in enterocytes was significantly down-regulated by Cu stress alone (P<0·05). Pre-treatment with Glu before Cu exposure significantly depressed Cu-induced down-regulation of Nrf2 mRNA (P<0·05). Cu exposure significantly increased the relative mRNA expression levels of Keap1a in enterocytes (P<0·05). Pre-treatment of cells with Glu partially prevented the increase in Cu-induced relative mRNA expression of Keap1a (P<0·05).

Fig. 4 Relative expression (shows to be relative to Ctrl/Ctrl, which was set at 1·0, in arbitrary units) of catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), NF-E2-related nuclear factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) mRNA in enterocytes cultured with medium containing graded levels of Glu for 72 h, followed by exposure to 6 mg/l Cu for 24 h. Values are means of six replicates, with their standard errors. a,b,c,d Mean values with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , 0 mmol/l;
, 0 mmol/l; ![]() , 2 mmol/l;
, 2 mmol/l; ![]() , 4 mmol/l;
, 4 mmol/l; ![]() , 6 mmol/l;
, 6 mmol/l; ![]() , 8 mmol/l;
, 8 mmol/l; ![]() , 10 mmol/l;
, 10 mmol/l; ![]() , 12 mmol/l.
, 12 mmol/l.
Discussion
Cu is an essential nutrient, which has numerous functions in cellular biochemistry (such as a cofactor for many different enzymes)( Reference Lall 45 ). However, Cu at higher concentrations may be toxic. The toxicity of Cu is suggested to be mainly caused by oxidative stress in fish( Reference Bopp, Abicht and Knauer 46 ). It was demonstrated that Cu exposure could induce oxidative stress in zebrafish hepatocytes( Reference Sandrini, Bianchini and Trindade 14 ), rainbow trout gill cell( Reference Bopp, Abicht and Knauer 46 ) and carp enterocytes( Reference Jiang, Wu and Kuang 16 ). Oxidative damage in cultured cells has been assessed by LDH release( Reference Chung, Walker and Brown 47 ). The extent of cell damage was assessed by measuring the release of the cytosolic enzyme LDH from damaged cells to the bathing medium( Reference Iizuka, Sasaki and Hirai 48 ). The present study demonstrated that Cu exposure significantly increased LDH levels in the medium, indicating severe enterocyte damage. The colorimetric assay using MTS can rapidly quantify the cell viability of fish enterocyte( Reference Jiang, Shi and Zhou 44 , Reference Jiang, Shi and Zhou 49 ). AKP serves as an enterocyte differentiation marker and is considered to be involved in the absorption of nutrients( Reference Villanueva, Vanacore and Goicoechea 50 ). In the present study, Cu exposure significantly decreased cell viability and AKP activity. Interestingly, treatment of Cu-exposed enterocytes with some concentrations of Glu depressed LDH release, and elevated cell viability and AKP activity. MDA and PC contents were widely used as biochemical indicators of oxidative damage( Reference Kohen and Nyska 51 ). Exposure of enterocytes to 6 mg/l Cu was shown to cause a significant increase in MDA and PC contents, suggesting that Cu exposure caused lipid and protein oxidative damage in enterocytes, which may be attributed to the increase of ROS production. ROS are produced in response to metals( Reference Valko, Morris and Cronin 52 ). Increasing the levels of ROS can lead to severe cell injury or death( Reference Li, Hu and Zhu 53 ). Superoxide anions can cause a wide range of oxidative damage within the cell( Reference Kohen and Nyska 51 ). The present study showed that MDA and PC contents were decreased in cells treated with Glu in a dose-dependent manner. The result suggested that Glu may exert potent protection of the intestine against Cu-induced oxidative damage. To our knowledge, no study has investigated the protective effects of Glu against metal toxicity. This is the first report demonstrating that Glu could attenuate Cu-induced cellular damage.
To investigate the potential protective effects of Glu against Cu-induced oxidative damage, the antioxidant enzymes such as SOD, CAT, GPx, GST, GR and GSH content were determined. The SOD is the first enzyme to respond against O2 radicals and important endogenous antioxidants for protection against oxidative stress( Reference Winston and Di Giulio 54 ). CAT has been implicated as an essential defence against the potential toxicity of hydroxyl radicals( Reference David, Munaswamy and Halappa 55 ). The GSH-dependent enzymes (GST, GPx and GR) are able to counteract peroxidative damage( Reference Cabrini, Bergami and Fiorentini 56 ). GSH is the major endogenous antioxidant scavenger that protects cells from oxidative stress, and GSH/GSSG represents the major cellular redox buffer and therefore is a representative indicator for the redox environment of the cell( Reference Sies 57 ). In the present study, a significant decrease in SOD and GR activities and GSH content was found in cells exposed to Cu. The result demonstrated that Cu-induced enterocyte oxidative damage may be partly related to disturbance of the antioxidant system. Glu pre-treatment prevented a Cu-induced decrease of these antioxidant enzyme activities. This may suggest that the antioxidative effect of Glu may be attributed to its ability to maintain the activity of radical scavenging enzymes. A variety of citrate cycle intermediates such as fumarate, oxaloacetate, malate and succinate were shown to modulate lipid peroxide production by interacting with endogenous iron ions, thus adjusting the iron redox cycle and, subsequently, free-radical generation( Reference Puntel, Roos and Grotto 58 ). Glu can form α-ketoglutarate by deamination and enter into the citrate cycle to promote the production of citrate cycle intermediates( Reference Blachier, Boutry and Bos 3 ). Hence, the reduction in lipid peroxides with an increase in GSH on Glu pre-treatment could be because of the antioxidant effect of citrate cycle intermediates formed from Glu. Further, GSH was produced from exogenous Glu( Reference Reeds, Burrin and Stoll 2 ). However, Cu exposure significantly elevated CAT and GPx activities. Glu pre-treatment of enterocytes further increased GPx activities. The reason might be attributed to an adaptive mechanism against stress. The enterocytes were directly exposed to Cu; increased CAT and GPx activities were required to inhibit the Cu-induced oxidative stress. The ASA and AHR activity are two indexes used to evaluate the total capacity of scavenging superoxide and hydroxyl radical, respectively( Reference Jiang, Zheng and Zhou 22 ). To investigate how Glu inhibited the Cu-induced oxidative damage in fish, we determined the ASA and AHR activities. Our results showed that Cu exposure significantly decreased the ASA and AHR activities in enterocytes. Glu pre-treatment prevented the Cu-induced decrease of ASA and AHR activities in a dose-dependent manner. These results indicated that the protective effects of Glu on Cu-induced oxidative damage may, at least in part, be because of the increased ASA and AHR activities.
The activities of antioxidant enzymes can be affected by the mRNA levels in fish( Reference Jiang, Liu and Hu 27 ). To further elucidate whether Glu regulated antioxidant enzyme activities at the gene level in fish, the mRNA levels of antioxidant enzyme of enterocytes after being challenged against Cu were investigated. The results of the current study demonstrated that Cu exposure significantly increased mRNA levels of CAT and GPx in enterocytes. Glu pre-treatment blocked the increase in CAT mRNA expression induced by Cu. However, Glu pre-treatment further elevated mRNA expression of GPx gene induced by Cu. The antioxidant enzyme gene expression exhibited a same pattern with their respective enzyme changes. The regulation of antioxidant gene mRNA levels may result from activating the antioxidant-related signalling molecules. Nrf2 is a master regulator of the antioxidant response through regulating the transcription of antioxidant gene in fish, including SOD, CAT, GPx, GR and GST ( Reference Ma 26 ). The present study showed that Cu exposure down-regulated the Nrf2 gene expression in enterocytes. The result is very well in agreement with our previous report, which showed that Cu exposure markedly decreased the binding of nuclear Nrf2 to ARE( Reference Jiang, Liu and Jiang 59 ). In addition, the negative effects of Cu-induced antioxidant gene mRNA expression may be partly ascribed to a decrease in Nrf2 nuclear translocation. Keap1 is identified as an Nrf2-binding protein that prevents Nrf2 translocation to the nucleus and promotes the ubiquitination-proteasomal degradation of Nrf2( Reference Ma 26 ). Our study also showed that Cu induces the up-regulation of Keap1a mRNA level in enterocytes( Reference Jiang, Shi and Zhou 6 ). Glu pre-treatment elevated Nrf2 mRNA expression and blocked the increase in Keap1a mRNA expression( Reference Jiang, Shi and Zhou 6 ). These results suggested that the antioxidant effect of Glu is mediated at least in part by Nrf2 signalling pathways in fish. To date, no information is available about the effect of Glu on Nrf2 gene mRNA expression in fish enterocytes. The underlying mechanism needs further investigation.
On the basis of the beneficial effects of Glu against Cu-induced oxidative damage in the enterocytes, it was reasonable to hypothesise that Glu can protect fish against Cu-induced intestinal oxidative damage in vivo. The present study showed that Cu exposure could induce intestinal oxidative stress in grass carp. Similar results were observed that Cu exposure could induce oxidative stress in juvenile Epinephelus coioides intestine( Reference Wang, Long and Liu 60 ) and pacu Piaractus mesopotamicus liver( Reference Garcia, de Lima and Tie 61 ). The antioxidant enzyme activities of intestine were elevated in response to Glu supplementation. The GR, GPx and GST mRNA abundance in intestine was enhanced by Glu pre-treatment. The positive effects of Glu on antioxidant enzyme mRNA expression may be partly ascribed to promote Nrf2 nuclear translocation by down-regulating Keap1a mRNA expression. In the present study, a significant increase in mRNA levels of Nrf2 was observed in the grass carp intestine with Glu supplementation. In contrast, Glu pre-treatment significantly decreased Keap1a mRNA expression. These results were in agreement with the present study statements in vitro. Studies from rats also indicated that Glu supplementation alleviates oxidative damage induced by isoproterenol( Reference Sivakumar, Babu and Srinivasulu Shyamaladevi 7 ). However, it is of interest that the in vivo GPx activity in intestine was decreased by Cu exposure, which was the reverse of the pattern observed in enterocytes. The reason for these results is unclear. The differences may be because, in this study, the enterocytes were directly exposed to Cu, whereas the intestine is indirectly exposed to Cu. In other words, because the enterocytes were directly exposed to Cu, increased GPx activity was required to inhibit the Cu-induced oxidative stress. The previous study in grass carp showed that dietary Cu content (5·25 mg/kg), beyond requirement (4·78mg/kg), induced intestinal oxidative stress, but increased GPx activity( Reference Tang, Feng and Jiang 9 ). In addition, higher doses of Cu (6·70–8·33 mg/kg) induced intestinal oxidative stress and decreased the GPx activity at the same time( Reference Tang, Feng and Jiang 9 ). Therefore, the enterocytes directly exposed to a certain amount of Cu by culture in vitro and oral administration in vivo can both induce oxidative stress, as well as increase GPx activity in fish intestine.
In conclusion, Cu exposure could induce oxidative damage, resulting in lipid peroxidation, protein oxidation and antioxidant enzymes activity alterations in intestine and the enterocytes of grass carp. Cu exposure down-regulated the mRNA abundance of GR, GPx and GST in the intestine of grass carp. Dietary and medium pre-supplementation with Glu could alleviate Cu-induced oxidative damage in fish intestine and the enterocytes, respectively. The protective effects of Glu on Cu-induced oxidative damage are associated with up-regulating the expression of antioxidant enzymes gene by regulating mRNA abundance of signalling molecule Nrf2. It provides a theoretical basis for reducing oxidative stress of Cu in cultured fish by means of nutrition.
Acknowledgements
The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
This study was financially supported by the Youth Foundation Program of the Education Department of Sichuan Province, China (grant no. 14ZB0021) and the Applied Basic Research Programs of Science and Technology Commission Foundation of Sichuan Province, China (grant no. 2015JY0067).
J. J. and X.-Y. W. conducted the trial, performed the RT-PCR experiments and wrote the manuscript. Y. Z. and X.-Q. Z. contributed to the design of the study. L. F. and W.-D. J. assisted in the manuscript preparation. Y. L. assisted with all data analysis. P. W. assisted with the trail.
The authors declare that there are no conflicts of interest.












