The dramatic growth in the incidence of diabetes in recent years has led to increased efforts to find natural and safe strategies to control complications associated with the condition( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 ). An abnormal metabolic profile, including impaired fasting glucose, insulin and glycaemic control, is a strong predictor of diabetes. Recently, it has been found that patients( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 – Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 ) with type 2 diabetes show an alteration in their gut microbial composition( Reference Chin 4 ). This suggests that probiotics may provide a new and promising way of regulating glucose and glycaemic factors through modifying gut microflora.
Probiotics have been defined by the WHO as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’( Reference Gilliland, Morelli and Reid 5 ). It has been demonstrated that probiotics can regulate gut microflora, which has health benefits in terms of improving gut health( Reference Fooks and Gibson 6 , Reference Kaushik, Kumar and Duary 7 ) and regulating plasma lipids( Reference Guo and Zhang 8 ). Furthermore, probiotics may have a role in preventing CVD and other chronic diseases through increasing enzymatic antioxidant activity, decreasing lipid components, BMI and blood pressure( Reference Khalesi, Sun and Buys 9 , Reference Sun and Buys 10 ). The multiple effects of probiotics raise questions as to whether they could aid the treatment of people with diabetes and associated risk factors.
Previous studies have assessed the effect of probiotics on metabolic profiles in people with hyperlipidaemia( Reference Asemi, Zare and Shakeri 11 ) and healthy adults( Reference Chang, Park and Jang 12 , Reference Jones, Martoni and Di Pietro 13 ), and a systematic review showed a moderate effect on glycaemic control in trials with a mixture of healthy and at-risk populations( Reference Ruan, Sun and He 14 ). However, the impact of probiotics on the glycaemic control of diabetes or associated risk factors is uncertain. For example, there is conflicting evidence about the effects of probiotics on lowering glucose and glycaemic components( Reference Jones, Martoni and Di Pietro 13 ), with some studies finding them effective( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 – Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 ) while others not( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Chang, Park and Jang 12 , Reference Ivey, Hodgson and Kerr 15 ). It is possible that multiple factors may confound the beneficial effects of probiotics, such as diabetic condition, single v. multiple species of probiotics, dosage use of probiotics, duration of probiotic consumption and probiotics in capsule or milk form. These factors need to be systematically examined to fully determine the effects of probiotics on lowering glucose and glycaemic factors in diabetes and its risk factors. The objective of the present meta-analysis was to synthesise results from randomised placebo-controlled studies on the effects of probiotics consumption in lowering glucose and glycaemic components in trials including both diabetes and risk factors of diabetes. These components included glucose, HbA1c, insulin and homoeostasis model assessment-estimated insulin resistance (HOMA-IR).
Methods
Literature search
The review protocol was registered at Prospero International Prospective Register of Systematic Reviews (Registration ID=CRD42014013606 PROSPERO 2014 website: http://www.crd.york.ac.uk/PROSPERO/printPDF.php?RecordID=13606&UserID=7309). The research team collected all pertinent studies published in English from 2000 to June 2015 through a systematic search of the following databases: PubMed, Scopus and the Cochrane Library. In addition, citation lists of all relevant articles and previous reviews were searched. Where a study had unreported data, the authors were contacted to determine whether the information was available. A combination of the following key words was used to locate relevant studies: Probiotics AND glycaemic factor or glucose OR insulin OR homeostasis model assessment and diabetes or diabetic risk factors. The title and abstract were first screened for relevance. The full text of the relevant articles was retrieved for further reading and quality assessment. Two researchers extracted data independently using a standard form and then resolved differences by discussion. The literature search and presentation of results were undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Identified articles were subsequently imported into EndNote reference package.
Inclusion criteria
The research team developed consensus on the four criteria used to determine the inclusion and exclusion of studies, and were as follows:
-
(1) Criterion 1: randomised placebo-controlled trials involved participants with diabetes or associated risk factors, including diabetes, abnormal glucose or HbA1c or insulin levels, overweight, obesity or the metabolic syndromes;
-
(2) Criterion 2: all trials involved human participants;
-
(3) Criterion 3: participants in the sample were randomly allocated into intervention (probiotics consumption) and control (placebo) groups, and all publications were quantitative research method based;
-
(4) Criteria 4: one or more of the following factors were included as outcome variables: glucose, HbA1c, insulin and HOMA-IR.
Where there were multiple publications and companion papers from the same population, only those papers with the largest sample and longest duration of intervention were included. In the event when there were multiple groups in one study, only the probiotics group and placebo group were included.
Data extraction and quality assessment
Extraction of data and assessment of studies according to the above-mentioned criteria were independently conducted by two researchers. The PRISMA flow chart was used to present a summary of the review (see Fig. 1). A pre-piloted data form was used to extract data from the selected randomised-controlled studies. Outcome variables extracted included glucose, HbA1c, HOMA-IR and insulin. Data were retrieved about the effects of probiotics on diabetes and groups with risk factors of diabetes and the characteristics of probiotics intake, including duration of probiotics intake, dosage of probiotics use, probiotics in milk or in capsule forms, single-strain probiotics intake and multiple strains of probiotics. Groups with risk factors of diabetes were defined as participants who had hypertension, hyperlipidaemia, obesity or other types of clinical illnesses. Data on glucose were converted to mmol/l when they were reported as mg/dl using the online converter: http://www.endmemo.com/medical/unitconvert/Glucose.php. The assessment of the abstracted articles was discussed with a view to gaining consensus. Disagreements were resolved through discussion.

Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart of the included studies.
The Physiotherapy Evidence Database (PEDro) tool was chosen to assess the quality of the abstracted articles (see Table 1)( Reference Verhagen, de Vet and de Bie 16 ). This tool categorises the quality of the evidence into three levels (high, 8 or more points; moderate, 4–7 points; low, 3 points or less) based on ten factors including the following: (1) random allocation of subjects into groups, (2) concealed randomisation, (3) similarity of baseline information between groups, (4) blinding in relation to subjects, assessors, (5) blinding in relation to researchers, (6) blinding in relation to assessors, (7) low attrition rate, (8) use of ‘intention to treat’ analysis, (9) use of variability measures such as standard deviation and/or standard error and (10) between-group comparison results. One point is allocated for each item present in the study. Studies assessed as low quality were excluded from the review( Reference Verhagen, de Vet and de Bie 16 ).
Table 1 Characteristics of included clinical trials

P/C, probiotic group/control group; CFU, colony-forming units; PEDro, Physiotherapy Evidence Database; T2DM, type 2 diabetes mellitus patients; DB, double blinded; PC, placebo controlled; P, parallel; HOMA-IR, homoeostasis model assessment-estimated insulin resistance; SB, single blinded; OB, obese subjects; OW, overweight subjects; HTN, hypertensive.
Statistical analysis
The comprehensive meta-analysis for pooled mean difference and effect size was used to analyse the mean differences( 17 ). The effect measures pooled mean difference with a 95 % CI was calculated to describe the effects of probiotics on the outcome variables. Subgroup analyses were used to determine possible sources of heterogeneity. Studies with values <50 % were considered to have low heterogeneity. Random-effect models were used to take into account between-study variation.
The detailed effect of probiotics was further explored using subgroup analysis to identify the sources of heterogeneity in relation to effects of (1) group with diabetes v. participants with risk factors of diabetes, (2) duration of probiotics intake (<8 weeks v. ≥8), (3) dosage level (109 colony-forming units (CFU) or more v. less than 109 CFU use and fermented dairy products), (4) medium type (milk-based v. capsule-based); (5) single strain v. multiple strain and (6) age of the participants (<50 v. ≥50 years). A multivariate meta-regression method was used to analyse the effects of probiotics due to multiple factors. Publication bias was assessed using funnel plots and Egger’s regression test. A P value of more than 0·05 in the Egger’s regression test suggests that there is no publication bias( Reference Egger, Smith and Schneider 18 ). Sensitivity analyses were conducted to assess whether the inferences overly depended on a particular study.
Results
In the initial search, 547 articles were identified from the key databases (220 from PubMed, 326 from Scopus and one from Cochrane Library) (see Fig. 1) and imported into EndNote. After removing duplicate studies, the remaining 211 were screened for relevance through their title and abstract, and then assessed according to the eligibility criteria previously stated. This step resulted in thirty-five articles. Further review using the PEDro tool resulted in twenty-three papers being excluded (ten because of a lack of placebo groups, six because of not focusing on diabetes or associated risk factors, two because of the language being other than English, four because of to low quality and two because of use of non-probiotic products). The remaining eleven papers were included in the final quantitative analysis, with ten having high quality (8 points or more) and one with moderate quality (5–7 points). Table 1 shows the final list of included studies, with their summary characteristics and quality assessment results.
Description of the included studies
A total of eleven randomised-controlled studies examining the effects of probiotics on the glucose and glycaemic factors among healthy participants and participants with diabetes or diabetic risk factors were included in the analysis (see Table 1), representing a total sample of 614 subjects. Among these studies, nine were double blinded( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Asemi, Zare and Shakeri 11 , Reference Ivey, Hodgson and Kerr 15 , Reference Gøbel, Larsen and Jakobsen 19 – Reference Sharafedtinov, Plotnikova and Alexeeva 24 ) and two were single blinded( Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 , Reference Barreto, Colado Simão and Morimoto 25 ). All studies were blinded to assessors. Samples were drawn from seven countries including Australia, Brazil, Denmark, Ireland, Iran, India and Russia. Participant characteristics included diabetes( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 , Reference Asemi, Zare and Shakeri 11 , Reference Mohamadshahi, Veissi and Haidari 21 , Reference Ostadrahimi, Taghizadeh and Mobasseri 22 ), obesity and overweight( Reference Ivey, Hodgson and Kerr 15 , Reference Gøbel, Larsen and Jakobsen 19 , Reference Lindsay, Kennelly and Culliton 20 ), hypertension( Reference Sharafedtinov, Plotnikova and Alexeeva 24 ) and the metabolic syndrome( Reference Barreto, Colado Simão and Morimoto 25 ). All studies measured one or more of the following outcome variables: glucose, HbA1c, HOMA-IR and insulin. The summary description of the study characteristics is presented in Table 1.
Effects on fasting glucose
All studies reported the effects of probiotics on fasting glucose (see Fig. 2(a)). The average reduction of glucose was −0·50 mmol/l (range of 0·09 to −1·29 mmol/l) in the probiotics group compared with 0·13 mmol/l (range of 1·90 to −1·20 mmol/l) in the control group. The pooled effect was −0·52 mmol/l (95 % CI −0·92, −0·11 mmol/l; P=0·01) with a heterogeneity level I 2=94 %, P<0·001.

Fig. 2 Forest plots on (a) glucose, (b) HbA1c, (c) insulin and (d) homoeostasis model assessment-estimated insulin resistance.
Subgroup analyses revealed significant subgroup effects on glucose (Table 2). In these analyses, a probiotic diet resulted in decreased glucose only in trials on diabetes, with a pooled mean difference of −1·46 mmol/l (95 % CI −1·67, −1·26 mmol/l; P<0·001). The heterogeneity level was I 2=0 %, P=0·66. There was no significant pooled mean difference and heterogeneity in trials among participants with other health conditions, with the pooled mean difference of −0·20 mmol/l (95 % CI −0·58, 0·19 mmol/l, Z=−1·59; P=0·11), I 2=92 %, P<0·001. Multivariate meta-regression was further conducted to confirm the effects of probiotics on glucose in participants with diabetes when multiple factors including dosage of probiotics consumption, form of probiotics and number of strains were analysed simultaneously (Table 4). There was a statistically significant effect of probiotics on glucose and diabetes with an estimated coefficient of −1·28 (95 % CI −3·82, −1·25; P=0·03). In addition, the capsule form of probiotics consumption had more significant effects with an estimated coefficient of –2·61 (95 % CI −4·74, −0·47; P=0·02). The total variances explained by the four variables were 100 %, of which diabetes status and form of probiotics explained a large proportion of the variances (43 and 48 %, respectively) (Fig. 2(a)).
Table 2 The subgroup analyses of the effect of probiotics on glucose and HbA1c by probiotics administration criteria (Mean differences and 95 % confidence intervals)

CFU, colony-forming units.
Effect size significance: * P<0·05, ** P<0·01, *** P<0·001.
Effects on HbA1c
Six studies reported HbA1c values among 348 participants (see Fig. 2(b)). The average reduction of HbA1c was 0·48 (range −0·3 to −1·21) % in the probiotics group compared with the increase of 0·05 (range 0·30 to −0·24) % in the placebo group. There was a statistically significant reduction in HbA1c with a pooled mean difference of −0·32 % (95 % CI −0·57, −0·07 %, Z= 2·49; P=0·01) and significant heterogeneity (I 2=83 %, P<0·001). Subgroup analysis (Table 2) was conducted, and it revealed that probiotics had a significant effect on the reduction of HbA1c in four diabetic trials among 218 participants( Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 , Reference Asemi, Zare and Shakeri 11 , Reference Mohamadshahi, Veissi and Haidari 21 , Reference Ostadrahimi, Taghizadeh and Mobasseri 22 ), with a pooled mean difference of −0·52 % (95 % CI −0·71, −0·33 %; P<0·001) and heterogeneity (I 2=5 %, P=0·37). There was no significant effect of probiotics on the reduction of HbA1c in trials with other health conditions and no measurable heterogeneity (I 2=0 %, P=0·34) (Table 2).
Effects on insulin
Eight studies, with 412 participants, reported the effects of probiotics on fasting insulin (see Fig. 2(c)). Probiotics had no statistically significant effect on fasting insulin levels in terms of a reduction in the probiotics group with a pooled mean difference of −0·48 µIU/ml (95 % CI −1·34, 0·38 µIU/ml; P=0·27) and significant heterogeneity (I 2=92 %, P<0·001). To assess the source of heterogeneity, subgroup analysis (Table 3) identified that probiotics had a significant effect on the reduction of insulin in the three diabetes trials involving 148 participants( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 , Reference Asemi, Zare and Shakeri 11 ), with a pooled mean difference of −0·40 µIU/ml (95 % CI −0·59, –0·21 µIU/ml; P<0·001) and significant heterogeneity (I 2=96 %, P<0·001). There was also significant reduction in the trials among participants with other health conditions with a pooled mean difference of −1·11 µIU/ml (95 % CI −1·37, −0·85 µIU/ml) and significant heterogeneity (I 2=56 %, P<0·001) (Table 3).
Table 3 The subgroup analyses of the effect of probiotics on insulin and homoeostasis model assessment-estimated insulin resistance (HOMA-IR) by probiotics administration criteria (Mean differences and 95 % confidence intervals)

CFU, colony-forming units.
Effect size significance: *** P<0·001.
Effects on homoeostasis model assessment-estimated insulin resistance
Eight studies reported HOMA-IR results among 242 subjects (see Fig. 2(d)). Probiotics had no statistically significant effect on the reduction in HOMA-IR (pooled effect of –0·44, 95 % CI −1·57, 0·70; P=0·45). Subgroup analysis (Table 3) revealed that probiotics had a significant effect on the reduction of HOMA-IR in three diabetic trials among 138 participants with a pooled mean difference of −1·54 (95 % CI −1·95, −1·13; P<0·001) and there was no significant heterogeneity (I 2=9 %, P=0·29). There was no significant effect of probiotics on the reduction of HOMA-IR in other trials with a pooled mean difference of 0·31 (95 % CI −0·20, 0·81; P=0·23) with no measurable heterogeneity (I 2=0 %, P=0·51) (Table 3).
Subgroup, sensitivity and publication bias
Subgroup analysis found that the effects of probiotic diet on glucose were statistically significant when it was in capsule form (P<0·05) and with multiple strains. Consumption of probiotics with milk and single-strain consumption did not result in a significant reduction in glucose. Consumption of probiotics resulted in a significant reduction in glucose regardless of dosage level and consumption duration (Table 2).
Subgroup analysis found that the effects of probiotics diet on HbA1c and insulin were statistically significant when it was based on multiple strains of probiotics. Consumption of probiotics with a single strain did not result in a significant reduction in HbA1c or insulin levels (Tables 2 and 3). Furthermore, the effects of probiotic diet on insulin were statistically significant when it was in capsule form. Consumption of probiotics in milk form did not result in a significant reduction in insulin (Table 3).
Sensitivity analyses revealed that no particular study significantly affected the summary effects for glucose, HbA1c, insulin and HOMA-IR. The results for glucose, HbA1c, insulin and HOMA-IR showed minimal asymmetry, which indicates minimal publication bias. Visual inspection of funnel plots (Fig. 3) showed no clear evidence of publication bias with regard to effects on glucose, HbA1c, insulin and HOMA-IR. Findings from the Egger’s regression test supported a finding that there was no publication bias for glucose (P=0·85), HbA1c (P=0·10), insulin (P=0·57) and HOMA-IR (P=0·28) (Table 5).

Fig. 3 Funnel plots on (a) glucose, (b) HbA1c, (c) insulin and (d) homoeostasis model assessment-estimated insulin resistance.
Discussion
Findings from this review indicate that probiotics consumption resulted in an overall reduction in glucose and HbA1c, but not in insulin and HOMA-IR, in trials with both diabetic participants and those with risk factors of diabetes. There were statistically significant reductions in glucose, HbA1c, insulin and HOMA-IR among participants with diabetes, with a large effect size, but not in glucose, HbA1c, HOMA-IR among those participants with risk factors of diabetes. This indicates that probiotics consumption has larger significant effects on reducing glucose metabolism in the diabetes population compared with populations who did not have elevated glycaemic levels. A similar result has also been observed in animal studies( Reference Al-Salami, Butt and Fawcett 26 – Reference Yadav, Jain and Sinha 28 ).
One of the possible mechanisms by which this effect occurs is through the impact of probiotics on changing intestinal microbiota( Reference Chin 4 , Reference Larsen, Vogensen and van den Berg 29 ). Following probiotics consumption, people with type 2 diabetes had balanced intestinal microbiota. This effect might have been caused by the SCFA that are produced from probiotics consumption( Reference Haleh Sadrzadeh-Yeganeh, Ibrahim Elmadfa and Abolghasem Djazayery 30 ) or decreased blood glucose due to increased gliclazide bioavailability( Reference Al-Salami, Butt and Fawcett 26 ).
Probiotics are effective in suppression of the progression of streptozotocin-induced diabetes( Reference Yadav, Jain and Sinha 28 ). Streptozotocin has the ability to selectively kill pancreatic β cells, which can result in a decrease in endogenous insulin release and increase of glucose intolerance( Reference Wilson, Patton and McCord 31 ). Probiotics consumption containing Lactobacillus acidophilus and Lactobacillus casei can delay the progression of streptozotocin-induced diabetes by suppressing the increase in glucose intolerance and blood glucose and maintaining the insulin levels. This indicates that probiotic consumption may have an anti-diabetic effect through a role in protecting pancreatic b-cells from damage( Reference Yadav, Jain and Sinha 28 ).
Improvements in antioxidant stress level through probiotics consumption may also indirectly affect insulin level and glucose homoeostasis( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 ). The distinct effect of probiotics consumption in relation to participants with diabetes may be because probiotics consumption can balance microflora in the gastrointestinal tract to normal levels, as well as change the intestine microbiota so that the elevation in insulin levels is delayed or prevented. In addition, it can also regulate abnormal glucose homoeostasis of participants with diabetes to the normal level. Although the exact mechanism in humans remains unclear, it is likely that multiple factors contribute to the effects of probiotics on diabetic patients( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 ).
The number of strains used in the included studies was related to the effects of probiotics on the glucose and glycaemic factors. Seven trials for glucose, five trials for HbA1c (five out of six) and six trials (six out of eight) for insulin used multiple strains. It was found that trials with multiple strains had statistically significant reductions in HbA1c and insulin levels, whereas trials with single strains did not have significant reductions in HbA1c and insulin levels. The possible mechanism might be that these multiple strains regulate multiple systems through the production of SCFA in the gut, leading to reductions in exogenous cholesterol and LDL and glucose( Reference Pereira and Gibson 32 , Reference Wong, de Souza and Kendall 33 ). This result is similar to the finding that trials with multiple strains had better effects as compared with those with a single strain on blood pressure in a recent meta-analysis( Reference Khalesi, Sun and Buys 9 ). Owing to the limited number of trials with multiple strains, further studies are warranted to confirm these findings.
The form of probiotics was also related to the effect of probiotics on the outcome variables. Five trials (five out of eleven) for glucose and five trials (five out of eight) for insulin used the capsule form of probiotics. It was found that trials using capsules had significant reductions in glucose and insulin, whereas trials using the milk form did not have significant reduction in glucose and insulin. The possible reason might be that the trials using capsules might have resulted in better digestion of the product, and therefore led to higher levels of bioactivity, which may account for this result. The dosage level and duration of probiotics consumption did not have any effects. Four trials (four out of six) for HbA1c used the milk or yoghurt form of probiotics( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Ivey, Hodgson and Kerr 15 , Reference Mohamadshahi, Veissi and Haidari 21 , Reference Ostadrahimi, Taghizadeh and Mobasseri 22 ). It was found that trials using milk or yoghurt had a significant reduction in HbA1c. It is possible that the milk or yoghurt form may keep more bacteria alive in the tract to balance the HbA1c. The replacement of sugar in milk or yoghurt by probiotics does not account for the reduction in HbA1c, as both the probiotics and placebo groups had the similar ingredients, except for the addition of probiotics in the probiotics group. This finding suggests that the reduction of HbA1c is not due to the reduction of sugar levels in milk or yoghurt, but is a treatment effect of the probiotics per se. We do not know the effect of insulin v. probiotics on the reduction of glucose, HbA1c, insulin and HOMA-IR, as the studies reviewed in this meta-analysis excluded patients using insulin( Reference Ejtahed, Mohtadi-Nia and Homayouni Rad 1 , Reference Mazloom, Yousefinejad and Dabbaghmanesh 3 , Reference Asemi, Zare and Shakeri 11 , Reference Mohamadshahi, Veissi and Haidari 21 , Reference Ostadrahimi, Taghizadeh and Mobasseri 22 ). However, the findings cannot be accounted for by the effect of other anti-diabetic medications rather than probiotics. Despite the fact that some patients were using anti-diabetic medication in a number of studies, the probiotic and placebo groups were randomised and showed similar baseline results on demographic factors, anthropometry, glycaemic characteristics and medication use, thus ruling out the hypothesis that the treatment effect was due to medication rather than probiotics.
None of the studies in this meta-analysis reported any adverse side-effects related to the mediums used (fermented milk and capsules), indicating that they were safe to use. In addition, all the studies had <15 % attrition rate over the course of the intervention, suggesting that the subjects had excellent level of compliance with the consumption of probiotics. Probiotics consumption may have provided additional health benefits in addition to medication use.
Strengths and limitations
This is the first meta-analysis study on randomised-controlled trials (RCT) relating to the effect of probiotics on glucose and glycaemic factors. The strength of this study is that all studies published since 2000 to 2015 have been included in the meta-analysis, and where there were unreported data the authors were contacted to provide it. Therefore, all available data were included( Reference Khalesi, Sun and Buys 9 ). All included studies in this review were placebo-controlled trials; thus, the effect of intervention due to probiotics consumption could be examined. In addition, the results of these analyses may be viewed as accurate, as we were able to adjust for multiple variables using meta-regression on glucose when conducting subgroup analyses.
A number of limitations of this study should be noted. The use of strains of probiotics was not homogeneous across studies and some studies used strains that could sensitively regulate glucose metabolism, whereas others were not in the included clinical trials. Owing to the limited number of included trials, it was not possible identify the effect of specific strains of probiotics on the reduction of glucose, HbA1c, insulin and HOMA-IR. Further exploration of such subgroup differences within the context of RCT will entail a meta-analysis to identify the effect of specific strains on the reduction of glucose, HbA1c, insulin and HOMA-IR. We also recognise that studies published in languages other than English were not included, and thus it is unknown whether results of these studies would have had effects on our meta-analysis results.
Implications for treatment and future research
The findings of this meta-analysis suggest that probiotics may play an important role in the future prevention of diabetes and reduction of risk factors of diabetes. However, further research is needed to confirm its benefits in the prevention and treatment of diabetes, particularly in relation to form, variability of probiotic strains and specification of strains. Nevertheless, there is a recognised and important role played by probiotics and considerable potential benefits in managing a number of risk factors of diabetes. Furthermore, inadequate data sources in studies for insulin and HOMA-IR meant that we could not differentiate between subgroup differences such as duration of probiotics use, dosage, single-strain v. multi-strain effects and specific strain effects. Furthermore, RCT involving long-term interventions with single v. multiple strains of probiotic supplement regimens are required to further clarify these findings.
Future research using a RCT with a large sample size is needed to confirm that such alternative nutrition regimens are effective with regard to the reduction of glycaemic factors. Similarly, it is necessary to identify and characterise known and unknown strains of probiotics, and identify strain-specific health outcomes to optimise treatment effects. This will enhance our understanding of the application of probiotics in diabetes prevention and treatment. It is possible that probiotics can be used as an adjunct method for glycaemic control in addition to medication intake for patients with diabetes. There is potential in the field of translational diabetic medicine to further examine the role probiotics play as an important dietary supplement for the prevention of diabetes and the risk factors. Further research is needed on whether probiotics have a treatment effect, in addition to pharmaceutical drug administration, and to determine whether probiotics should be included as a functional food and treatment option for diabetes.
Table 4 Meta-regression analysis (Mean differences and 95 % confidence intervals)
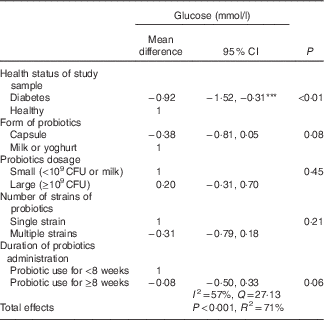
CFU, colony-forming units.
*** Effect size significance: P<0·001.
Table 5 Egger’s regression analysis on publication bias
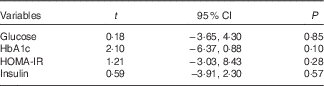
HOMA-IR, homoeostasis model assessment-estimated insulin resistance.
Conclusions
In summary, the findings of the present study suggest that probiotic supplementation use is effective in improving glucose metabolism. The effect of probiotics use on glucose is more effective when it is used in the capsule form and with multiple strains, and it is more effective in participants with impaired glucose and insulin resistance levels. With the improvement of multiple risk factors of diabetes, probiotics may provide a potential avenue to improve glucose metabolism, which may lead to a reduction in diabetes complications and its rising incidence.
Acknowledgements
The authors acknowledge Michelle Carty’s assistance for the data search and extraction procedure.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. S. designed the study, formulated research questions, collected data, analysed the data and wrote the article. N. J. B. collected data, evaluated the quality of the study, interpreted the data and edited the article.
The authors declare that there are no conflicts of interest.











