Both protective and detrimental effects of ethanol on insulin sensitivity have been widely reported(Reference Joosten, Beulens and Kersten1–Reference Kao, Puddey and Boland6). The majority of researchers have suggested that the differential effects of ethanol on insulin sensitivity are primarily due to the dosage of ethanol consumed(Reference Bell, Mayer-Davis and Martin7–Reference Zilkens, Burke and Watts11). In addition, a few researchers have proposed that drinking pattern also plays an important role in mediating the effects of ethanol(Reference Dorn, Hovey and Muti12–Reference Trevisan, Dorn and Falkner18). However, the ethanol consumption pattern is quite complex and includes the frequency of ethanol consumption in addition to the food setting. The present study was designed to investigate the influence of daily ethanol consumption frequency on insulin sensitivity in a high-fat (HF) diet setting. To the best of our knowledge, this is the first study that focused on daily ethanol consumption frequencies, but not in a long time period.
Over the past few years, the number of studies that have investigated the effects of ethanol in combination with a HF diet has increased. The underlying reason for this increase is that alcohol consumption is often accompanied by intake of a HF diet. To date, reports on the effects of ethanol plus a HF diet on insulin sensitivity remain controversial. Some studies have shown that a combination of alcohol consumption and HF diet resulted in decreased glucose uptake in skeletal muscle and in adipose tissue, leading to a high incidence of diabetes(Reference Wilkes, DeForrest and Nagy19, Reference Wilkes and Nagy20). In contrast, we and others have found that ethanol consumption improved insulin resistance induced by a HF diet(Reference Fromenty, Vadrot and Massart3, Reference Feng, Gao and Guan21–Reference Paulson, Hong and Holcomb23). Similar to the present and Hong's results, Fueki et al. (Reference Fueki, Miida and Wardaningsih24) found that regular alcohol consumption improved insulin resistance in healthy Japanese men, independent of obesity. In addition to the unclear effect of ethanol and HF diet on insulin sensitivity, the underlying mechanisms of this effect are also obscure. Here, we evaluated the insulin sensitivity of HF diet-fed rats after ethanol treatments with different frequencies and explored potential mediating mechanisms by determining the expression levels of AMP-activated protein kinase (AMPK)α, PPARγ and GLUT4. In addition, we also measured adiponectin, a known insulin sensitiser and upstream activator of AMPK, in both adipose tissue and sera.
Experimental methods
Animal feeding
Initially, forty-eight male Wistar rats (weight, 160–180 g; age, 4–6 weeks) were acclimatised to a HF diet for 1 week. Based on energy content, the HF diet consisted of 59 % fat from lard, 24 % carbohydrate and 17 % protein. The acclimatised rats were randomly divided into four groups according to weight, and they received ethanol with varying administration patterns: ad libitum consumption of tap water without ethanol (controls, C); twice daily administration of ethanol (E1, 5 g/kg per d); continuous drinking of ethanol (E2, 5 g/kg per d); once daily administration of ethanol (E3, 5 g/kg per d). The animals in groups E1 and E3 received ethanol via a gastric tube. Body weights were monitored and ethanol volumes were adjusted weekly. Unfortunately, a portion of the animals in group E3 died within the first 2 months, and we could only provide the complete data for groups C, E1 and E2 in the present study.
All rats were purchased from the Laboratory Animal Center of Shandong University (Jinan, China). During the period of treatment, rats were housed in individual cages in a temperature-controlled room (24°C) on a 12 h light–12 h dark cycle. Water was available ad libitum. The animal study was approved by the Shandong University Institutional Animal Care and Use Committee.
Oral glucose tolerance test
An oral glucose tolerance test was carried out at the end of 8 and 18 weeks. After overnight fasting, rats received a glucose solution (2 g/kg body weight) via a gastric tube. Blood glucose levels were measured from whole blood samples obtained by tail bleeding at 0, 30, 60 and 120 min after the glucose load was administered. Blood glucose (BG) concentrations were determined using a OneTouch SureStep Meter (Life Scan, Milpitas, CA, USA). The area under the curve (AUC = 1/4 BG (0 min)+1/2 BG (30 min)+3/4 BG (60 min)+1/2 BG (120 min)) was calculated to assess glucose tolerance.
Determination of plasma ethanol concentration
Blood samples were obtained from the inferior vena cava 40 min after gastric tube administration of ethanol in groups E1 and E3 and after the removal of ethanol in group E2. Plasma ethanol concentrations were determined with a dry chemical method (Johnson & Johnson, New Brunswick, NJ, USA).
Tissue collection
Most rats in group E3 died within the first 2 months of the experiment. The remaining five rats in group E3 were killed after 8 weeks of feeding; the rats in groups E1 and E2 were killed at the end of week 18. After a 10 h fast, animals were anaesthetised with an intraperitoneal injection of sodium pentobarbital (0·1 ml/100 g body weight), and blood samples were obtained from the inferior vena cava for chemical analyses, including determination of glucose, insulin and adiponectin levels. The epididymal and perirenal fat pads were rapidly removed and weighed for the calculation of the relative adipose tissue weight compared with body weight. The epididymal adipose tissues were frozen in liquid N2 for mRNA and protein analyses.
Biochemical analysis and evaluation of insulin sensitivity
Blood glucose levels and insulin concentrations were measured using the glucose oxidase method and RIA (Northern Bioengineering Institute, Beijing, China), respectively. Adiponectin concentrations in both adipose tissue and sera were, respectively, measured using an ELISA kit (adiponectin; Bionewtrans Pharmaciutical Biotechnology Company Limited, Franklin, MA, USA), and then total adiponectin contents in adipose tissue of each rat were calculated according to adipose tissue weight. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula(Reference Matthews, Hosker and Rudenski25):
RNA extraction and RT-PCR
Total RNA was extracted from epididymal adipose tissues using the standard Trizol RNA isolation method. The quality of RNA was checked by using the DU640 nucleic acid analyser (Beckman, Fullerton, CA, USA). Reverse transcription of 4 μg RNA from each sample was carried out using the RevertAid™ First Strand cDNA Synthesis Kit (no. K1622; Fermentas, Beijing, China) according to the manufacturer's instructions.
PCR amplification was carried out as described previously(Reference Feng, Gao and Guan21, Reference Feng, Song and Guan26). All primers were synthesised by Shanghai Sangon Biotechnology Corporation (Shanghai, China), and the sequences were as follows: AMPKα1, 5′-ggg atc cat cag caa cta tcg-3′ (sense) and 5′-ggg agg tca cgg atg agg-3′ (antisense), accession no. NM_019142; AMPKα2, 5′-cat ttg tgc aag gcc cct agt-3′ (sense) and 5′-gac tgt tgg tat ctg cct gtt tcc-3′ (antisense), accession no. NM_023991; GLUT4, 5′-ggg ctg tga gtg agt gct ttc-3′ (sense) and 5′-cag cga ggc aag gct aga-3′ (antisense), accession no. NM_012751; PPARγ, 5′-tgt gga cct ctc tgt gat g-3′ (sense) and 5′-cat tgg gtc agc tct tgt ga-3′ (antisense), accession no. EV468317; glyceraldehyde-3-phosphate dehydrogenase, 5′-tgg tgg acc tca tgg cct ac-3′ (sense) and 5′-cag caa ctg agg gcc tct ct-3′ (antisense), accession no. XM_344448.
Western blotting
Total proteins were extracted from adipose tissues by using radio-immunoprecipitation assay lysis buffer supplemented with 1 mm-phenylmethylsulfonyl fluoride and Western blotting was carried out as described previously(Reference Feng, Gao and Guan21, Reference Feng, Song and Guan26). The primary antibodies were bought from Cell Signaling Company, Danvers, MA, USA (total AMPKα and phosphorylated AMPKα) and Abcam Limited, Cambridge, UK (PPARγ and GLUT4), respectively.
Data analysis
The data shown represent a minimum of three independent experiments. All values are presented as means and standard deviations. Data were analysed with SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA). After comparison by ANOVA, a least significant difference statistical test was performed for post hoc comparisons, with P < 0·05 considered to be statistically significant.
Results
Fasting glucose levels, fasting insulin concentrations and homeostasis model assessment of insulin resistance
Statistics were not performed on the data obtained at the end of the 8th week (Table 1) because of the small number of rats. However, despite equal total daily ethanol dosages, HOMA-IR was obviously reduced in group E1 and was slightly decreased in group E2 compared with group C. In contrast, HOMA-IR in group E3 was obviously increased. Despite administration of the same ethanol dosage each day, the different administration frequencies led to markedly different blood ethanol concentrations, which were 183 mg/l in group E1, only 43 mg/l in group E2 and 970 mg/l in group E3. As shown in Fig. 1, there was a U-shaped relationship between HOMA-IR and ethanol concentrations.
Table 1 Characterisation of the rats* (8 weeks)
(Mean values and standard deviations for five animals per group)
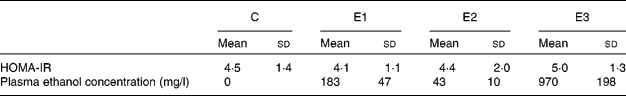
C, control; E1, rats that received ethanol twice per d; E2, rats that received ethanol continuously; E3, rats that received ethanol once per d; HOMA-IR, homeostasis model assessment of insulin resistance.
* Rats received a high-fat diet only (group C) supplemented with ethanol once (group E3) or twice (group E1) daily (total 5 g/kg) via a gastric tube, or with ethanol by drinking (group E2, total 5 g/kg daily) for 8 weeks.

Fig. 1 Relationship between homeostasis model assessment of insulin resistance (HOMA-IR) and ethanol concentrations. Initially, forty-eight male Wistar rats were randomly divided into four groups according to weight, and they received ethanol with varying administration patterns: ad libitum consumption of tap water without ethanol (controls, ×); twice daily administration of ethanol (5 g/kg per d, ▲); continuous drinking of ethanol (5 g/kg per d, □); once daily administration of ethanol (5 g/kg per d, ○). The data were obtained at the end of 8 weeks.
After the rats were fed the HF diet for 18 weeks (Table 2), elevated levels of fasting glucose and fasting insulin were observed in group C, but these had been decreased by 7·5 and 23·4 %, respectively, in group E1. However, the fasting plasma glucose and fasting insulin levels of group E2 were not statistically significant compared with those of group C. Accordingly, insulin resistance, which was evaluated by HOMA-IR, was observed in HF diet-fed rats, but was ameliorated with ethanol administration; the value of HOMA-IR was reduced by 30·8 % in group E1 and 9·2 % in group E2 (Fig. 2(a)).
Table 2 Characterisation of the rats (18 weeks)‡
(Mean values and standard deviations)

C, control; E1, rats that received ethanol twice per d; E2, rats that received ethanol continuously; E3, rats that received ethanol once per d; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance.
Mean values were significantly different from those of group C: * P < 0·05, ** P < 0·01.
† Mean values were significantly different from those of group E1 (P < 0·05).
‡ Rats received a high-fat diet only (group C) supplemented with ethanol twice daily (total 5 g/kg) via a gastric tube (group E1) or with ethanol by drinking (group E2; total 5 g/kg daily) for 18 weeks.

Fig. 2 Different daily ethanol consumption frequencies restore insulin resistance induced by a high-fat diet differently. A total of thirty-six male Wistar rats fed with a high-fat diet were divided into three groups: ad libitum consumption of tap water without ethanol (controls, C); twice daily administration of ethanol (E1, 5 g/kg per d); continuously drinking of ethanol (E2, 5 g/kg per d). The oral glucose tolerance test (OGTT) was carried out after an 18-week feeding period. Blood glucose levels were measured from samples obtained by tail bleeding at 0, 30, 60 and 120 min after the glucose load (2 g/kg body weight). The area under the curve (AUC = 1/4 BG (0 min)+1/2 BG (30 min)+3/4 BG (60 min)+1/2 BG (120 min)) was calculated to assess glucose tolerance (b). At 4 d after the OGTT, all rats were anaesthetised and blood samples were obtained from the inferior vena cava for the determination of glucose and insulin concentrations. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: fasting plasma glucose (mmol/l) × fasting insulin (microunits/ml)/22·5 (a). Values are means, with standard deviations represented by vertical bars (n 12).
Body weights and fat masses
The body weight and fat masses of rats at 18 weeks are shown in Table 2. Ethanol administration lessened the weight gain from the HF diet by 8·5 % in group E1 and by 3 % in group E2. Coincident with the reductions in body-weight gain, the epididymal and perirenal fat masses were reduced by 12·1 and 11·4 % in group E1, respectively, but no significant change was observed in the fat pad masses of group E2 relative to group C.
The area under the curve of the oral glucose tolerance test
An oral glucose tolerance test was carried out on rats after feeding a HF diet for 18 weeks, and the AUC was calculated. As shown in Fig. 2(b), the AUC in group E1 was reduced by 10·2 % relative to that in group C (P < 0·05 v. C), and it was not significantly different between groups E2 and C.
Adiponectin levels
The concentrations of adiponectin in sera and adipose tissue were recovered towards normal by 35·3 % (P < 0·01 v. C) and 24·5 % (P < 0·01 v. C), respectively, in group E1, but were only recovered by 15·7 % (P < 0·05 v. C) and 10·9 % (P < 0·05 v. C), respectively, in group E2 (Table 2). Correlation analysis results showed an intimate correlation between the tissue and serum levels of adiponectin (r 0·572, P < 0·01).
AMP-activated protein kinase activity
It is known that adiponectin is an activator for AMPKα. In parallel with the changes in adiponectin levels, the ratio of AMPKα (phosphorylated AMPKα):total AMPKα in rats that received ethanol administration twice per d and in those that drank ethanol continuously increased by 97·6 % (P < 0·01 v. C) and 17 % (P>0·05 v. C), respectively, compared with that of group C (Fig. 3(b)). Ethanol consumption had no influence on either AMPKα1 or α2 mRNA expression (Fig. 3a).

Fig. 3 Different daily ethanol consumption frequencies ameliorate AMP-activated protein kinase (AMPK) activation, but not expression in different degrees. After feeding the rats for 18 weeks, we determined (a) mRNA levels of AMPKα1 and α2 isoforms by RT-PCR and (b) protein levels of total AMPK (T-AMPK) and phosphorylated AMPK (pAMPK) by Western blotting. Values are means, with standard deviations represented by vertical bars (n 12). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
PPARγ expression
Relative to group C, PPARγ mRNA levels were increased by 62·9 % (P < 0·01 v. C) in group E1, but only by 20 % (P>0·05 v. C) in group E2. In accordance with increased PPARγ gene transcript abundance, PPARγ protein expression was increased by 43·8 % (P < 0·01 v. C) in group E1 and by 11·3 % (P>0·05 v. C) in group E2 (Fig. 4).

Fig. 4 Different daily ethanol consumption frequencies restore PPARγ expression differently. After the rats were fed for 18 weeks, using RT-PCR and Western blotting, we determined PPARγ expression both in (a) mRNA and (b) protein levels. PPARγ mRNA levels were normalised by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and protein levels were normalised by β-actin. Values are means, with standard deviations represented by vertical bars (n 12). E1, rats that received ethanol twice per d; E2, rats that received ethanol continuously; E3, rats that received ethanol once per d.
GLUT4 expression
In parallel with the augmentation of AMPK activation and PPARγ expression, both GLUT4 mRNA and protein expression were significantly increased by 38·3 and 12·7 %, respectively, in group E1 compared with those in group C (both P < 0·01 v. C). However, these levels were only elevated by 8·3 and 5·6 %, respectively, in group E2 relative to group C (both P>0·05 v. C; Fig. 5).

Fig. 5 Different daily ethanol consumption frequencies improve GLUT4 mRNA and protein expression differently. After feeding the rats for 18 weeks, we determined GLUT4 (a) mRNA levels by RT-PCR, (b) protein levels by Western blotting and (c) immunofluorescence ( × 200). GLUT4 mRNA levels were normalised by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and protein levels were normalised by β-actin. Values are means, with standard deviations represented by vertical bars (n 12). E1, rats that received ethanol twice per d; E2, rats that received ethanol continuously; E3, rats that received ethanol once per d.
Discussion
Previously, studies have shown that varying dosages, categories and drinking patterns of ethanol consumption resulted in different effects on insulin sensitivity(Reference Joosten, Beulens and Kersten1–Reference Kao, Puddey and Boland6). We presumed that all of these factors could lead to different plasma ethanol concentrations, which could subsequently result in differential effects on insulin sensitivity. Therefore, plasma ethanol concentration could be the underlying factor that determines ethanol action. In the present study, rats received ethanol at the same dosage of 5 g/kg per d, which is equivalent to an ethanol consumption of 48 g/d for a person whose body weight is 60 kg. At this same total daily ethanol dosage, variation of the daily ethanol consumption frequency led to markedly different effects. A daily ethanol administration frequency of twice per d was more beneficial than the continuous drinking pattern in the improvement of the adverse effect of a HF diet on insulin sensitivity. However, we did not verify whether this conclusion would also apply to daily ethanol dosages other than 5 g/kg per d. In fact, according to the initial design of the study, a group in which rats received ethanol once daily (group E3) at the dosage of 5 g/kg per d was also included. Unfortunately, a portion of the rats in group E3 died within the first 2 months of the study and only five rats survived to 8 weeks. Thus, we could not provide complete data for this group. However, after only 8 weeks of feeding a HF diet, we found that HOMA-IR was ameliorated in the groups that were administered ethanol via a gastric tube twice per d and by continuous drinking. In contrast, HOMA-IR was worsened in the group that was administered ethanol administration once per d. Thus, the twice-daily administration pattern showed a more beneficial effect on HF diet-induced insulin resistance than the once-daily or continuous drinking patterns.
Based on the present data, HOMA-IR was not positively related to the frequencies of ethanol consumption. Therefore, we determined the plasma ethanol concentration because this could be a mediating factor that influences the effect of ethanol on insulin sensitivity. Whereas there must be a fluctuation in ethanol concentration after the intake of ethanol, it was difficult for us to monitor these dynamic changes in ethanol concentrations due to the limitation of rat blood volume. Usually, ethanol will reach a peak concentration within 35–40 min after ingestion. Therefore, we chose to determine the peak concentration. We found that the plasma ethanol concentration was highest in group E3, lowest in group E2 and moderately elevated in group E1. We observed a U-shaped relationship between plasma ethanol concentration and insulin resistance. In other words, both low and high ethanol concentrations were associated with high insulin resistance, whereas moderate ethanol concentration was associated with improved insulin sensitivity in the setting of a HF diet. Based on these results, we deduced that there must be a concentration range at which ethanol can exert a beneficial effect on insulin sensitivity. Beyond this ethanol concentration range, a positive action might not be observed, and, on the contrary, a negative effect might be instead observed.
Our previous studies and the present study have shown that the intake of ethanol at certain dosages and frequencies can ameliorate HF diet-induced insulin resistance in rats, which is in agreement with the results of Dixon et al. (Reference Dixon, Dixon and O'Brien9) and Gigleux et al. (Reference Gigleux, Gagnon and St-Pierre27). These studies found that moderate alcohol intake decreased the risk of diabetes in extremely obese individuals. Furthermore, Hong et al. (Reference Hong, Smith and Harvey22) proposed several possible mechanisms by which ethanol could improve insulin sensitivity, such as by inhibiting gluconeogenesis (production of glucose by the liver), decreasing inflammation, increasing the production of factors that improve insulin sensitivity and increasing the production of insulin by the pancreas. In the present study, we found that ethanol improved HF diet-induced insulin resistance via increased adiponectin and PPARγ levels in both adipose tissue and sera, which led to enhanced AMPK activity and GLUT4 expression in adipose tissue. Previously, we have reported that ethanol influences insulin sensitivity via the AMPK pathway(Reference Feng, Gao and Guan21, Reference Feng, Song and Guan26), which was confirmed in the present study. We and others have found that the HF diet can significantly reduce plasma adiponectin levels(Reference Hu, Liang and Spiegelman28–Reference Arita, Kihara and Ouchi30) and that particular patterns of ethanol consumption can relieve this HF diet-induced inhibition of adiponectin to some extent, which is in accordance with other reports(Reference Joosten, Beulens and Kersten1, Reference Abel and Feher31–Reference Imhof, Plamper and Maier33). Adiponectin is a known agonist of AMPK(Reference Whitehead, Richards and Hickman34–Reference Yoon, Lee and Chung36); thus, a proposed hypothesis for the mechanism by which ethanol affects AMPK and GLUT4 levels is through an effect on adiponectin levels. This mechanism will be useful for the development of new drugs for the treatment of HF diet-induced insulin resistance, such as ethanolic products and treatments involving adiponectin or other AMPK agonists. In fact, some studies have demonstrated that adiponectin treatment can reverse insulin resistance that is associated with obesity(Reference Yamauchi, Kamon and Waki29).
The reported associations between ethanol consumption and insulin resistance have been conflicting. The first reason for the lack of a clear trend from these studies might be related to the complex effects of ethanol itself. The second reason is that the major factor that influences the effect of ethanol on insulin resistance has not been identified. Most researchers believe that the ethanol effect is associated with its dosage. In other words, light or heavy ethanol consumption leads to insulin resistance, whereas moderate ethanol consumption results in increased insulin sensitivity(Reference Bell, Mayer-Davis and Martin7–Reference Zilkens, Burke and Watts11). However, no universal definitions of light, moderate or heavy drinking exist because of the many factors that could affect the definition, including different ethanol contents, categories, drinking patterns and so on. Currently, most definitions are based on a certain number of drinks consumed within a specific time period(Reference Dufour37). A meta-analysis study defined light, moderate and heavy drinkers as those who consumed ethanol at < 6, 6–48 and >48 g/d, respectively(Reference Koppes, Dekker and Hendriks38). At least to some extent, we think that it is not reasonable to define drinkers solely according to ethanol dosage. Because even if the dosage and the frequency of ethanol intake is similar between individuals, the in vivo effect of ethanol would still be quite different due to the different metabolic ability of each individual's liver. The liver metabolic activity might be associated with race, somatotype, activities of hepatic enzymes and so on. In our pilot experiments and in the present study, we observed that the effect of ethanol was directly associated with plasma ethanol concentration, but not with ethanol dosage or drinking frequency. Thus, it might be more reasonable to define light, moderate and heavy drinkers according to plasma ethanol concentrations because, by doing so, many of the confounding factors, such as frequency and liver metabolic ability, can be eliminated.
Taken together, the present data show that a particular ethanol consumption pattern can improve insulin resistance induced by a HF diet, and this improvement is associated with a mechanism involving adiponectin and AMPK. Moreover, we found that a twice daily administration of ethanol was more beneficial than a continuous intake of ethanol at the total dosage of 5 g/kg per d. The differential plasma ethanol concentrations resulting from these administration patterns might be the key factor influencing the effect of ethanol on insulin sensitivity.
Acknowledgements
This study was supported by the grant from the National Natural Science Foundation of China (30940038 and 81000323) and the Natural Science Foundation of Shandong Province, China (Y2001C12, ZR2009CM008 and 2009ZRB14022). The authors acknowledge the expert technical assistance by teachers in the Central Laboratory and Experimental Animal Center of Provincial Hospital affiliated to the Shandong University. Partial data of the present study were presented at 44th EASD Annual Meeting (A-08-1890-EASD) and 45th EASD Annual Meeting (A-09-2096-EASD). This study was carried out in the Central Laboratory of Shandong Provincial Hospital, Shandong Province, China. L. F. and B. H. performed most of the work, such as feeding animals, performing experiments and writing the manuscript. R. W. provided help for the experiment designing. Q. L. and D. B. helped the first author determine plasma ethanol concentration. C. M. and G. S. helped L. F. prepare the animals. J. Z. and L. G. designed the experiment and supplied essential support of technology and fund. The authors declare that there is no conflict of interest.









