Taste is a decisive factor in the formation of human dietary behaviour. Taste can affect the enjoyment or rejection of certain types of food, and this may lead to an individual’s selective intake of nutritive compounds(Reference Duffy1). Therefore, the five types of tastes human can perceive – sweet, salty, sour, bitter and umami – not only result in the simple delight of eating but also have important effects on human dietary behaviour and health(Reference Duffy1).
Fat was considered to have no taste but was rather associated with only textural characteristics. However, recent findings have suggested that fat is a sixth type of taste(Reference Burgess, Melis and Scoular2–Reference Stewart, Newman and Keast5). Dietary fat is critical for health since the excessive intake of fat is a major concern in the context of many degenerative and metabolic diseases, including obesity, hypertension, type 2 diabetes mellitus and colon cancer(Reference Smit, Mozaffarian and Willett6). However, fat is still an important source of energy and a physiological vehicle for nonpolar compounds. Furthermore, fat in food could also modify the preference for the food due to its unique sensory traits, fattiness and creaminess(Reference Running, Mattes and Tucker7). Therefore, the factors related to fat perception and consumption are important in the food industry as well as in the clinical setting.
Earlier studies have suggested that proteins including cluster of differentiation 36 (CD36) and the G-protein-coupled receptor family are involved in the perception of fat. Among them, CD36 is a commonly studied genetic component in fat perception in relation to food intake(Reference Pioltine, de Melo and Santos8,Reference Ozdener, Subramaniam and Sundaresan9) . CD36 protein is located in the cellular membrane and has a primary role in the sensing of long-chain fatty acids by binding to various types of lipids, including cholesterol, phospholipids and lipoproteins(Reference Febbraio, Hajjar and Silverstein10,Reference Silverstein, Li and Park11) . For this reason, genetic variation in CD36 is associated with the oral sensing of and preference for dietary fat and the differentiation of dietary fatty acids. A genetic variation of rs1761667 G>A in CD36 was observed to be associated with a variation in the sensing of the taste of fat, fat consumption and differentiation of fatty acid types(Reference Melis, Sollai and Muroni12–Reference Karmous, Plesník and Khan15). Furthermore, the variation was also associated with body composition and obesity(Reference Melis, Carta and Pintus16). However, findings have been inconsistent: having the A allele mutation was associated with reduced fatty food intake only in obese Brazilian children(Reference Pioltine, de Melo and Santos8). The genetic variation also showed a significant association with obesity measures in African American adults(Reference Pepino, Love-Gregory and Klein14,Reference Keller17) but not in Malaysians(Reference Ong, Tan and Say18). These findings suggested that the effect of the CD36 genetic variation could differ by ethnicity and adiposity. However, the effect of the CD36 genetic variation has not yet been explored in the Korean population. Furthermore, fat is generally consumed in the form of food, not as a sole nutrient. The intensity of the fat taste affects not only the simple intake of fat but also foods rich in fat, as well as other types of foods and cooking methods. Therefore, to better understand the modifying role of CD36 in Koreans’ dietary intake, it is necessary to examine how this genetic factor influences fat consumption as well as overall related dietary behaviour.
This study aimed to examine whether genetic variation in CD36 is associated with dietary behaviour in Koreans, with a focus on fat consumption. As a genetic marker, rs1527479 T>C was used as a proxy of rs1761667 G>A. Using the data of the Ansan/Ansung Community Cohort Genome-Epidemiologic Study, analyses were performed to ascertain the effect of CD36 genetic variation on the intake of fat, macronutrients and selected food groups, as well as the frequency of oily food consumption in Koreans stratified by adiposity level. Since sex disparities clearly exist in health and dietary behaviour(Reference Joseph, Reed and Mennella19,Reference Westenhoefer20) , the study employed a sex-stratified approach.
Materials and methods
Study population
This study was conducted with data from the Ansan/Ansung Community Cohort Study, a part of the Korean Genome and Epidemiology Study. The characteristics of the Ansan/Ansung Community Cohort study and the Korean Genome and Epidemiology Study are described elsewhere(Reference Kim, Han and Ko21,Reference So, Choi and Joung22) . The materials used in this study were baseline data obtained from 2001 to 2002. Among a total of 8840 subjects (aged 40–69 years) whose genetic characteristics were analysed, subjects with no dietary data (n 290) or implausible total energetic intake (<2092 kJ/d (<500 kcal/d) or >20 920 kJ/d (>5000 kcal/d), n 77) were excluded. Additionally, subjects without anthropometric information, body composition (n 1679) or CD36 rs1527479 genotype data (n 175) were also removed from the data set. Finally, the remaining 3194 males and 3425 females were analysed for the study (Fig. 1). The Korean Genome and Epidemiology Study was conducted following a protocol approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention. All participants provided written informed consent prior to study commencement. This study was also approved by the Institutional Review Board (40525-201802-HR-121-01).
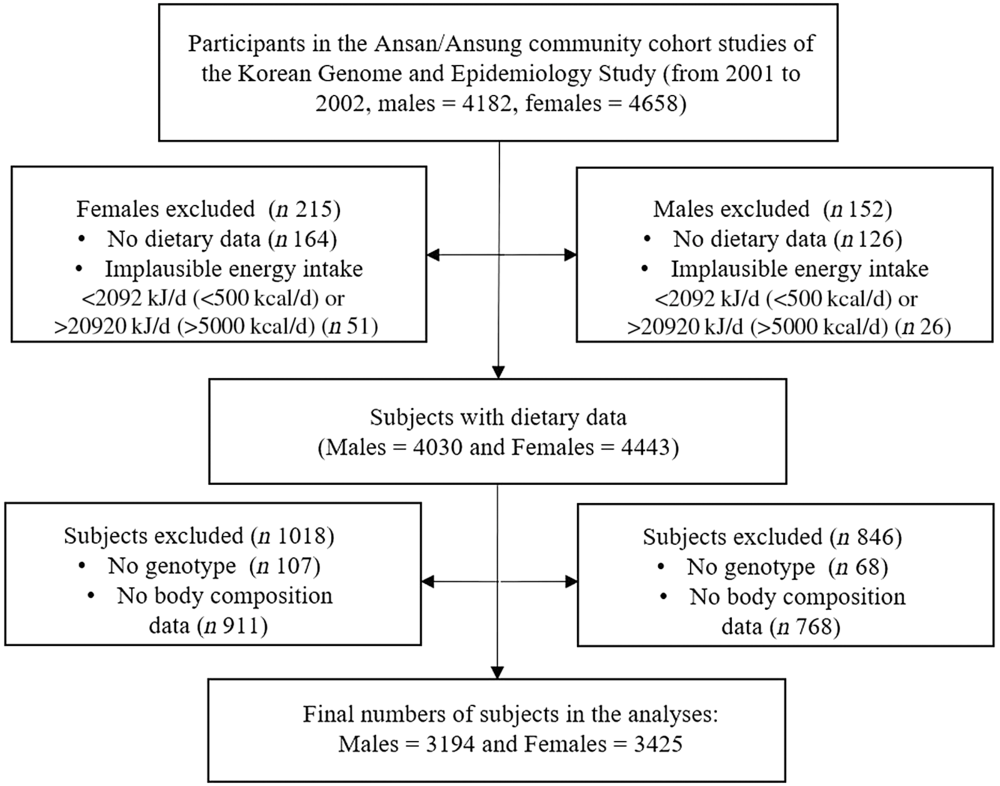
Fig. 1. Procedure for the selection of study subjects.
Collection of general characteristics and anthropometric data
General characteristics, including sociodemographic and lifestyle factors (i.e. age, sex, alcohol consumption, tobacco smoking, marital status, education level and physical activity level), of the study population were obtained by trained interviewers using a questionnaire. The use of tobacco or consumption of alcoholic beverages was classified into two levels: never and ever. Subjects’ physical activity levels were defined in the form of metabolic equivalents computed as the sum of metabolic equivalents for five levels of action (1 for sedentary, 1·5 for very light, 2·4 for light, 5·0 for moderate and 7·5 for intense activities)(Reference Kim, Kwak and Lim23,Reference Ainsworth, Haskell and Whitt24) . The education level was classified into four levels: elementary school or less (≤6 years), middle school (7–9 years), high school (10–12 years) and college or higher (≥13 years). Marital status (cohabitation) was grouped according to the presence or absence of a partner. Body size (weight and height) was estimated to the nearest 0·1 kg and 0·1 cm, respectively, using a stadiometer. BMI was computed as weight (kg) divided by the squared height (m2).
Collection and assessment of dietary intake and behaviour data
To collect the dietary intake data, a validated FFQ with 103 food items was employed(Reference Ahn, Kwon and Shim25). Study participants marked the frequency of their consumption of each food based on nine response options (never or barely, 1 time/month, 2–3 times/week, 1–2 times/week, 3–4 times/week, 5–6 times/week, 1 time/d, 2 times/d or ≥3 times/d) and three differential serving sizes (small, medium or large). To estimate the intake of seasonal foods (i.e. fruits), participants were also asked to record the period of consumption (3, 6 or 9 months or a year). Nutritional intake was estimated using the Food Composition Table, Korea (7th edition). To investigate the influence of CD36 rs1527479 on Korean males’ dietary intake, the 103 food items were grouped by taking into account Koreans’ dietary culture: carbohydrate foods, carbohydrate- and fat-rich foods, sweets, protein-rich foods, dairy products, meats, seafood, fatty foods, all vegetables, green vegetables, cruciferous vegetables, seaweeds, all fruits and citrus fruits. Additionally, participants recorded the frequency of consumption of fried food based on five levels (rarely, 1–2 times/month, 1–3 times/week, 4–6 times/week or every day). To investigate the preferred type of oil for seasoning and cooking, the commonly used oils in Korean cuisine (for seasoning, sesame oil and perilla oil; for cooking, soyabean oil, maize oil, olive oil and butter) were presented; however, participants were still freely able to write in a response if the preferred or commonly used oil was not listed in the questionnaire.
Genotype assessment and selection of proxy marker
Genomic DNA specimens were obtained from participants’ peripheral blood. The genotype was determined using the Affymetrix genome-wide human SNP array 5.0 (Affymetrix Inc.). The quality control of the genetic data obtained was performed following Bayesian robust linear modelling with the Mahalanobis distance algorithm. Samples were excluded if they presented low quality, including genotyping call rate <96 %, excessive heterozygosity, sex and ethnic mismatch, or cryptic relatedness. Genetic loci were excluded if they possessed a call rate <95 %, a low minor allele frequency (MAF) of <0·01 or deviated from Hardy–Weinberg equilibrium (P < 1 × 10–6)(Reference Cho, Go and Kim26,Reference Rabbee and Speed27) . As genotype result for rs1761607 was not included in the data, the analyses were performed using a proxy marker. The LDlink analyses suggested that rs1527479 is located near and highly associated with the target rs1761667 (r 2 1, D′ = 1, about 27·6 Kb downstream). This was confirmed in Japanese population data because no Korean data have been reported yet. Therefore, rs1527479 was selected as a genetic proxy marker(Reference Machiela and Chanock28).
Statistical analyses
The differences in general information among individuals with the CD36 rs1527479 genotypes were determined using generalised linear models and χ 2 tests accounting for the type of variables. Food and nutritional intake data were adjusted for total energetic intake using Willett’s residual method and were then included in the analyses(Reference Willett, Howe and Kushi29). The comparisons of dietary and nutritional intake and CD36 rs1527479 genotypes were performed using generalised linear models, either with or without covariates. The post hoc comparisons between those three genotypes were performed with Tukey’s technique. All continuous variables, including dietary and anthropometric data, were log-transformed prior to inclusion in the statistical models for better normality. The frequency of consuming oily foods was also transformed to determine the yearly frequency (e.g. rarely = 0, 1–2 times/month = 18, 1–3 times/week = 104, 4–6 times/week = 260, everyday = 365, etc.) and was then log-transformed prior to the analyses. The association between the CD36 genotype and the mainly used type of seasoning and cooking oil was investigated using χ 2 tests. All statistical studies were performed with SAS version 9.4 (SAS Institute Inc.). Two-tailed P values <0·002 were recognised as statistically significant to correct for multiple test issues following Bonferroni’s rule (0·002 = 0·05/24 dietary-related variables examined).
Results
Table 1 presents the descriptive information of the study population, taking into account the BMI and CD36 rs1527479. Approximately 41·8 and 44·1 % of males and females, respectively, were defined as having obesity. In males with or without obesity, the MAF for the C allele was 0·29 and 0·30, respectively. In females with or without obesity, the MAF was 0·31 and 0·29, respectively. The MAF of rs1527479 in Koreans has not yet been reported. However, the MAF values in Han Chinese and Japanese individuals were 0·34 and 0·22, respectively, which did not deviate much from that of Koreans according to the current study(Reference Auton, Brooks and Durbin30). The statistical analyses suggested that CD36 rs1527479 genetic variation had no meaningful association with the subjects’ age, living area, alcohol consumption status, tobacco smoking status, marital status, education level or physical activity in all subgroups, taking into account sex and obesity level.
Table 1. Descriptive data of the study population by CD36 rs1527479 genotype and level of obesity in males and females
(Mean values and standard deviations; numbers and percentages)
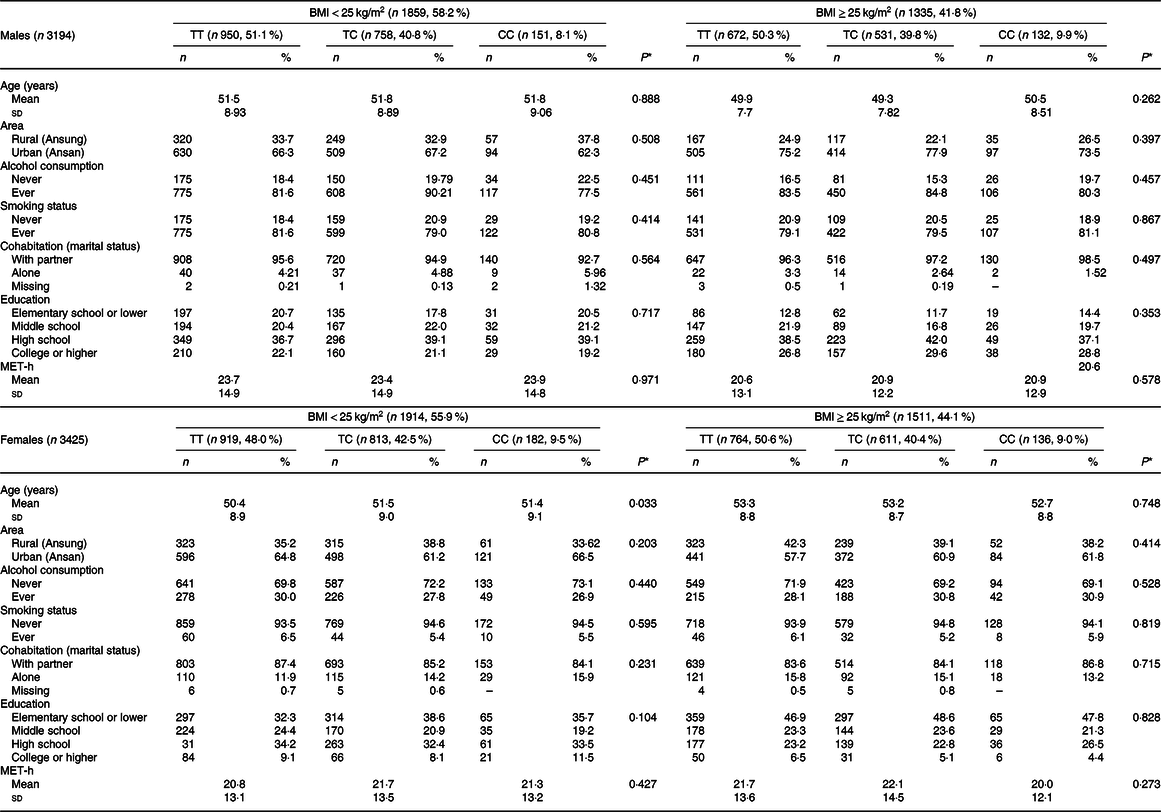
MET-h, metabolic equivalents.
* P values for age and metabolic equivalents were from generalised linear models, otherwise from χ 2 tests among three genotypes.
To examine whether CD36 genetic variation may influence the intake of total energy and macronutrients, statistical analyses were performed. The findings suggested that CD36 rs1527479 genetic variation was not associated with total energy, fat, cholesterol, protein or carbohydrate intake or the percentage of energy content obtained from those three macronutrients (Table 2). However, the analyses regarding the association between CD36 genotype and food intake revealed interesting findings (Table 3). In the group of males with obesity, CD36 rs1527479 showed an association with the intake of vegetables. In the subjects with obesity, cruciferous vegetable intake was lower in individuals with the CC genotype: for the TT, CT and CC genotypes, the levels were 218·9 (SD 132·3), 226·5 (SD 125·5) and 192·1 (SD 116·2) g/d, respectively (P = 0·0004). This cruciferous vegetable intake and genotype association were still significant when the covariates were adjusted in the statistical models (adjusted P = 0·0015). However, the association was not observed in the non-obese subjects or females. In females without obesity, the genotype appeared to influence the intake of green vegetables, but the statistical significance was limited.
Table 2. Total energy and macronutrient intakes for each CD36 rs1527479 genotype group by level of obesity in males and females
(Mean values and standard deviations)
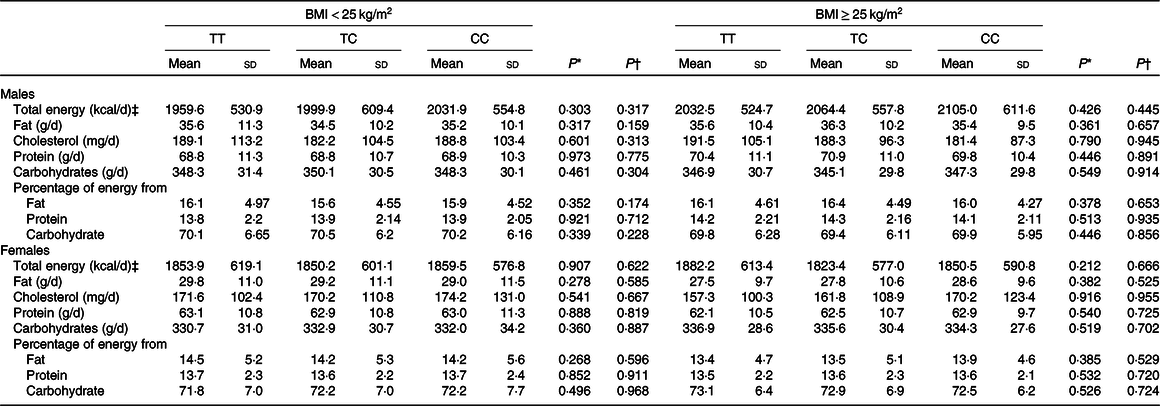
* P values were from crude generalised linear models.
† P values were from generalised linear models with the covariates including area, age, BMI, cohabitation, education, alcohol consumption, tobacco smoking, physical activity level and total energy intake.
‡ To convert energy values from kcal to kJ, multiply by 4·184.
Table 3. Intake of selected food groups in CD36 rs1527479 genotype groups by level of obesity (g/d)
(Mean values and standard deviations)
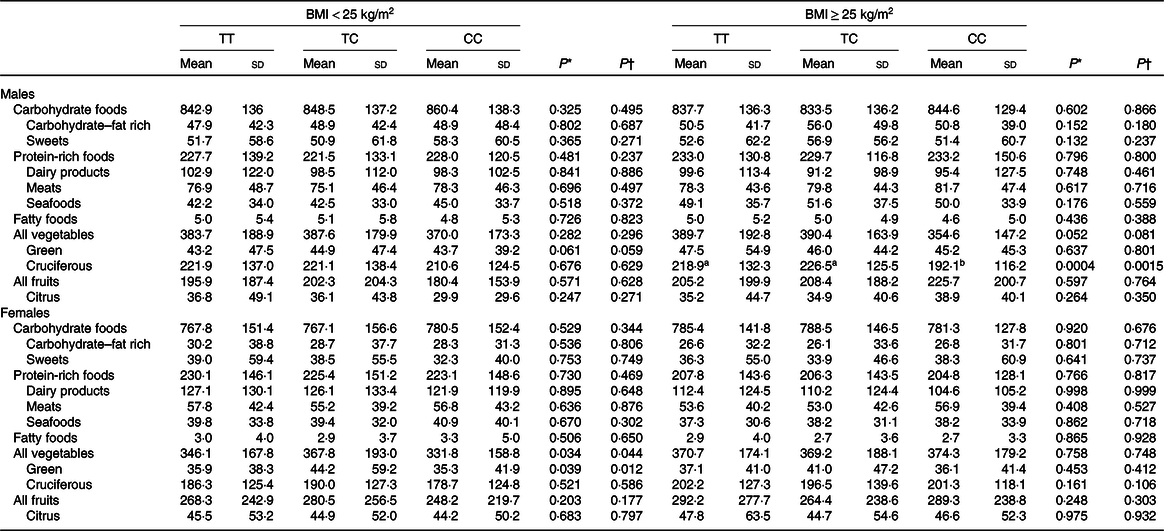
a,b Mean values within a row with unlike superscript letters were significantly different between genotypes (P < 0·05; determined by Tukey’s method).
* P values were from crude generalised linear models.
† P values were from generalised linear models with the covariates including area, age, BMI, cohabitation, education, alcohol consumption, tobacco smoking, physical activity level and total energy intake.
Last, Table 4 presents the results from the analyses showing that the CD36 rs1527479 genotype influences dietary behaviour regarding oil consumption. The findings suggested that in obese or non-obese groups of males and females, the rs1527479 genetic variation was not associated with fat-related dietary behaviours, frequency of fried food consumption or commonly used types of oils for seasoning and cooking.
Table 4. Dietary behaviour-related oil consumption in CD36 rs1527479 genotype groups by level of obesity in males and females
(Numbers and percentages)
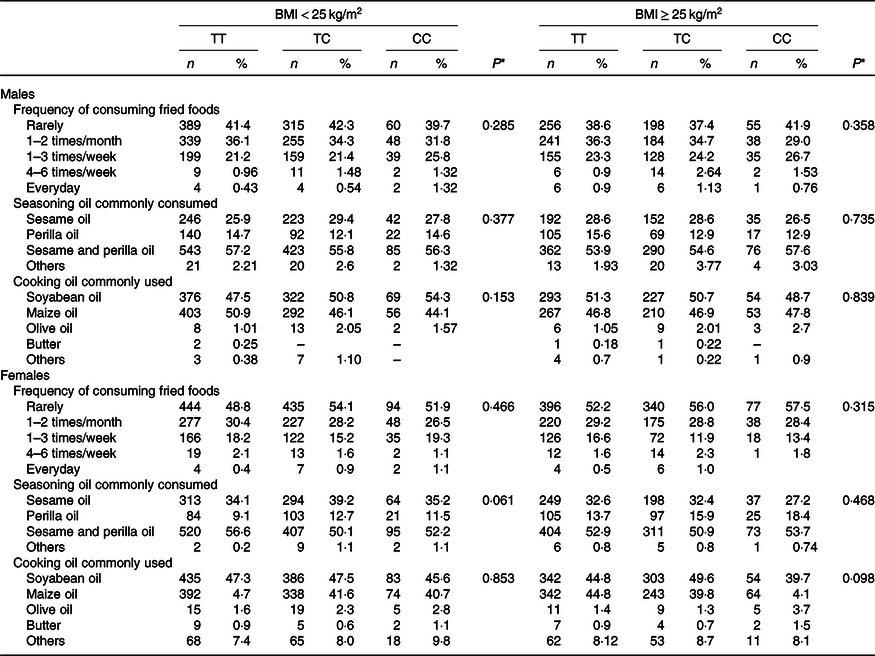
* P values for analyses of ‘Frequency of consuming fried foods’ were from generalised linear models. The frequency was converted to the times per year and applied for the statistical models. Covariates including area, age, BMI, cohabitation, education, alcohol consumption, tobacco smoking, physical activity level and total energy intake were adjusted in the statistical models. P values for analyses of ‘Seasoning oil commonly consumed’ and ‘Cooking oil commonly used’ were from χ 2 tests between types of oil and genetic groups. ‘Others’ were excluded from the analyses due to rarity.
Discussion
This study investigated whether the genetic variation rs1527479 T>C in CD36 is associated with fat and nutrition intake and related dietary behaviour in Koreans. Rs1527479 was selected as a proxy marker of rs1761667 G>A previously shown to represent the phenotypic changes in fat sensing. The findings suggested that CD36 genetic variation was not associated with fat consumption or related dietary behaviour but that it showed a significant association with vegetable intake.
CD36 rs1761667 G>A is an intronic mutation close to the 5’ flanking exon region(Reference Daoudi, Plesník and Sayed31). Earlier studies have suggested that rs1761667 in CD36 was associated with the perception of fat taste. In studies of Tunisian(Reference Mrizak, Sery and Plesnik13) and Algerian individuals(Reference Sayed, Šerý and Plesnik32), individuals with the AA mutant genotype showed lower intensity of fat perception and a preference for added fat and oil; hence, these individuals had higher fat intake than individuals with the genotype with the G allele(Reference Burgess, Melis and Scoular2). Additionally, studies have clearly suggested that the genetic variant was associated with clinical outcomes, including lower rates of hypertension, coronary artery disease and liver fibrosis(Reference Fujii, Hishida and Suzuki33–Reference Ramos-Lopez, Roman and Martinez-Lopez35). The association between the variation in CD36 and fat sensing may be explained as follows. CD36 mediates the relocation of selected fatty acids across the cellular membrane. Although rs1761667 is an intronic variation, experimental evidence has suggested that the variant results in reduced mRNA transcription and protein expression(Reference Love-Gregory, Sherva and Schappe36). This reduction could be associated with differences in the oral perception of the intensity of fat/fatty acid taste and intake(Reference Love-Gregory, Sherva and Schappe36). However, in this study of Korean males, the effect of the variation in CD36 was not evident in regard to fat and macronutrient intake, the preference for and frequency of fried food consumption or the commonly consumed types of oils. These conflicting findings regarding CD36 and dietary behaviour might be associated with diverse dietary cultures and fat intake levels in different ethnicities. In animal models, varied oral expression of CD36 was evident based on the fat content of the diets(Reference Martin, Passilly-Degrace and Gaillard37,Reference Zhang, Zhou and Ban38) . As alluded to above, the effect of CD36 genetic variation on dietary intake also differed according to ethnicity and adiposity level. The Korean population generally showed relatively lower fat intake than other populations. A traditional Korean diet could be defined as being composed mainly of vegetables and grains, with limited use of oil and high-fat fried foods(Reference Kim, Kim and Lee39). Animal fats are barely used for cooking and seasoning; rather, relatively small amounts of vegetable oils, including sesame, perilla and soybean oil, are generally used(Reference Kim, Kim and Lee39). In this study of Koreans over 40 years old, the average intake of fat and the percentage of total energy obtained from fat were only approximately 27–36 g/d and 13–16 %, respectively. This level of fat intake is much lower than that of other populations, showing the association of CD36 with fat intake(Reference Pioltine, de Melo and Santos8). Such differences in the type of dietary fat consumed and the intake level may be associated with the minimal effect of CD36 genetic variation in the Korean male population.
In the present study, CD36 genetic variation had a significant effect on cruciferous vegetable intake but not on fat consumption. Males with obesity and the CC genotype showed significantly lower cruciferous vegetable intake than those with the TT and CT genotypes. Cruciferous vegetables contain glucosinolate molecules with thiourea moieties, resulting in bitterness(Reference Cartea, Francisco and Soengas40). Earlier studies have reported that the bitterness genotype and phenotype defined by 6-n-propylthiouracil are associated with fat taste intensity. 6-n-propylthiouracil bitterness non-tasters were not able to distinguish the differences in the fat content of oil dressing(Reference Tepper and Nurse41) and had greater preference for high-fat oil dressing and daily discretionary fats(Reference Tepper and Nurse41,Reference Keller, Steinmann and Nurse42) . Studies have attempted to explain the association between bitterness and fat taste intensity. Individual bitterness phenotypes result from multiple genetic traits, including taste receptor 2 member 38 (TAS2R38) and carbonic anhydrase 6. The lower taste intensity is the consequence of the structural change in the proteins due to the individuals’ genetic characteristics and, hence, reduced differentiation of taste cells(Reference Keller17,Reference Bartoshuk, Duffy and Miller43) . Those less-differentiated cells with functionally altered proteins therefore could modify the sensing of overall taste, including bitterness and possibly the fat taste(Reference Keller17,Reference Tepper44) . A recent study also revealed a more direct relationship between CD36 fatty acid sensing and the bitterness phenotype(Reference Sollai, Melis and Mastinu45). In this Korean study, the CD36 genotype showed a significant association with cruciferous vegetable consumption. As alluded to above, fat has less significant meaning in Korean dietary culture than in other non-East Asian ethnicities. However, cruciferous vegetables account for a substantial amount, approximately 60 %, of the vegetables consumed by this Korean male population. Therefore, the effect of CD36 genetic variation may be evident in bitter-tasting cruciferous vegetable intake due to the link between the fat and bitter taste phenotypes. Additionally, studies have attempted to investigate the genetic factors associated with vegetable consumption because consuming a sufficient amount of vegetables is important because they are not only a great source of dietary fibre but are also high in nutritive compounds(Reference Manchali, Chidambara Murthy and Patil46). The TAS2R38 diplotype was known to be associated with cruciferous vegetable consumption in studies, but this association is still inconclusive in Koreans(Reference Choi, Lee and Oh47–Reference Choi49). The present findings suggest that the potential effect of CD36 genetic variation, not TAS2R38, is associated with vegetable intake in obese males. Overall, the present study could provide evidence that the putative link between bitterness and fat taste phenotypes and genotypes may play a role in Korean vegetable consumption.
Last, in the current study, the association between dietary intake and CD36 genetic variation differed according to sex and level of obesity: the effect of CD36 genetic variation was evident in only obese males but not in non-obese males or females. The association between CD36 polymorphisms and the intensity of fat perception has been evident mainly in females(Reference Mrizak, Sery and Plesnik13,Reference Sayed, Šerý and Plesnik32) . However, controversies remain. The association of the CD36 genotype with fat intensity varied between studies(Reference Burgess, Melis and Scoular2,Reference Ong, Tan and Say18) . Limited numbers of studies have also adopted a sex-stratified design or were performed in male subjects. Sex is a decisive factor in health behaviour, including dietary consumption. Males and females have different levels of health knowledge and willingness to adopt healthy behaviours(Reference Westenhoefer20). Males and females also experience different taste intensities from childhood(Reference Joseph, Reed and Mennella19). These sex-specific characteristics might result in the differential effect of the CD36 variant in dietary intake. Additionally, one experimental study verified that CD36 rs1761667 was associated with fatty acid metabolism and the circulating endocannabinoid levels involved in energy metabolism by regulating appetite, and these effects of CD36 varied by individual adiposity level (BMI)(Reference Melis, Carta and Pintus16). This may provide evidence on how the CD36 genetic variation has a differential effect on dietary behaviour according to the level of obesity. Given all these findings, the design of the study, milieu and biological factors interactively contributed to the association identified between Korean males’ dietary consumption and CD36 genotype in the current study. Further experimental and epidemiological studies are required to explain the sex- and adiposity-specific underlying mechanisms of the potential modifying effects of CD36 on dietary behaviour and health outcomes.
This study examined the association between CD36 and dietary behaviour in Koreans with the rs1527479 genetic variation as a modifying factor. Limited knowledge regarding genetic factors in Korean dietary behaviour is currently available; therefore, this study may provide preliminary evidence. However, the study could have limitations. First, this study was performed with the data of approximately 6600 subjects from the Ansan/Ansung Community Cohort Study, a representative large genome-epidemiological study cohort. However, the size of the study population might be relatively small, and the subjects were over 40 years old, which may not fully represent the characteristics of all Koreans. Second, the study provided only limited information regarding fat intake. The full information regarding fat consumption, such as detailed types and intake of fatty acids, could not be analysed in this study. Third, CD36 is a critical genetic locus for fat sensing and metabolism. However, taste perception is a highly complicated mechanism, and the effects of other genes and environmental factors were not considered in this study. Last, the dietary information was collected using a FFQ. This may be associated with an accuracy issue in capturing a small amount of food and nutrition intake, as well as recall bias(Reference Shim, Oh and Kim50). Therefore, findings must be interpreted with caution.
In conclusion, rs1527479 (a proxy of rs1761667) in CD36 was not associated with fat, macronutrient intake or fat-related dietary behaviour in Korean males. However, this genetic variation was associated with cruciferous vegetable intake in obese males. These findings may aid in the understanding of the role of CD36 in the dietary intake and behaviour of Koreans.
Acknowledgements
This study was conducted with bioresources from the National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea (18031403-01-01).
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (no. NRF-2018R1A1A1A05019155).
J. H. C. conducted this work and is responsible for the final content. J. H. C. conceived and designed the study, performed all analyses and wrote the manuscript.
The author declares no potential conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003748








