Gallic acid (GA) represents one of the most abundant phenolic compounds found in several fruits and medicinal plants(Reference Choubey, Varughese and Kumar1). In monogastric animals, plant polyphenols may exert detrimental effects on animal growth performance by reducing sugar and amino-acid (AA) absorption(Reference Biagia, Cipollini and Paulicks2,Reference Karasov, Meyer and Darken3) . However, their use in animal nutrition is appealing because of several health-promoting properties(Reference Girard, Thanner and Pradervand4,Reference Girard and Bee5) . It is known that the absorption of both exogenous and endogenous nutrients can be affected by the epithelial membrane integrity, as determined by the expression of tight-junction proteins, such as occludin, claudin and zonula occludens(Reference Chelakkot, Ghim and Ryu6,Reference Ballard, Hunter and Taylor7) . Moreover, intestinal AA absorption is also regulated by specific AA transporters located at the luminal or basolateral sides of intestinal epithelial cells(Reference Biolley, Tretola and Bee8). Phenolic compounds, together with other extracellular stimuli, can modulate both tight-junction proteins and AA transporter expression(Reference Chelakkot, Ghim and Ryu6–Reference Biolley, Tretola and Bee8). This latter property is of great interest for human and animal well-being, considering that AA are effectors of epithelial cell function(Reference Kong, Zhang and Zhang9). Peptides and AA are actively absorbed in the intestine, and the major site of their absorption is the jejunum(Reference Broer10). In the present study, we focused on l-glutamate (l-Glut), l-arginine (l-Arg), l-lysine (l-Lys) and l-methionine (l-Meth) AA. These four AA were chosen to investigate the three main categories of AA transporters present in the vertebrate intestinal-brush-border membrane(Reference Broer10) – namely, anionic (i.e. l-Glut), cationic (i.e. l-Arg and l-Lys) and neutral (i.e. l-Meth) AA transporters. Uptake of anionic AA, such as l-Glut, is strictly Na+-dependent in the small intestine, where the high-affinity glutamate transporter excitatory-amino-acid transporter-3 (EAAT3) is expressed(Reference Kanai and Hediger11). In mammals, cationic AA are mainly absorbed by system y+ activity, which exerts an Na+-independent uptake of l-Arg, l-Lys and l-ornithine (l-Orn). A cationic-amino-acid transporter-1 (CAT-1) is the transporter with the highest affinity for l-Arg and l-Lys, and it is abundantly expressed by the small-intestinal epithelium(Reference Palacin, Fernandez and Chillaron12,Reference Hatzoglou, Fernandez and Yaman13) . By contrast, the uptake of neutral AA across the apical membrane of intestinal epithelial cells is almost entirely Na+-dependent(Reference Broer10). The major apical neutral AA transporter in the intestine is the B(0)AT1 (SLC6A19) system, which co-transports one Na+ per AA(Reference Camargo, Makrides and Virkki14).
Prior studies demonstrated morphological and functional similarities between the intestinal porcine enterocyte cell line (IPEC)-J2 and intestinal epithelial cells(Reference Schierack, Nordhoff and Pollmann15,Reference Deglaire and Moughan16) . For this reason, we used the IPEC-J2 cell line as a well-established in vitro model of nutrient absorption and compared the data against those obtained from porcine jejunum tissue ex vivo. Although several polyphenolic compounds have been investigated for their ability to affect growth performance in monogastric animals, little is known about the effects of GA on intestinal integrity and nutrient absorption. The present study therefore aims to evaluate GA’s effect on intestinal-barrier integrity and the absorption of the stated AA in monogastric animals.
Methods
Tissue recovery for ex vivo experiments
The Swiss Cantonal Committee for Animal Care and Use approved all procedures involving animals (2018_30_FR).
Swiss Large White pigs housed at Agroscope animal facilities were used. Only females were selected to avoid the sex effect. The pigs were reared in pens with slatted metal floor, with ad libitum access to a low-crude-protein grower-finisher diet and drinking water. Ambient temperature was maintained at around 28°C. No welfare-related interventions were needed prior to, during or after the experiment since no unhealthy conditions were observed, based on animal appearance, body functions, environment quality and behaviours. Animal were slaughtered at 171 (sd 2·8) d of age at the research station abattoir after being fasted for approximately 15 h, according to the Swiss Cantonal Committee procedures.
Intestinal segments from the third-metre distal to the pylorus were removed within 15 min after exsanguination. The intestinal content was removed with cold saline solution (4°C) and tissues were further stored in a serosal buffer solution (see the following). Before mounting in the Ussing chamber device (Physiologic Instruments), tissues were stripped of outer muscle layers. Each experiment started within 30 min from the tissue recovery. The intestinal tissues used for the ex vivo experiments were collected by pigs bred in standard conditions without any experimental nutritional intervention. After slaughtering, some samples were collected for quality analysis and genotyping and the rest of the carcass entered the normal market chain of pig meat. The collection of the jejunum for our study was a complementary analysis.
A minimum of seven independent Ussing chamber experiments were performed. Each experiment was carried out using intestinal tissues from one pig mounted in eight different chambers. In each experiment, two chambers were randomly assigned to an experimental group and control group. Consequently, a minimum of seven biological and fourteen technical replicated per group were used.
The sample size was justified by statistical power analysis using SYSTAT version 13.2. The main parameter used was Arg-induced ΔIsc in jejunum tissues. These were the other parameters: statistical model = one-way ANOVA, number of groups = three, standard deviation = 0·13, significance level = 0·05, power threshold = 80 % and effect size = 0·97. The power analysis resulted in a sample size of five.
Intestinal porcine enterocyte cell line--J2 cell culture conditions
IPEC-J2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 supplemented with 10 % porcine serum, 50 U/ml penicillin and 50 U/ml streptomycin. Cells were differentiated as described previously(Reference Schmidt, Kohrt and Brown17). Briefly, cells were seeded in 12 mm inside-diameter polycarbonate-filter inserts with pores of 0·4 µm diameter (Corning GmbH) and 80 000 cells/cm2 density. The basolateral and apical compartments were filled with 1·5 and 0·5 ml of culture medium, respectively. Cells were cultured in these conditions for 10 d after confluence was reached. Upon differentiation, the porcine serum concentration was reduced to 1 %. After 24 h of serum reduction, transepithelial resistance (TEER) was measured with an EVOM2 epithelial voltohmmeter (World Precision Instruments). Because TEER values exceeding 200 Ω × cm2 are generally considered acceptable(Reference Vergauwen, Verhoeckx, Cotter and Lopez-Exposito18), only monolayers with a TEER above 200 Ω × cm2 were used for absorption experiments. If not used for Ussing chamber experiments, differentiated cells were treated directly on transwells. At the end of the treatments, the medium was replaced with fresh 1 % porcine serum-containing DMEM/Ham’s F12, the TEER was measured and cells were harvested for further analysis.
Experimental procedures
To evaluate AA transport across intestinal epithelial cells, differentiated IPEC-J2 monolayers (exposed area of 0·33 cm2) or jejunum tissue (exposed area of 1 cm2) were mounted on an Ussing chamber. Differentiated cells were treated for 24 h with GA at concentrations of 0 (untreated, T0), 5 (T5), 25 (T25) or 50 (T50) µmol/l before to be mounted on the Ussing chambers. The chambers were filled with 4 ml Krebs–Ringer buffer (115 mmol/l NaCl, 2·4 mmol/l K2HPO4, 0·4 mmol/l KH2PO4, 1·2 mmol/l CaCl2, 1·2 mmol/l MgCl2 and 25 mmol/l NaHCO3 −). The serosal buffer (pH 7·4) also contained 10 mmol/l glucose as an energy source that was osmotically balanced with 10 mmol/l mannitol in the mucosal buffer (pH 7·4). Buffers were continuously perfused with a 95 % O2 and 5 % CO2 gas mixture. The temperature was maintained at 37°C by a circulating water bath. When using jejunum tissue, indomethacin was added in both the mucosal and serosal buffers at a final concentration of 0·01 mmol/l. Using a computer-controlled device, the transepithelial potential difference (TEER) and short-circuit current (Isc) were continuously monitored. Cells’ monolayers were voltage clamped at 0 mV by an external current after correction for solution resistance. Under these conditions, the absorption of cations and the secretion of anions resulted in an increase in Isc. Cells were equilibrated for 20 min before the mucosal addition of 5 mmol/l l-Glut, followed by the addition of l-Arg, l-Meth and l-Lys AA at the same concentration. The corresponding equimolar concentration of mannitol was added into the serosal compartment. At the end of the experiment, forskolin (10 µmol/l) was added in the serosal compartment to test cell viability.
AA uptake on tissues was performed with a similar protocol: after a stabilisation period of 20 min, the tissues were challenged for 30 min with 5 (T5) or 25 mmol/l (T25) of GA or with Krebs–Ringer buffer (T0) as a control. This incubation time was chosen to test the acute effects of GA and because the Ussing chamber ex vivo study compel to complete the experiments within 2–3 h, before the appearance of first signs of tissue suffering.
Following the equilibration phase, 10 mmol/l l-Glut was added to the mucosal buffer, followed by the addition of l-Arg at the same concentration. The addition of l-Meth and l-Lys ex vivo did not led to any electrical response in both experimental and control groups. Given that was not possible to perform a proper data analysis, they have been excluded from the experiments (data not showed). Each addition was kept in an equilibrated osmotic condition by the addition of equimolar (10 mmol/l) mannitol on the serosal side. Each AA was added 15 min after the previous addition, and forskolin (10 µmol/l) was added in the serosal compartment at the end of the experiment to test tissue viability.
Apoptosis in intestinal porcine enterocyte cell line-J2 cells
For this experiment, IPEC-J2 cells were seeded in six well plates containing coverslips in each well for cell adherence. After 24 h, cell monolayers were treated as previously described. Cells incubated with medium and without GA (T0) were used as the untreated control. After incubation, the culture medium was removed, and the cells were washed with PBS and fixed with 1 % paraformaldehyde. The IPEC-J2 cells were then evaluated by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling Assay kit (Roche Applied Science) for apoptosis according to the manufacturer’s instructions and as previously described(Reference Jung, Hu and Saif19). IPEC-J2 cells treated only with trypsin (10 μg/ml) were also tested as a positive control for the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assay.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from the cells using an RNeasy Mini Kit (Macherey-Nagel), following the manufacturer’s instructions. The extracted RNA concentrations were measured with a NanoDrop spectrophotometer (Witec). The RNA integrity was assessed by the Qsep100 system (Lab-Gene Instruments). Synthesis of cDNA was performed starting from 250 ng RNA using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific). The PCR was then performed using the KAPA SYBR FAST Universal Kit (Sigma-Aldrich). The primers for determination of the mRNA expression of claudin-1 (CLDN1), occludin (OCLN) and zonula occludens-1 (ZO-1) (Table 1) were used at a final concentration of 200 nmol/l with a PCRmax Eco real-time PCR device (PCRmax). Glyceraldehyde 3-phosphate dehydrogenase was used as a housekeeping gene for data normalisation. The amplification profile was composed of an activation step of 5 min at 95°C followed by forty cycles of two-step amplification (5 s at 95°C and 20 s at 60°C). Relative expression was determined using the ΔΔCt method with efficiency correction(Reference Pfaffl20). All the primers were designed using Primer-BLAST(Reference Ye, Coulouris and Zaretskaya21).
Table 1. Primers used for real-time PCR
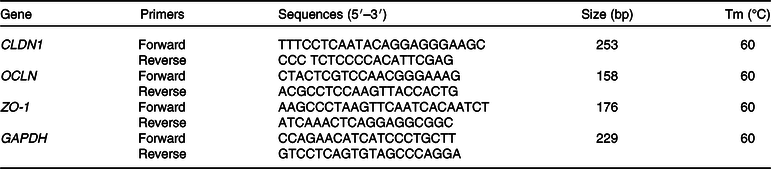
CLDN1, claudin-1; OCLN, occluding; ZO-1, zonula occludens-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Tm, melting temperature.
Western blot
After treatment, the cells and tissues were lysed in CelLytic M and CelLytic MT, respectively, complemented with protease inhibitors. After centrifugation at 12 000 g at 4°C for 10 min, the protein concentration in the supernatant was determined using an Asys UVM 340 microplate reader (Biochrom). Twenty microgram of total protein extracts was denatured at 95°C for 5 min, separated via 7 or 10 % SDS-PAGE gel (polyacrylamide: 30 % w/v, acrylamide: 0·8 % w/v and bisacrylamide (37·5:1)); 1·5 m Tris HCl, pH 8·8; SDS 0·4 %; ammonium persulfate 10 % and tetramethylethylenediamine 0·01 %. Proteins were then blotted on a WesternBright polyvinylidene difluoride membrane (Witec) at 90 V for 90 min using the Bio-Rad Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked for 60 min at room temperature with 5 % bovine serum albumin. The following primary antibodies were used to incubate the membranes at 4°C overnight: anti-ZO-1 (Ab216880, Abcam), anti-OCLN (Ab31721, Abcam), anti-CLDN1 (Ab129119, Abcam), anti-EAAT3 (Ab124802, Abcam), anti-CAT-1 (sc33087, Santa Cruz) and vinculin (V4505, Sigma-Aldrich). All except the vinculin were diluted 1:1000 in 0·1 % Tween 20, in PBS containing 5 % bovine serum albumin. Only the vinculin was diluted 1:2000. For all the primary antibodies, the secondary antibody was goat-anti-rabbit (A9169, Sigma-Aldrich) diluted 1:1000 in 0·1 % Tween 20, in PBS containing 5 % milk powder, whereas goat-anti-mouse (DC02L, Merck) diluted 1:3000 in the same buffer was used as the secondary antibody for the vinculin. Chemiluminescence signals were detected using a Quantum-Kit (Witec). Western blots were quantified by measuring the intensity of the correctly sized bands using a Syngene G:BOX (Syngene). The ratio of the intensities of the protein bands of interest v. the housekeeping protein was calculated for each filter, and the ratios from different Western blot filters were used to determine protein abundance.
Reagents
If not otherwise stated, all the chemicals used in the present study were purchased by Sigma-Aldrich Chemie. DMEM/Ham’s F12 culture medium was purchased by Amimed (Bioconcept Ltd) and the fetal bovine serum, penicillin and streptomycin were purchased by Chemie Brunschwig. All oligonucleotides cited in this work were synthesised by Microsynth.
Statistical methods
The data were analysed using IBM SPSS Statistics version 24 (SPSS) and are presented as mean values with their standard errors of means. The results have been obtained in a minimum of three independent experiments, and for Ussing chamber experiments, in a minimum of seven independent assays. The data were tested for normality with the Shapiro–Wilk test. The statistical analysis for in vitro Ussing chamber experiments was performed with the non-parametric Mann–Whitney U test, as the data were not normally distributed. The results of the Ussing chamber experiments using intestinal jejunum segments were tested with a mixed linear model in which the pig was considered a random effect and which was calculated using the Bonferroni correction. Differences between the control and treatment groups were considered significant when P < 0·05.
Results
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling staining revealed that gallic acid-treated intestinal porcine enterocyte cell line-J2 cells may not undergo apoptosis
When IPEC-J2 cells were treated with T5, T25 and T50 for 24 h, most or large numbers of cells failed to show terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling-positive signals (data not showed), indicating that no apoptotic processes take place in treated cells.
Effects of gallic-acid concentrations on small-intestinal epithelial cells’ integrity and on claudin-1, occludin and zonula occludens-1 mRNA expression in small-intestinal epithelial cells
The TEER value of IPEC-J2 cells decreased (−36 %, P = 0·03) when cells were incubated with T50 (Fig. 1) compared with T0. However, no differences in TEER values were observed in cells incubated with T5 or T25 compared with the control (Fig. 1).
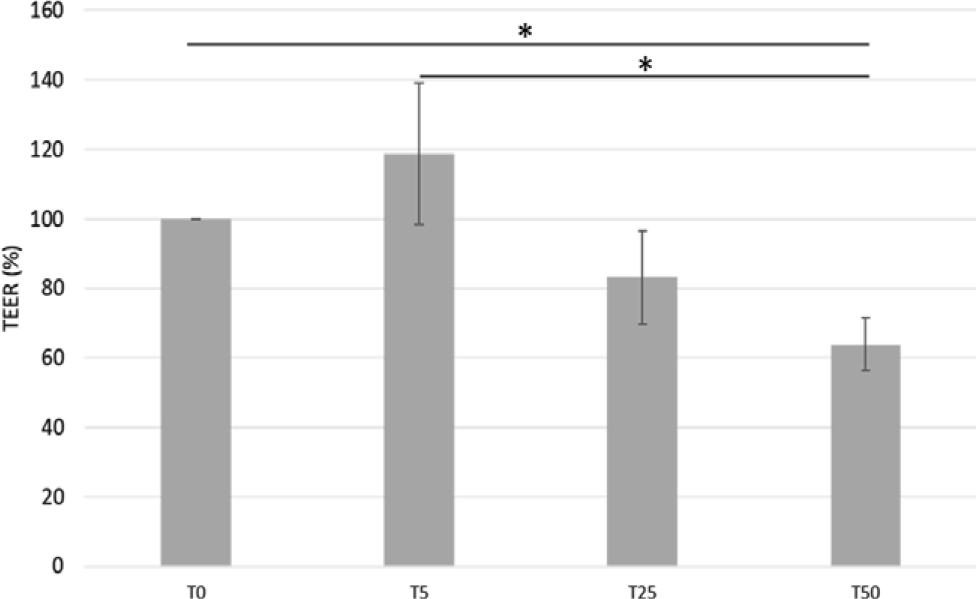
Fig. 1. Effects of gallic acid (GA) concentrations on intestinal porcine enterocyte cell line J2 (IPEC-J2) transepithelial resistance (TEER) values. Data are expressed as percentage of control (untreated IPEC-J2 cells). TEER 100 % corresponds to a 622 Ω × cm2 TEER. Values are means, with their standard errors represented by vertical bars. * P < 0·05. T0, GA at 0 μmol/l; T5, GA at 5 μmol/l; T25, GA at 25 μmol/l; T50, GA at 50 μmol/l.
Accordingly, incubation with T50 reduced (−66 %, P = 0·02) protein expression of CLDN1 compared with untreated cells and cells incubated with T5 and T25 (Fig. 2(a)). Moreover, regardless of the GA treatment, protein expression of ZO-1 and OCLN was unaffected compared with T0 (Fig. 2(a)). No effects of GA concentrations on tight-junction-protein mRNA expression levels have been observed in small-intestine epithelial cells, with the exception of a tendency (P = 0·07) of decreased CLDN1 mRNA expression in T50 compared with T0 (Fig. 2(b)).
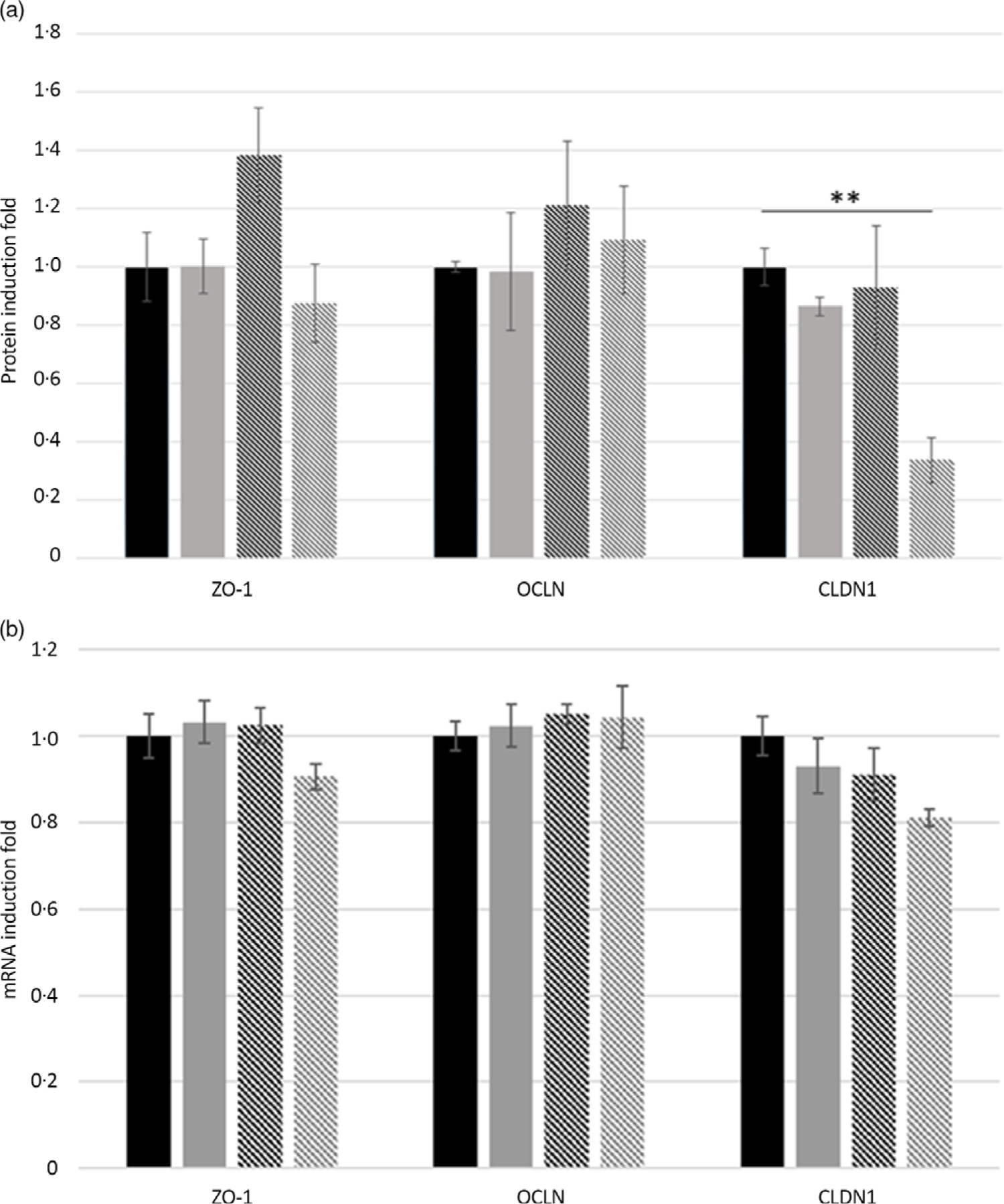
Fig. 2. Effects of gallic acid (GA) concentrations on tight-junction protein expression in intestinal porcine enterocyte cell line J2 (IPEC-J2) cells. Data are expressed as fold change of control (untreated IPEC-J2). (a) Zonula occludens-1 (ZO-1), claudin-1 (CLDN1) and occludin (OCLN) protein-induction fold in IPEC-J2 cells incubated for 24 h with GA at 0, 5, 25 or 50 μmol/l (T0, T5, T25 or T50). (b) Effects of GA concentrations on CLDN1, OCLN and ZO-1 expression in the small-intestinal epithelial cell line (IPEC-J2). Cells were cultured for 24 h in medium containing T0, T5, T25 or T50. CLDN1, OCLN and ZO-1 mRNA expression levels were detected by real-time PCR. Values are means, with their standard errors represented by vertical bars. Results are from four different experiments. ** P < 0·01. ![]() , T0;
, T0; ![]() , T5;
, T5; ![]() , T25;
, T25; ![]() , T50.
, T50.
Given the results obtained with cells with T50 treatment, this concentration of GA was not further tested on tissues.
Effects of gallic acid on amino-acid uptake in intestinal porcine enterocyte cell line-J2 cells
Because the highest concentration of GA (T50) had detrimental effects on TEER in IPEC-J2 cells compared with untreated cells (Fig. 1), corresponding data are not shown. As a consequence of L-AA addition to the apical buffer during the experiment, the Isc changed, indicating a transepithelial net-ion transfer (Table 2). Specifically, incubation for 24 h with T5 increased l-Arg (+83 %, P = 0·018) and l-Lys (+41 %, P = 0·013)-induced ΔIsc in IPEC-J2 cells. Given the increased uptake of the two cationic AA, l-Arg and l-Lys, we examined the protein expression levels of CAT-1 in IPEC-J2 cells incubated for 24 h with T5 and T25. However, no significant differences between treated cells and the control were found (Fig. 3).
Table 2. Amino-acid-induced ΔIsc (µA) in IPEC-J2 cells incubated with different concentrations of gallic acid (GA)
(Mean values with their standard errors)
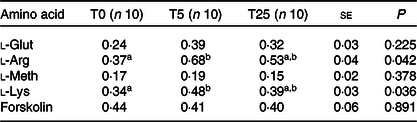
Isc, short-circuit current; IPEC-J2, intestinal porcine enterocyte cell line J2; T0, GA at 0 μmol/l; T5, GA at 5 μmol/l; T25, GA at 25 μmol/l; l-Glut, l-glutamate; l-Arg, l-arginine; l-Meth, l-methionine; l-Lys, l-lysine.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
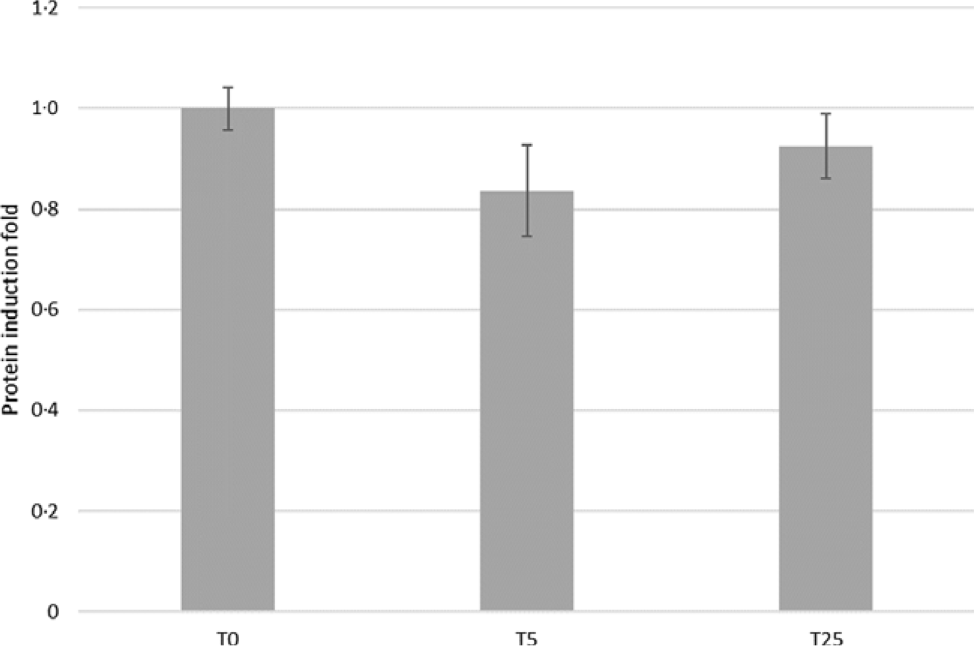
Fig. 3. Effects of gallic acid (GA) concentrations on cationic-amino-acid transporter-1 protein expression in intestinal porcine enterocyte cell line J2 (IPEC-J2) cells. Cells were incubated for 24 h with GA at 0, 5 or 25 μmol/l (T0, T5 or T25). Values are means, with their standard errors represented by vertical bars.
Effects of gallic acid concentrations on jejunum epithelial cells’ integrity
TEER values after 30 min of incubation with GA (T5, T25 and T50) never dropped below 80 % compared with the control tissues (Fig. 4). In the jejunum, T25 and T50 decreased the protein expression of ZO-1 (−46 and −49 %, P = 0·04 and P = 0·02, respectively) and CLDN1 (−48 and −27 %, respectively, P < 0·001) compared with T0. Treatment with T50 showed a higher (+21 %, P = 0·02) CLDN1 protein expression compared with T25 and a lower (−53 %, P < 0·001) OCLN protein expression compared with T0. Similarly, T5 decreased (−33 %, P < 0·001) CLDN1 protein expression compared with untreated jejunum segments, but it increased the protein expression of OCLN compared with the T25 (+46 %, P = 0·002) and T50 (+72 %, P < 0·001) groups (Fig. 5).
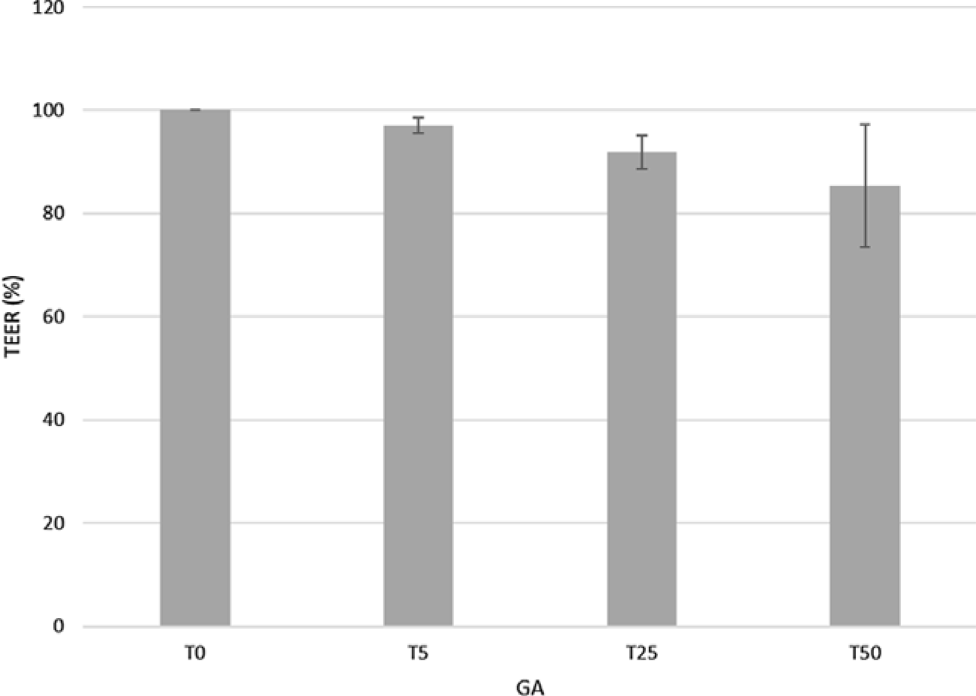
Fig. 4. Effects of gallic acid (GA) at 5, 25 or 50 μmol/l (T5, T25 and T50) on transepithelial resistance (TEER) values (%) compared with untreated jejunum samples. TEER 100 % corresponds to a 58·2 Ω × cm2 TEER. Values are means, with their standard errors represented by vertical bars.
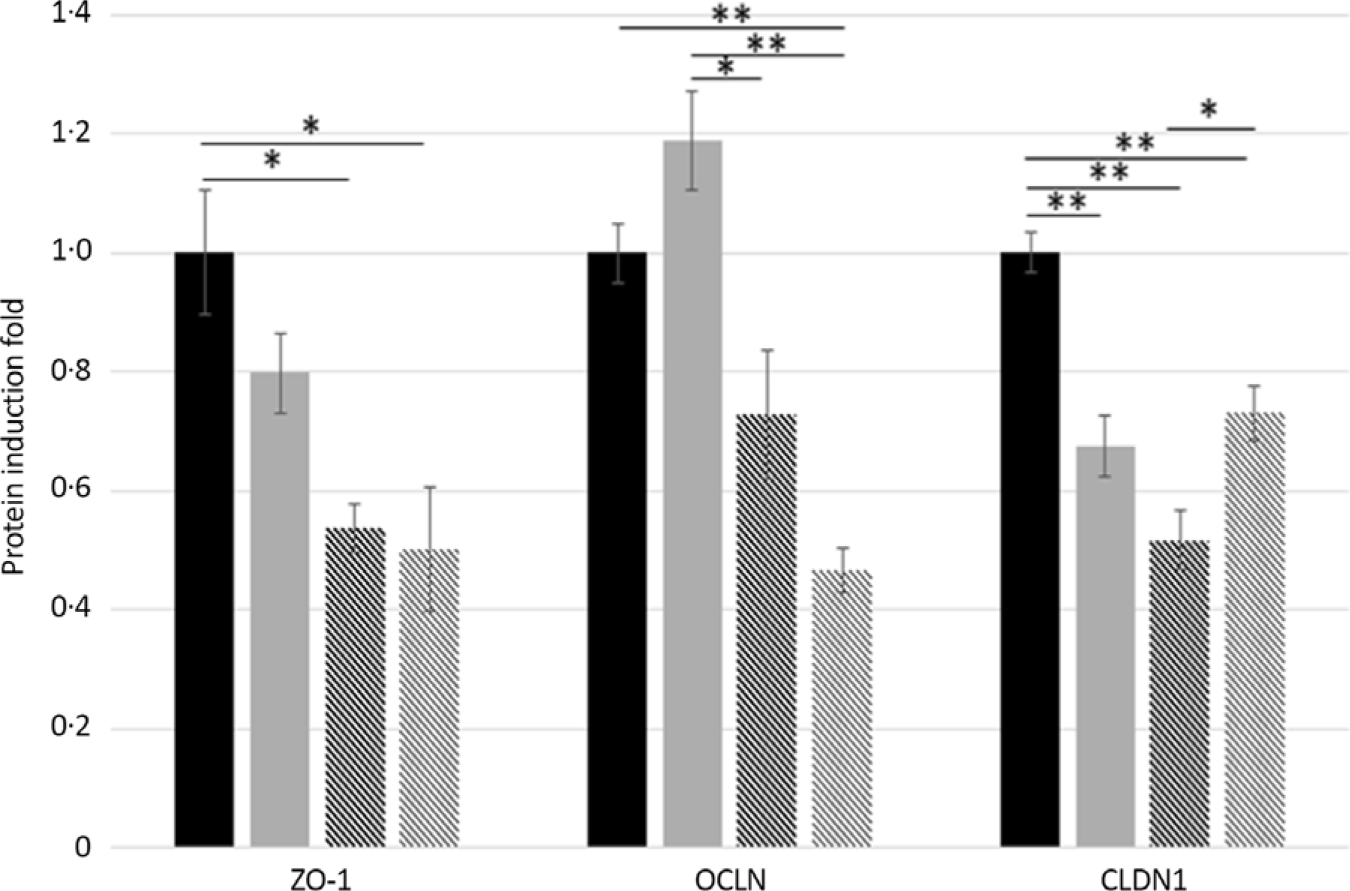
Fig. 5. Effects of gallic acid (GA) concentrations on tight-junction protein expression in pig jejunum. Zonula occludens-1 (ZO-1), claudin-1 (CLDN-1) and occludin (OCLN) protein-induction fold in pig jejunum segments incubated for 30 min with GA at 0, 5, 25 or 50 μmol/l (T0, T5, T25 or T50). Values are means, with their standard errors represented by vertical bars. * P < 0·05, ** P < 0·001. ![]() , T0;
, T0; ![]() , T5;
, T5; ![]() , T25;
, T25; ![]() , T50.
, T50.
Effects of gallic acid on amino-acid uptake in pig jejunum
In order to compare the in vitro effects of GA to ex vivo conditions, we tested the effects of three GA levels on l-Glut and l-Arg in pig jejunum intestinal segments. Here, we did not investigate the uptake of l-Meth because no effect was observed in the IPEC-J2 experiments. Moreover, the same cationic AA transporter is involved in the uptake of l-Arg and l-Lys. Incubation of jejunum segments with T25 led to a higher l-Glut- and l-Arg-induced Isc compared with untreated tissues (+85 and +109 %, P = 0·02 and P = 0·01, respectively). The results are summarised in Table 3.
Table 3. l-Glut- and l-Arg-induced ΔIsc (µA) in jejunum samples incubated for 30 min with two different concentrations of gallic acid (GA) compared with untreated tissue (T0)
(Mean values with their standard errors)

l-Glut, l-glutamate; l-Arg, l-arginine; Isc, short-circuit current; T5, GA at 5 μmol/l; T25, gallic acid at 25 μmol/l.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Western blot analysis of proteins extracted from jejunum segments and incubated for 30 min with T5 and T25 showed that, despite a higher l-Glut uptake in tissues treated with T25, no significant differences were observed in the EAAT3 protein expression between treated tissues and the control (Fig. 6). Conversely, the increased l-Arg uptake in jejunum segments incubated with T25 was confirmed by a higher (+20 %, P = 0·03) CAT-1 protein expression (Fig. 6).
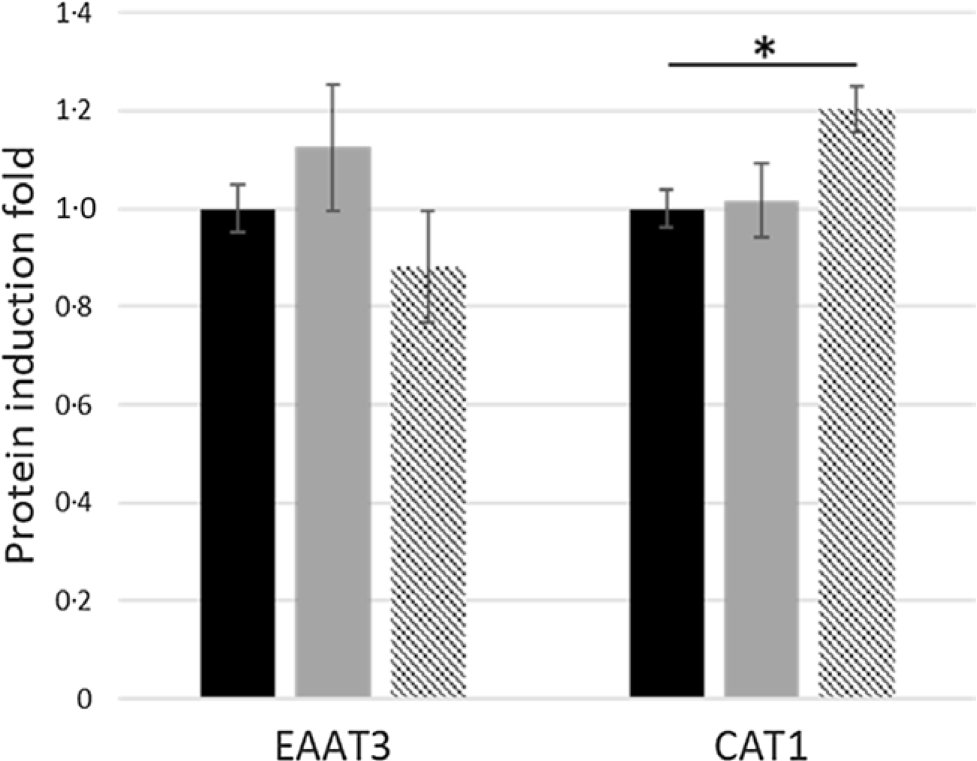
Fig. 6. Effects of gallic acid (GA) concentrations on excitatory-amino-acid transporter-3 (EAAT3) and cationic-amino-acid transporter-1 (CAT-1) protein-induction fold in pig jejunum segments incubated for 30 min with GA at 5 or 25 μmol/l (T5 or T25) v. control tissues (T0). Values are means, with their standard errors represented by vertical bars. * P < 0·05. ![]() , T0;
, T0; ![]() , T5;
, T5; ![]() , T25.
, T25.
Discussion
To date, there are limited studies about the effect of GA on intestinal integrity and AA absorption.
The literature demonstrated that plant polyphenol can affect growth performance in monogastric animals(Reference Karasov, Meyer and Darken3–Reference Girard and Bee5,Reference Tretola, Maghin and Silacci22) . To assess the biological effects of GA, it is important to acquire knowledge about its modulatory effects on intestinal epithelial functionality. Several attempts have been made to develop models based on human intestinal cell lines that mimic the functional and morphological organisation of intestinal epithelium. IPEC-J2 cells are porcine intestinal enterocytes that mimic the human physiology more closely than any other cell line of non-human origin. They are therefore ideal tools with which to study epithelial transport and the effect of nutrients on a variety of widely used parameters reflecting epithelial functionality (i.e. TEER). IPEC-J2 cells undergo a culture process of spontaneous differentiation that leads to the formation of a polarised monolayer with low TEER when porcine serum is added to the culture medium, enabling comparison with the in vivo situation(Reference Vergauwen, Verhoeckx, Cotter and Lopez-Exposito18,Reference Zakrzewski, Richter and Krug23) . However, in vitro, ex vivo and in vivo cells or tissue can respond differently to environmental stimuli (e.g. diet)(Reference Vergauwen, Verhoeckx, Cotter and Lopez-Exposito18,Reference Cheli, Giromini and Baldi24) . The results obtained with IPEC-J2 cells were compared with those obtained with ex vivo pig jejunum to evaluate the two models against each other. In addition, the Ussing chamber is an efficient model for studying intestinal transport and modulation of tight junctions(Reference Bergmann, Rogoll and Scheppach25). Concentrations applied in in vitro and ex vivo studies commonly range from low µmol/l to mmol/l. In the present study, polyphenol concentrations were chosen according to the concentrations found in human large intestine(Reference Kahle, Kraus and Scheppach26). However, in several human studies plasma metabolites concentration rarely exceed nmol/l(Reference Manach, Williamson and Morand27).
Even if the polyphenol concentration in vivo could be lower, it has to be considered that the cells are consistently exposed to it. Thus, elevated in vitro doses can be useful to ‘facilitate’ an outcome, but such results must be extrapolated with care.
In the IPEC-J2 model, GA at a concentration of 50 µmol/l did not affect cell viability, as demonstrated by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assay. It markedly decreased TEER to approximately 60 % of the initial value, however, suggesting that the integrity of the IPEC-J2 monolayer was affected, whereas a lower concentration of GA did not significantly affect TEER compared with the control. On the other hand, the highest concentration of GA decreased TEER to approximately 80 % of the initial value in stripped porcine jejunum, suggesting that the native porcine jejunum is less susceptible to the action of GA than IPEC-J2 cells. A reason for this could be the different incubation time between tissues and IPEC-J2 cells. In contrast to the in vitro model, the ex vivo Ussing chamber experiment does not allow to choose long incubation times without having detrimental effects on tissue viability. However, it was useful to investigate acute effects of GA on pig jejunum. The extrapolation of in vitro data to the in vivo situation is always problematic. However, we speculate that our ex vivo model could represent a good compromise(Reference Cheli, Giromini and Baldi24). Another possible reason for the differences observed between the in vitro and ex vivo models is the different complexity between the two tested models. The intestinal mucosa is characterised by the presence of villi, which constitute the anatomical and functional unit for nutrient absorption. They consist of an epithelial layer, the lamina propria and the muscularis mucosa. IPEC-J2 alone does not fully represent the morphology and physiology of the gastrointestinal tract, the latter consisting of a number of other cell types, such as goblet cells, which do not form tight junctions(Reference Srinivasan, Kolli and Esch28). The primary function of the goblet cells is to provide a mucus layer involved in both protection and nutrient uptake by secreting mucins.
By including these cells, the strength of the barrier function of such models is altered, rendering them more permeable and physiological(Reference Srinivasan, Kolli and Esch28). Consequently, we speculate that IPEC-J2 cells are more sensitive to the action of GA in TEER than the pig jejunum, enhancing the effects of GA on the intestinal epithelial cells’ integrity compared with ex vivo models. In both in vitro and ex vivo models, however, our results showed that incubation with T50 had an adverse effect on intestinal integrity. By contrast, lower GA concentrations had no detrimental effects on the TEER of intestinal epithelial cells’ integrity in vitro, but they influenced the protein expression of specific tight-junction proteins and increased the uptake of selected AA. Our study indicates some similarities between IPEC-J2 and tissue models, as far as the sensitivity of AA uptake to acute GA exposure. In IPEC-J2, cationic AA uptake (l-Lys and l-Arg) increased following exposure to T5, whereas in jejunum, cationic AA uptake (l-Arg) increased in tissues incubated with T25. Additionally, treatment with T25 increased l-Glut uptake in jejunum. At first glance, these results suggest a lesser anti-nutritional effect with GA compared with tannic acid, which decreased nutrient uptake in mouse intestine(Reference Karasov, Meyer and Darken3).
The increased AA uptake observed in our study suggests that GA does not strongly interact with luminal membrane or mucin proteins. A strong interaction, in fact, should lead to a partial blockage of nutrient absorption across the brush-border membrane(Reference Karasov, Meyer and Darken3). In our study, we found that T5 and T25 increased cationic AA uptake in IPEC-J2 and pig jejunum, respectively. For this reason, we speculate that GA could present less anti-nutritional effects in monogastric animals compared with other natural polyphenols used to counteract afflictions like post-weaning diarrhoea. There is increasing evidence that CAT-mediated transport can be an important determinant of cationic-AA-related reactions(Reference San Martín and Sobrevia29). In mammals, four different cationic-AA transport systems have been identified (y+, y+L, b0,+ and B0,+). Of these, the y+ system (Na+-independent uptake of l-Lys, l-Arg, l-Orn and l-histidine) is expressed on both the apical and basolateral membranes of absorptive small-intestinal epithelial cells. Four proteins (CAT-1, CAT-2a, CAT-2b and CAT-3) are known to possess y+ system activity. Of these, CAT-1 is the only one abundantly expressed by the small-intestinal epithelium, possessing a high affinity for l-Lys and l-Arg(Reference Hatzoglou, Fernandez and Yaman13). The relative steady-state expression levels of CAT-1 are considered proportional to CAT-1 activity(Reference Fernandez, Yaman and Mishra30), indicating that the content of CAT-1 likely represents relative CAT-1 activity. In our study, even when GA increased cationic AA uptake in both IPEC-J2 and pig jejunum, it was only in tissues where we observed a corresponding increase in CAT-1 protein expression. This difference could be due to the varying incubation times for the two models (24 h for IPEC-J2 and 30 min for jejunum). Indeed, it was observed that, in the presence of a specific stimulus, the induction of translation of the CAT-1 mRNA is transient, decreasing to baseline levels after 18 h, which highlights that timing is important in evaluating the expression of genes during cellular adaptation processes(Reference Hatzoglou, Fernandez and Yaman13). It will therefore be necessary for future studies on AA uptake to take into account the timing of cellular response for polyphenolic-compound incubation.
Glutamate represents one of the major constituents of human dietary protein and it is considered the precursor of several non-essential AA(Reference Janeczko, Stoll and Chang31). Moreover, it attenuates oxidative stress, inflammation and permeabilisation of intestinal epithelium when stressed with mycotoxin deoxynivalenol(Reference Wu, Xiao and Ren32). In the present study, we observed an increased l-Glut-induced Isc in jejunum incubated with T25, but no effects on EAAT3 protein expression were observed, suggesting that a different transporter could be accountable for the increased glutamate transport induced by GA.
Conclusions
The results obtained in the present study demonstrate that GA at lower concentrations (<50 µmol/l) has no detrimental effect on TEER neither in IPEC-J2 cells nor in jejunum. However, the protein expression of specific tight-junction proteins may be affected. In addition, GA positively affected the uptake of selected cationic AA by intestinal epithelial cells, probably mediating CAT-1 expression regulation. Ex vivo, GA also improved the uptake of l-Glut, but further studies are necessary to clarify the mechanisms involved in such nutrient-uptake regulation. A comparison between in vitro and ex vivo models highlighted some important differences regarding evaluation of the effects of GA on intestinal-membrane integrity, where in vitro models seem to be more sensitive than native intestinal segments to GA although different experimental conditions have been applied. Similarities in the evaluation of GA effects on nutrient uptake have been observed, however, even if a higher-intensity response after luminal AA addition in Ussing chamber experiments made data analysis and interpretation more clear ex vivo compared with the in vitro model.
Acknowledgements
The authors would like to thank Claudine Biolley, Tamara Gobet, Carmen Vonnez and Bernard Dougoud for their precious help with laboratory analysis and tissue collection.
The presented work was totally financed by ordinary budget of Agroscope.
Conceptualisation: G. B., P. S. and M. T.; methodology: G. B., P. S. and M. T.; formal analysis: M. T.; investigation: M. T.; resources: G. B. and P. S.; data curation: G. B., P. S. and M. T.; writing – original-draft preparation: M. T.; writing – review and editing: G. B., P. S. and M. T.; supervision: G. B. and P. S. All the authors have read and agreed to the published version of the manuscript.
There are no conflicts of interest.












