Nasopharyngeal carcinoma (NPC) is a rare neoplasm in most countries (<1×10−5 person-years), but a much higher incidence has been observed in southern China (20−50×10−5 person-years)( Reference Yu and Yuan 1 – Reference Jia, Huang and Liao 3 ). Infection with the Epstein–Barr virus (EBV) is considered a key cause of NPC( Reference Yu and Yuan 1 ), but the ubiquity of EBV suggests that other cofactors, such as diet, may have a role in NPC aetiology and may contribute to the remarkable geographic variation( Reference Macsween and Crawford 4 , Reference Yoshizaki, Kondo and Wakisaka 5 ). However, the role of diet has scarcely been investigated.
There is much evidence suggesting that DNA hypomethylation may interfere with DNA replication and repair and activate numerous cancer-related genes, each of which can predispose an individual to develop neoplasia (e.g. NPC)( Reference Suzuki, Maruyama and Yamamoto 6 , Reference Challouf, Ziadi and Zaghdoudi 7 ). The one-carbon metabolism pathway plays an important role in regulating DNA methylation( Reference Christman, Sheikhnejad and Dizik 8 ) and mediating nucleotide synthesis( Reference Choi and Mason 9 ). Thus, a low methyl supply may induce global DNA hypomethylation and aberrant DNA synthesis, thus promoting carcinogenesis( Reference Christman, Sheikhnejad and Dizik 8 ).
Dietary factors involved in the one-carbon metabolism pathway mainly include carbon donors (e.g. folate, choline and betaine), intermediate-product methionine, and enzymatic cofactors (e.g. vitamins B6 and B12). Recently, we reported protective associations between NPC risk and dietary intake of choline and betaine( Reference Zeng, Xu and Liu 10 ). Like choline and betaine, folate is an important methyl-group donor. Epidemiological studies have noted inverse associations between dietary intake or circulating levels of folate and the risk for cancer in the breasts( Reference Zhang, Ho and Chen 11 ), colon( Reference Schernhammer, Giovannuccci and Fuchs 12 ), ovaries( Reference Harris, Cramer and Vitonis 13 ), oesophagus and stomach( Reference Xiao, Freedman and Ren 14 ) and pancreas( Reference Lin, An and Wang 15 ). Thus, it is reasonable to speculate that folate may reduce NPC risk. However, only one case–control study of 198 NPC Italian cases focused on NPC risk and found a null association( Reference Polesel, Negri and Serraino 16 ). Although many other studies have found an inverse association between dietary( Reference Matsuo, Rossi and Negri 17 – Reference Arthur, Duffy and Sanchez 23 ) or blood( Reference Almadori, Bussu and Galli 24 – Reference Almadori, Bussu and Galli 27 ) folate and the risk for head and neck cancer, the numbers of NPC cases in these studies were generally <100. In addition, all of these studies were conducted among populations with dietary habits and NPC risks different from those of the Chinese in south China( Reference Yu and Yuan 1 – Reference Jia, Huang and Liao 3 ). Thus, further study of the folate–NPC association in high-risk Chinese adults is necessary. In addition, only a few studies of limited numbers of NPC cases have assessed the effects of vitamin B6 ( Reference Polesel, Negri and Serraino 16 , Reference Negri, Franceschi and Bosetti 19 , Reference Bravi, Bosetti and Filomeno 20 ), B12 ( Reference Arthur, Duffy and Sanchez 23 – Reference Raval, Sainger and Rawal 25 , Reference Almadori, Bussu and Galli 27 ) and methionine( Reference Pelucchi, Talamini and Negri 28 ) on the risk for head and neck cancer, and their results have been inconsistent.
Hence, the primary purpose of this study was to evaluate the associations between four key dietary nutrients in the folate-mediated one-carbon cycle (folate, vitamins B12 and B6 and methionine) and NPC risk among the Chinese in south China. We explored the modifying effects of some potential risk factors, such as smoking and alcohol consumption, which may interact with folate to alter methylation levels( Reference Halsted, Villanueva and Devlin 29 ).
Methods
Study population
We conducted a 1:1 matched case–control study in Guangdong Province, China( Reference Liu, Dai and Xu 30 ). Between July 2009 and March 2011, patients newly diagnosed (within 3 months) with histologically confirmed epithelial cell carcinoma of the nasopharynx hospitalised at the Sun Yat-sen University Cancer Center were identified. Patients aged 30–75 years and resident in the Guangdong Province for over 10 years were eligible. We excluded those with a family history of NPC, with substantial self-reported changes in dietary habits over the previous 5 years, with chronic diseases (e.g. diabetes, other cancers) that may lead to dietary habit change, and with cognitive impairment. Potential eligible patients from the Department of Nasopharyngeal Carcinoma and Department of Radiotherapy were tracked through electronic medical records and made an appointment with for face-to-face interviews within 1 week.
Controls matched by age (±3 years), sex and household type (urban/rural) were identified from the following four departments of Sun Yat-sen University Ophthalmic Center – Department of Ocular Trauma, Department of Glaucoma, Department of Corneal Disease and Department of Fundus – where they had been concurrently hospitalised within 1 week for ocular disease. The recruitment flow of the controls was similar to that of cases. The same selection criteria for the NPC cases were applied to the control subjects, except for history of NPC.
Written consent was obtained from each participant, and the Ethics Committee of the School of Public Health of Sun Yat-sen University approved the study protocol.
Data collection
The participants were interviewed in person by experienced interviewers with relevant medical knowledge using a structured questionnaire( Reference Liu, Dai and Xu 30 ). Demographic and socio-economic status (SES) were collected, along with occupational and domestic exposure to toxic substances, smoking history, alcohol consumption, history of chronic disease(s), medical history, physical activity over the past year and habitual dietary consumption. For obtaining data on exposure to toxic substances, we interviewed participants if they had never- or ever-exposure to one of the following factors over 1 year: loud noise, heat, dust environment, radiation, pesticides, heavy metals, organic solvents and cooking fumes. Height and weight were measured, and BMI (in kg/m2) was calculated. Each interviewer completed an equal number of case and control subject interviews. Additional NPC-related information was obtained from the medical records, such as histological type, tumour node metastasis (TNM) stage and EBV viral capsid antigen (VCA)-IgA and early antigen (EA)-IgA antibody titres.
Assessment of dietary intake
Dietary intake was evaluated using a validated FFQ containing seventy-eight items of commonly eaten foods in Guangdong( Reference Liu, Dai and Xu 30 ). The participants were asked the frequency (never, per year, month, week or day) and amount (in bowls, grams, boxes, cups, etc.) of food intake for each food item during the year before diagnosis (for cases) or before the interview (for controls). Coloured pictures of different portion sizes of food were provided to help quantify the food consumed. Daily intakes of dietary energy and nutrients (including folate, vitamin B6, vitamin B12 and methionine) were estimated using the China Food Composition Table ( Reference Yang, Wang and Pan 31 ). The reproducibility and validity of the FFQ have been reported previously( Reference Zhang and Ho 32 , Reference Zhang, Pan and Li 33 ). The correlation coefficients for dietary folate, vitamin B6, vitamin B12 and methionine intakes were 0·35, 0·26, 0·50 and 0·36 when comparing the second FFQ and 18-d dietary records, and were 0·60, 0·57, 0·60 and 0·49 when comparing the two FFQ( Reference Zhang, Ho and Chen 11 , Reference Zhang and Ho 32 ).
Statistical analysis
The analysis was performed using SPSS (version 17.0; SPSS Inc.). The differences between cases and controls were determined using the t test for continuous data and the χ 2 test for categorical data. All of the statistical tests were two-sided and considered statistically significant when P<0·05.
The dietary intake of nutrients was adjusted for total energy using the residual method( Reference Willett, Howe and Kushi 34 ). Energy-adjusted intakes of folate-mediated one-carbon metabolism-related nutrients were grouped into quartile 1 to quartile 4 (Q1−Q4) based on control subjects by sex. Sex-specific cutoffs were then applied to the cases. The lowest quartile (Q1) served as the reference group. Univariate (crude) and multivariate (adjusted) conditional logistic regression models were used to assess the associations between the quartiles of intake and NPC risk, and OR and 95 % CI were calculated. Crude adjusted OR (95 % CI) were obtained without further adjustment of covariates; adjusted OR (95 % CI) were obtained after covariates had been adjusted for age, BMI, occupation, marital status, educational level, household income, smoking status, drinking status, exposure to potentially toxic substances, multivitamin supplementation, chronic rhinitis history, physical activity and daily energy intake (log-transformed) for each nutrient. The multivariate-adjusted model was treated as the final model for conclusion. The criteria for entry and non-entry of these confounders were P<0·05 and P>0·10, respectively, using the forward stepwise method.
Stratified analyses were conducted to determine whether the folate–NPC associations were modified according to potential risk factors, including sex (women/men), smoking (yes/no), alcohol drinking (yes/no) and exposure to toxic substances (yes/no). We used unconditional logistic regression models to assess the associations because these factors were not matched between NPC patients and control subjects. The potential interactions were also examined via multiplicative interaction terms in the multivariate unconditional logistic regression models. We also conducted sensitivity analyses to explore whether the results were significantly changed after excluding those who used multivitamins using unconditional logistic regression models.
Results
Study participants
Among 1063 cases and 800 controls, 461 cases (mean age: 47·2 years; 23·9 % women) and 186 controls (mean age: 48·5 years; 24·7 % women) were excluded because of the following reasons: discomfort with completing the questionnaire (twenty cases and seven controls); refusal to participate (260 cases and forty-one controls); change of dietary habits (forty-nine cases and sixty-eight controls); language difficulties (twelve cases and thirteen controls); and having left the hospital (120 cases and fifty-seven controls). We further excluded two cases who reported implausible energy intake (<2928 or >17 572 kJ/d for men; <2092 or >14 644 kJ/d for women (<700 or >4200 kcal/d for men; <500 or >3500 kcal/d for women)) in the data analysis. In addition, fourteen controls were excluded because of 1:1 match to cases. Finally, 600 (56·4 %) cases and 600 (75 %) controls were included in the analyses. The majority of the 600 cases were in late-stage NPC (stages III and IV) (85 %), had undifferentiated carcinoma (95 %) and were seropositive for EBV VCA-IgA (93 %) and EA-IgA (75 %). The controls included 249 (41·3 %) cases of ocular fundus disease, 111 (18·5 %) cases of glaucoma, 107 (17·8 %) cases of ocular trauma and 133 (22·2 %) cases of other ocular diseases.
The demographics, lifestyle characteristics and selected NPC risk factors of the cases and controls are presented in Table 1. Compared with the controls, the cases were more likely to have higher BMI, education level, meat and fish intake, be married, and have a history of chronic rhinitis, but to have a lower activity level.
Table 1 Comparison of the demographics, lifestyle characteristics and selected nasopharyngeal carcinoma risk factors of cases and controls (Mean values and standard deviations; numbers and percentages)

MET, metabolic equivalent.
* Physical activity included daily occupational, leisure time, and household chores activities, evaluated by MET-h/d.
† Smoker included current smoker and ex-smoker who had smoked at least 1 cigarette/d for at least 6 months earlier.
‡ Alcohol drinker included current drinker and ex-drinker who had consumed wine at least once a week for at least 6 consecutive months.
§ Exposure to potential toxic substances included ever being exposed to one of the following substances over the past year: heat, organic solvents, pesticides, heavy metals, smoke from burning incense, anti-mosquito coils, new furniture or decoration and radiation.
The NPC cases consumed less folate (268 v. 295 μg/d; P<0·001) and vitamin B6 (1·06 v. 1·14 mg/d; P<0·001), but more vitamin B12 (2·51 v. 2·30 μg/d; P=0·004) and methionine (1·63 v. 1·57 g/d; P=0·013), compared with controls (Table 2). In addition, cases consumed higher meat and fish, but lower energy, vegetable and fruit, compared with controls (Table 2).
Table 2 Comparison of dietary intake between nasopharyngeal carcinoma cases and controls (Means, medians and interquartile ranges (IQR))

* Energy-adjusted intake.
Both vegetables and cereal were the main source of folate (33·3 and 24·0 %) and vitamin B6 (23·3 and 40·6 %); meat (52·9 %) and fish (23·5 %) were the major source of vitamin B12; and cereal (34·6 %) and meat (29·5 %) were the major source of methionine.
Correlations between folate-mediated one-carbon metabolism-related nutrients
The intakes of folate, vitamin B6, vitamin B12 and methionine by the controls were all significantly correlated with each other, except between vitamin B12 and folate or vitamin B6 among women. The correlations were highest between folate and vitamin B6 and lowest between folate and vitamin B12 for both men and women (Table 3).
Table 3 Spearman’s rank correlation coefficients (r S) of the association between dietary energy-adjusted folate, vitamin B6, vitamin B12 and methionine intake among controls, divided by sexFootnote †
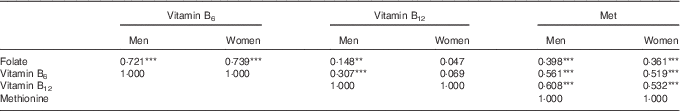
**P<0·01, ***P<0·001.
† Adjusted for age and household type.
Folate-mediated one-carbon metabolism-related nutrients and risk for nasopharyngeal carcinoma
Univariate analyses showed dose-dependent and inverse associations between NPC risk and dietary intake of folate and vitamin B6 (both P trends<0·001), but a positive relationship with methionine intake (P trend=0·031). After adjustment for covariates, significant associations remained for dietary folate and vitamin B6 intake (Table 4). Compared with the lowest quartile of intake, the OR for quartiles 2–4 were 0·66, 0·52 and 0·34 (P trend<0·001) for folate, 0·72, 0·55 and 0·44 (P trend<0·001) for vitamin B6, 0·76, 1·01 and 1·03 (P trend>0·05) for B12, and 1·10, 1·38 and 1·33 (P trend>0·05) for methionine.
Table 4 Risk for nasopharyngeal carcinoma for quartiles (Q) of dietary folate, vitamin B6, vitamin B12 and methionine intake in Guangzhou, China (Medians and numbers; odds ratios and 95 % confidence intervals)

* Crude adjusted OR (95 % CI): without further adjustment; adjusted OR (95 % CI): covariates adjusted for age, BMI, occupation, marital status, educational level, household income, smoking status, drinking status, exposure to potentially toxic substances, multivitamin supplementation, chronic rhinitis history, physical activity and daily energy intake (log-transformed) for each nutrient. Multivitamin supplementation was not adjusted for multivitamin supplement use.
We also examined whether the folate–NPC relationship varied according to sex, smoking, alcohol consumption and exposure to toxic substances (Table 5). The favourable association between folate intake and NPC risk was more substantial among the participants without (v. with) toxic substance exposure (P interaction=0·014). There were no significant interactions after stratification by sex, smoking and alcohol consumption (all P interactions>0·50). In addition, sensitivity analyses indicated that the results were not considerably changed after excluding those who used multivitamins (online Supplementary Table S1).
Table 5 Risk for nasopharyngeal carcinoma for quartiles (Q) of dietary energy-adjusted folate intake by sex and potential risk factors in Guangzhou, ChinaFootnote * (Numbers, odds ratios and 95 % confidence intervals)

* See Table 3 for the covariates.
Discussion
This case–control study investigated the association between dietary nutrients involving the one-carbon metabolism pathway (e.g. folate, vitamins B6 and B12 and methionine intake) and the risk for NPC in a high-risk population in southern China. Dietary folate and vitamin B6 intakes were inversely related to NPC risk. A null association was observed for vitamin B12 and methionine.
Many studies have found protective associations between the intake of folate and vitamin B6 and the risk for cancer in the breasts( Reference Zhang, Ho and Chen 11 ), colon( Reference Schernhammer, Giovannuccci and Fuchs 12 ), ovaries( Reference Harris, Cramer and Vitonis 13 ) and other organs( Reference Xiao, Freedman and Ren 14 , Reference Lin, An and Wang 15 ). For example, from a case–control study with 581 pairs of breast cancer cases and controls conducted in the same geographic area as our study, Zhang et al. ( Reference Zhang, Ho and Chen 11 ) reported that folate and vitamin B6 intakes were inversely associated with breast cancer risk. A combined analysis of the data from the Nurses’ Health Study and the Health Professional Follow-up cohort showed an inverse association between the intake of folate and vitamin B6 and colon cancer risk( Reference Schernhammer, Giovannuccci and Fuchs 12 ). Consistent with these findings, our study, which had a relatively larger number of NPC cases (600 cases), showed that both folate and vitamin B6 intake were inversely associated with NPC risk. To our knowledge, only one previous hospital-based case–control study has focused on the relationship between dietary folate or vitamin B6 intake and NPC risk. It showed a non-significant association, possibly due to the limited study size of only 198 NPC cases( Reference Polesel, Negri and Serraino 16 ). Our findings and other related studies support a protective association between folate and vitamin B6 and the risk for NPC and certain other cancers. However, because of the scarcity of available evidence, further prospective studies are needed to confirm these protective associations.
A possible mechanism linking nutrients in the one-carbon metabolism pathway with cancer risk is their effect on the maintenance of nuclear and mitochondrial genome integrity( Reference Christman, Sheikhnejad and Dizik 8 , Reference Choi and Mason 9 ). In this pathway, carbon donors (e.g. folate, choline and betaine) can convert a one-carbon moiety to homocysteine to generate methionine, and then produce S-adenosyl methionine (SAM). SAM is the universal methyl donor required to prevent uracil misincorporation into DNA and hypomethylation of DNA, and subsequently to maintain the methylation patterns in DNA that determine gene expression and chromosome conformation( Reference Choi and Mason 9 ). Diets deficient in methyl donors (e.g. folate) may lead to decreased tissue SAM, global DNA hypomethylation and ultimately hepatic tumourigenesis in rodents( Reference Davis and Uthus 35 ).
Vitamin B6 and B12 play an important role as co-enzymes in one-carbon metabolism( Reference Christman, Sheikhnejad and Dizik 8 ). Vitamin B6 mediates the conversion of uracil to thymidylate to regenerate 5,10-methylenetetrahydrofolate( Reference Christman, Sheikhnejad and Dizik 8 ). In addition, vitamin B6 may influence NPC risk through its antioxidant properties, as studies have reported that vitamin B6 is effective at scavenging free radicals that can promote carcinogenesis( Reference Matxain, Padro and Ristila 36 ). Vitamin B12 is needed for methionine synthase and is responsible for catalysing the methylation of homocysteine to methionine( Reference Christman, Sheikhnejad and Dizik 8 ). However, we failed to find any significant association for vitamin B12 in our study, probably because of the significantly lower levels of vitamin B12 (2·30 μg/d) compared with other nutrients (e.g. 294·6 μg/d (folate), 1·14 mg/d (vitamin B6) and 1·57 g/d (methionine)). The resulting larger random measurement error for vitamin B12 may have led to greater variation and probably attenuated the associations to the null( Reference Beaton 37 ).
As mentioned above, methionine is also an intermediate methyl donor in one-carbon metabolism. However, a null association was observed between methionine intake and NPC risk in our study, similar to the results found in some studies on breast cancer( Reference Shrubsole, Jin and Dai 38 ), oral and pharyngeal cancer( Reference Pelucchi, Talamini and Negri 28 ) and lung cancer( Reference Takata, Cai and Beeghly-Fadiel 39 ), but not in other studies on ovarian cancer( Reference Harris, Cramer and Vitonis 13 ) and breast cancer( Reference Stevens, McCullough and Sun 40 ). The inconsistent findings across studies may be partly due to differences in the type of cancer, study design and population, sample size, method of measuring dietary nutrients and adjustments for covariates. In addition, two sources of methionine can be used in vivo: dietary and biotransformational. Pelucchi et al. ( Reference Pelucchi, Talamini and Negri 28 ) suggested that if methionine levels are low more folate is used as methyltetrahydrofolate to form methionine.
As reported in our previous article( Reference Zeng, Xu and Liu 10 ), we observed that the folate–NPC association was more significant among those without exposure to toxic substances (e.g. organic solvents, pesticides, heavy metals, smoke from burning incense and new furniture or decor) than among those with exposure (P interaction=0·014). Our findings provided evidence that exposure to toxic substances might mitigate the favourable effects of folate intake. The biological rationale for this finding is unclear. It is possible that toxic substances decrease DNA methylation( Reference Fonnum and Lock 41 ), and hence more folate is needed to achieve a favourable effect. Further studies are warranted to confirm these findings and to elucidate the pathophysiological mechanisms.
Our study has important strengths, including a relatively large sample size providing higher power to detect associations and allowing potential confounders to be taken into account; the evaluation of dietary habits using a reproducible and validated FFQ; portion size assessment using visual aids; the completion by each interviewer of an equal proportion of interviews for both cases and controls to minimise information bias; and matching on key confounders to minimise confounding.
Potential limitations of our study should also be considered. First, we could not rule out the possibility of selection bias due to the hospital-based case–control study design, although we reduced the potential by selecting participants from the two comparable hospitals with the same catchment area in southern China. In addition, the dietary habits of hospital-based controls may differ from those of the source population. However, we included only those control subjects with ocular diseases that might not change their dietary habits. Further, the use of hospital control subjects tends to reduce the differential report on diet( Reference D’Avanzo, La Vecchia and Katsouyanni 42 ). Second, a case–control design is unlikely to establish temporal links, although we minimised possible reverse causation by excluding participants with essential changes in dietary habits over the past 5 years and including only newly diagnosed cases (within 3 months). Third, the small number of participants among cases (56·4 %) and controls (75·0 %) might reduce the generalisibility of the findings. We found that the age and sex of included cases and controls were comparable to those who did not participate. However, we could not further compare other characteristics because of lack of information for non-participants. Fourth, recall bias in dietary assessment using an FFQ is difficult to avoid in a case–control study. However, misclassification in dietary evaluation is unlikely to differ between cases and controls because of minimal public awareness of the cancer-preventing effects of these nutrients. Fifth, we did not determine the internal levels of these nutrients in the body, and the relatively less precise assessment by the FFQ may have attenuated any associations. Sixth, in the present study, we only assessed dietary nutrient intakes and each multivitamin supplement, but no data were available for individual supplements, and most common Chinese multivitamins contained folate, vitamin B6 and B12. Thus, we could not exclude the possibility of potential misclassifications of the intakes of some nutrients. Seventh, the differences in SES between the cases and controls suggested that they might have different lifestyles that might affect the NPC risk or modify the studied associations. However, after adjustment for SES, the associations were not considerably changed (Table 5), and stratified analyses indicated no significant interactions between folate or vitamin B6 and SES (occupation, marital status, education level and household income) (P interaction range: 0·236–0·988, data not shown), suggesting that these SES factors did not significantly influence the associations found. Finally, we could not exclude the potentially confounding effects of other nutrients or food components coexisting with vitamin B6 and folate.
In conclusion, our findings suggest protective associations between the risk for NPC and dietary folate and vitamin B6 intake, especially among patients who have not been exposed to toxic substances. Considering the pitfalls of a hospital-based case–control study, confirmation of our results by other large well-designed studies, especially large-scale cohort studies, is warranted.
Acknowledgements
The authors are grateful to all the participants, and to all the doctors and nurses in two university-affiliated hospitals (The Cancer Center and Ophthalmic Center) of Sun Yat-sen University who assisted in the implementation of the study.
This study was supported by the 5010 Program for Clinical Researches of Sun Yat-sen University, Guangzhou, People’s Republic of China (2007032). The sponsor had no role in the design, analysis or writing of this article.
Y.-m. C. conceived and designed the study, and critically revised the manuscript; F.-f. Z. analysed the data and wrote the paper. Y.-t. L., X.-l. L., Y.-y. F., X.-l. Z. and C.-h. X. carried out the study and data cleansing, and participated in paper-writing.
There are no conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515004146








