β-Hydroxy-β-methylbutyrate (HMB) is a five-carbon organic acid, produced in vivo from leucine(Reference Van Koevering and Nissen1). Leucine has been shown to be a regulator of protein metabolism(Reference Frexes-Steed, Lacy and Collins2) and previous studies in human subjects have shown that dietary supplementation with 3 g of calcium HMB (CaHMB)/d attenuates the loss of muscle mass and function in various conditions such as resistance exercise training, cancer and AIDS(Reference Nissen and Sharp3–Reference Clark, Feleke and Din7). The mechanisms for these improvements are not yet clear. However, recent in vitro studies have shown that HMB stimulates protein synthesis through mammalian target of rapamycin phosphorylation, which is central to controlling translation initiation(Reference Eley, Russell and Baxter8, Reference Eley, Russell and Tisdale9). This effect on protein synthesis appears to be shared with the anabolic effects of amino acids (particularly leucine) and insulin(Reference Eley, Russell and Tisdale10, Reference Little and Phillips11). In addition, HMB attenuates the induction of the ubiquitin–proteosome proteolytic pathway caused by proteolysis-inducing factor, lipopolysaccharide and angiotensin II, resulting in a decrease in muscle protein breakdown(Reference Smith, Wyke and Tisdale12–Reference Russell and Tisdale14).
Human studies have shown a positive effect of resistance exercise on muscle protein synthesis as early as 1–2 h post-exercise and lasting up to 48 h(Reference Phillips, Tipton and Aarsland15, Reference Dreyer, Fujita and Cadenas16). Studies have also shown that the timing of nutrient availability is critical for maximal post-exercise stimulation of protein synthesis as well as blunting of protein breakdown(Reference Rennie17). The most optimal time for delivery of nutrient appears to be within 2 h post-exercise. Dreyer et al. (Reference Dreyer, Drummond and Pennings18) demonstrated that the ingestion of a leucine-rich nutrient solution within 1 h of post-exercise recovery resulted in significant enhancement of the mammalian target of rapamycin signalling pathway and muscle protein synthesis. As a dietary supplement, HMB has been used as the mono-hydrated Ca salt, whose empirical formula is Ca(HMB)2·H2O. The dissociation curve of CaHMB is identical to that of calcium acetate(Reference Sousa, Abumrad and Martins19), resulting in peak plasma HMB levels ranging from 60 to 120 min after ingestion depending upon the dosage given. Time to peak plasma levels after a typical 1 g dosage was 2 h(Reference Vukovich, Slater and Macchi20), thus requiring CaHMB to be taken before exercise for maximal benefit. Hence, a more efficient delivery system resulting in increased peak plasma HMB coupled with increased retention would allow for consumption of HMB either during or immediately after exercise.
Methods and materials
Subjects
We performed two longitudinal studies, each consisting of four males and four females (ages 19–29 years old). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Iowa State University Institutional Review Board. A written informed consent was obtained from all the subjects before participation. Due to the nature of the treatments, neither the subjects nor the researchers could be blinded.
Treatments
The same treatments were given to the subjects in both study groups. Treatments were given in random order to each subject with at least 1 week washout period between treatments. Each subject was instructed to fill out a 3 d diet record before each treatment. The CaHMB capsules were obtained from a commercial supplement manufacturer (Optimum Nutrition, Aurora, IL, USA) and were confirmed to contain 0·8 g of HMB by HPLC analysis. The HMB free acid gel was prepared at Metabolic Technologies' laboratory. Briefly, the HMB free acid (approximately 0·8 ml), containing an equivalent amount of HMB as the CaHMB capsule (0·8 g), was adjusted to pH 4·5 with K2CO3. Food-grade orange flavours and sweeteners were then added for a total dosage volume of 1·2 ml. The CaHMB capsule was taken with 355 ml of water; the free acid gel treatments were either swallowed (FASW) or held sublingual for 15 s and then swallowed (FASL). FASW consisted of expelling the entire dose of free acid into the mouth, followed with 355 ml of water. FASL subjects were instructed to place the gel under the tongue and hold the dosage for 15 s before swallowing; they then rinsed and followed the dose with 355 ml of water. In all cases, the water was completely consumed within 5 min of the dosage.
Study design
Both studies were performed after a 10–12 h overnight fast. An intravenous catheter was inserted into a forearm vein using sterile procedures and a pre-ingestion blood sample was drawn (time 0). In study 1, we obtained blood samples at 2, 5, 10, 15, 25, 35, 45, 60, 90, 120 and 180 min after ingestion, while in study 2, we also obtained additional blood samples at 360, 720 and 1440 min after the ingestion of the supplement.
Plasma was separated and samples were stored at − 80°C for analysis of HMB concentration. In addition, a portion of the pre-ingestion and 180 min blood samples (study 1) and samples from 1440 min (study 2) were used for measurements (LabCorp, Kansas City, MO, USA) of glucose, uric acid, blood urea nitrogen, creatinine, Na, K, Cl, CO2, P, protein, albumin, globulin, albumin:globulin ratio, total bilirubin, direct bilirubin, alkaline phosphatase, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, Fe-binding capacity, unsaturated Fe-binding capacity, Fe, Fe saturation, total cholesterol, TAG, HDL, LDL and cholesterol ratio. A complete blood count was also performed before and after each treatment. Subjects also completed a brief questionnaire to report any physical symptoms (such as nausea, headache) they may have experienced during the experiment.
The subjects were provided with a standardised lunch after the 180 min blood sampling and instructed to eat this at approximately 240 min post-ingestion. Following the 720 min blood sample, subjects were instructed to eat a normal evening meal before 22.00 hours. Subjects were refrained from any strenuous physical activity or exercise during each testing period, and diet and activity patterns were replicated for each treatment testing period. Subjects reported back to the laboratory the following morning for the fasted 1440 min blood sample. A urine collection container was provided and the subjects collected all urine produced during the 24 h experimental period. The urine container was stored refrigerated when not being used. Urine volumes were measured and samples were stored at − 80°C until analysed for HMB.
β-Hydroxy-β-methylbutyrate analysis
Plasma and urine HMB were analysed by GC/MS, as previously described(Reference Nissen, Van Koevering and Webb21).
Statistical analysis and calculations
Areas under the curves (AUC) were calculated for each subject using the trapezoidal method that sums the area above the baseline(Reference Urso, Blardi and Giorgi22). Half-life of plasma HMB was calculated for study 2 using the following equations:
The peak plasma concentrations for each subject were used for calculating C peak. Trough concentrations, C trough, were the concentrations measured at 720 min because the 720 min plasma concentrations by treatment were not significantly different from the baseline. T peak was the time at which C peak was measured and T interval is 720 (time at C trough) minus T peak. The extracellular fluid compartment was assumed to be 20 % of body weight and calculated using equation 3 below(Reference Guyton and Hall23). The plasma clearance of HMB was then calculated by multiplying the extracellular fluid compartment, V d, by the elimination constant, K el, as shown in equation 4(Reference Thalhammer, Schenk and Burgmann24).
The data were analysed using a cross-over design with Proc GLM in SAS (SAS Institute, Cary, NC, USA)(25). For the timed sampling of plasma HMB, a repeated-measures polynomial model was used. The model included subject, order and treatment main effects and time × treatment interaction, where appropriate. For other parameters, Proc GLM was also used with subject, order and treatment main effects, and the P values are reported for the treatment main effect. Statistical significance was determined for P < 0·05.
Results
Subject characteristics
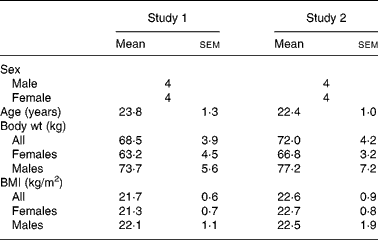
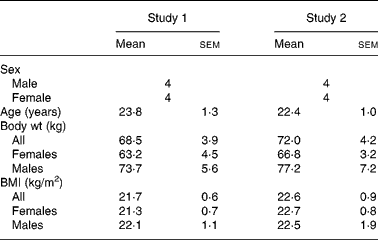
Table 1 shows the subject characteristics for studies 1 and 2. Each study was balanced for sex and each treatment group maintained steady weights throughout the three testing periods. BMI for the males and females in both studies was in the normal body type range. In each study, all the subjects completed all the three testing protocols. No adverse treatment effects were reported in either study, during or after ingestion of the free acid form.
Table 1 Subject descriptors
(Mean values with their standard errors)
Plasma kinetics of β-hydroxy-β-methylbutyrate
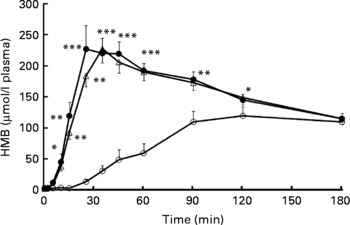
Plasma HMB levels measured after treatment administration in study 1 are shown in Fig. 1. CaHMB taken by capsule resulted in peak plasma HMB of 131 (sem 10) μmol/l, whereas HMB taken either by free acid gel FASW or by FASL delivery resulted in significantly greater (259 (sem 24) and 231 (sem 20) μmol/l, respectively, P < 0·0001) and earlier (33·1 (sem 4·6) and 36·3 (sem 1·3) min, respectively) peaks in plasma HMB compared with CaHMB by capsule (at 121·9 (sem 15·6) min, P < 0·0001). At 180 min, plasma HMB for all delivery methods was still elevated above the baseline, and there were no treatment differences among the three groups.

Fig. 1 Plasma β-hydroxy-β-methylbutyrate (HMB) concentrations in study 1. HMB was administered orally as either CaHMB in a gelatin capsule (-○-), HMB free acid by swallowing (-●-) or HMB free acid by 15 s of sublingual administration (-△-). Values are expressed as means with their standard errors, four males and four females. *P < 0·05, **P < 0·001, ***P < 0·0001 for free acid treatments v. CaHMB capsules.
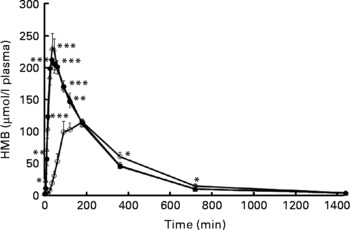
Study 2 was conducted to examine the plasma HMB for a 24 h period as well as to measure urinary losses during this same time period. Similar to study 1, Fig. 2 shows a rapid increase and significantly greater plasma HMB with the free acid gel forms, FASW and FASL, than with CaHMB in capsule form. All levels were back to basal by 720 min.

Fig. 2 Plasma β-hydroxy-β-methylbutyrate (HMB) concentrations in study 2. HMB was administered orally as either CaHMB in a gelatin capsule (-○-), HMB free acid by swallowing (-●-) or HMB free acid by 15 s of sublingual administration (-△-). Values are expressed as means with their standard errors, four males and four females. *P < 0·05, **P < 0·01, ***P < 0·0001 for free acid treatments v. CaHMB capsules.
Table 2 lists peak plasma HMB concentrations (C peak), time to peak plasma HMB concentrations (t peak) and plasma HMB half-life. Delivery of HMB via FASW or FASL resulted in identical and greater HMB plasma peaks (P < 0·0003) in shorter time periods (P < 0·0001). The levels attained were 239 (sem 16) μmol/l achieved at 41·9 (sem 5·8) min for FASW and 248 (sem 20) μmol/l at 38·8 (sem 2·6) min for FASL. These contrast with lower plasma peaks achieved with CaHMB administered by capsule (131·2 (sem 6·0) μmol/l) attained at longer time (135 (sem 17) min). These kinetics were associated with shorter HMB half-lives for FASW and FASL (2·50 (sem 0·13) and 2·51 (sem 0·14) h, respectively) compared with 3·17 (sem 0·22) h for CaHMB capsule delivery (P < 0·004).
Table 2 Plasma kinetics of β-hydroxy-β-methylbutyrate (HMB) in studies 1 and 2 and urinary excretion and HMB retention in study 2
(Mean values with their standard errors)

AUC, area under the curve.
* Treatment mean values were significantly different.
Whole body retention of β-hydroxy-β-methylbutyrate
The AUC for plasma HMB following the three treatments for both studies are also shown in Table 2. In study 1, HMB administered in free acid gel form resulted in doubling of the plasma AUC compared with CaHMB (P < 0·0001); there were no differences between HMB free acid gel delivered by FASW or by FASL. Similarly, the 24 h period AUC for HMB administered as the free acid gel were significantly greater (approximately 15 %) for FASW and FASL than for CaHMB administered in capsules (P < 0·001). Despite the significant increases in peak plasma HMB and AUC, urinary HMB losses were identical and accounted for 18 (sem 3)% and 17 (sem 3)% of the doses ingested for FASW and FASL v. 15 (sem 2)% for CaHMB (P>0·18). There was an approximately 25 % increase in HMB clearance with the free acid gel form compared with the CaHMB form (P < 0·003).
Safety studies
There were no differences in blood chemistry and haematology between the two studies. Treatment with HMB did not result in differences in any of the measured parameters for blood chemistry and for blood haematology parameters (The supplementary material (tables from study 2) for this article can be found at http://www.journals.cambridge.org/bjn).
Discussion
It is clear from the present studies that oral or sublingual administration of HMB in free acid gel form resulted in earlier and greater peaks in plasma HMB compared with HMB administered as the Ca salt (CaHMB). Free acid gel administration of HMB resulted in a significant increase (+15 %) in the AUC for plasma HMB without any major changes in urinary losses over the 24 h period. Consequently, there was a significant increase in the clearance and utilisation of the HMB dosage (approximately 25 %) attesting to the improved efficiency of this new form of delivery of HMB.
The plasma HMB kinetics following CaHMB administration seen in the present study agree with those previously reported by Vukovich et al. (Reference Vukovich, Slater and Macchi20). Both the studies showed congruent peak and time to peak plasma levels when a 1 g dosage of CaHMB was given. Interestingly, the delivery of an equivalent amount of HMB as free acid in a gel form, swallowed or sublingual, almost doubled the peak concentrations. While a greater dosage of CaHMB has been shown to increase plasma HMB level over that of a 1 g equivalent dose of HMB free acid gel, the time to peak plasma level for the large dosage of CaHMB was still greater than that seen in the present studies for the free acid in gel form(Reference Vukovich, Slater and Macchi20). These data support the concept of a more rapid absorption of the free acid form as compared to that of CaHMB, most probably occurring at the most proximal sites of the gastrointestinal tract, favouring absorption of hydrophilic compounds in the sublingual mucosa(Reference Madhav, Shakya and Shakya26) and acidic compounds in the stomach(Reference Gupta, Bhandari and Sharma27). On the other hand, it is likely that the absorption of CaHMB occurs more distally in the duodenum and proximal jejunum where most of the Ca absorption occurs(Reference Bouillon, Van and Carmeliet28).
Several studies have supported the use of HMB as a nutritional supplement during exercise. HMB has been shown to enhance protein synthesis(Reference Eley, Russell and Tisdale9) and decrease muscle damage and protein breakdown(Reference Nissen, Sharp and Ray5, Reference Jówko, Ostaszewski and Jank29, Reference Knitter, Panton and Rathmacher30). It therefore seems advantageous to achieve higher plasma HMB levels and higher retention rates during exercise than those previously reported(Reference Nissen, Sharp and Ray5, Reference Vukovich, Slater and Macchi20, Reference Nissen and Abumrad31). It is clear from our studies that the combination of significantly higher peak plasma concentrations and AUC, coupled with no differences in urinary excretion over the measurement period, indicates greater retention of HMB. The shorter half-lives and higher clearance rates from the plasma compartment are also indicative of the increased utilisation of the HMB free acid gel forms. From the present study, it is impossible to tell if this was direct uptake by skeletal muscle or further metabolism. Previous studies, however, showed a direct effect of HMB intake on reduction of muscle protein degradation as measured by 3-methylhistidine release(Reference Nissen, Sharp and Ray5); we, therefore, hypothesise that skeletal muscle accounts for a significant component of the increased clearance of HMB from the plasma compartment following free acid administration of HMB.
Several studies have shown a direct relationship between the levels of HMB achieved following ingestion and improved nitrogen retention. Nissen et al. (Reference Nissen, Sharp and Ray5) demonstrated a dose-dependent response to oral administration of CaHMB given twice daily at either 1·5 or 3 g/d. Ingestion of 3·0 g dose of CaHMB/d resulted in decreased release of creatine kinase, an indicator of muscle damage, and 3-methylhistidine, an indicator of muscle protein breakdown. Similar findings were reported by Gallagher et al. (Reference Gallagher, Carrithers and Godard32) who showed that administration of 6 g/d was more beneficial than 3 g/d in preventing muscle damage. Taken together, the above studies indicate a benefit to having more HMB available to the muscles during exercise. Therefore, using this new delivery method would optimise the delivery timing of the HMB dose to achieve maximum compliance and efficacy during exercise.
While our data demonstrate an improved kinetics in the delivery of HMB as a nutrient to the body, the present study, however, has several limitations. The study does not directly assess the influence of the efficient delivery of HMB on muscle protein kinetics during exercise. Many of the assumptions related to retention of HMB and utilisation by skeletal muscle are based on indirect data. However, previous studies have shown that exercise and post-exercise feeding of amino acids, in particular branched-chain amino acids, have additive effects in stimulating protein synthesis through the mammalian target of rapamycin pathway(Reference Little and Phillips11, Reference Rennie17, Reference Rivas, Lessard and Coffey33). Similar to leucine, HMB, at a much lower dosage, has also been shown to stimulate protein synthesis through the mammalian target of rapamycin pathway(Reference Eley, Russell and Baxter8). Thus, having high levels of HMB immediately post-exercise should be beneficial in maximising the effect of exercise on protein synthesis. Based upon HMB's ability to increase lean mass with exercise, delivery of maximal HMB to tissues during and immediately post-exercise would result in a further increase in protein synthesis(Reference Nissen and Sharp3). Yet, additional studies are required to examine such direct effects and to determine the effect of HMB with/without exercise on protein synthesis.
In conclusion, HMB delivery by free acid gel results in a faster and greater peak in HMB plasma concentration as well as equally sustained concentration compared with CaHMB administered in a capsule. This form of delivery is equally safe as those presently and previously found with oral administration of CaHMB(Reference Nissen, Panton and Sharp34, Reference Gallagher, Carrithers and Godard35). These data offer the theoretical advantages of delivering a better dosing method based upon ease of administration, and achieving higher sustained HMB plasma concentration that could improve HMB availability and efficacy to tissues.
Acknowledgements
The present research was funded through a grant from the Institute for Physical Research and Technology, Iowa State University with matching funds from Metabolic Technologies, Inc., Ames, IA. The authors acknowledge Rachel Bell, Alison Glidden and Justin Scherff for their assistance in conducting the studies. J. C. F., S. M. B. and J. A. R. are employed by Metabolic Technologies, Inc., Ames, IA. H. F. A. had no conflicts of interest and R. L. S. received matching funds from Metabolic Technologies to perform the study. J. A. R. and S. M. B. designed the research. J. A. R., H. F. A. and R. L. S. conducted the research, including the sample analysis. J. C. F. analysed the data and wrote the paper. J. A. R. had primary responsibility for final content. All the authors read and approved the final manuscript.