Sarcopenia is associated with physical inactivity, poor nutritional status and genetic susceptibility(Reference Marty, Liu and Samuel1). Recent studies using non-invasive tests show that low skeletal muscle mass is associated with the presence of non-alcoholic fatty liver disease (NAFLD) and significant liver fibrosis(Reference Lee, Kim and Song2–Reference Xia, Chen and Wu5). Skeletal muscle is an important organ that affects whole-body insulin sensitivity, and low skeletal muscle mass may influence metabolic health through altered insulin-mediated glucose disposal(Reference Mesinovic, Zengin and De Courten6). Moreover, skeletal muscle may release a variety of myokines that influence other organs such as adipose tissue and liver(Reference Karstoft and Pedersen7). It has been suggested that myokines (e.g. IL-6, IL-15 and irisin) may mediate, at least in part, the protective effects of physical exercise against chronic diseases, such as type 2 diabetes, NAFLD and chronic vascular diseases(Reference Gomarasca, Banfi and Lombardi8).
Physical exercise may increase the expression of fibronectin type III domain-containing protein 5 (FNDC5), a membrane protein that is mainly expressed in skeletal muscle and is cleaved and released into the circulation as irisin(Reference Perakakis, Triantafyllou and Fernández-Real9). Some studies have recently indicated that the FNDC5 rs3480 G variant affects the stability and expression of FNDC5 and may be associated with protection from clinically significant fibrosis in patients with NAFLD(Reference Petta, Valenti and Svegliati-Baroni10,Reference Metwally, Bayoumi and Romero-Gomez11) . However, the impact of the FNDC5 rs3480 G variant on the histological features of NAFLD remains controversial. Petta et al. (Reference Petta, Valenti and Svegliati-Baroni10) have reported that the FNDC5 rs3480 G allele was associated with lower levels of significant fibrosis in NAFLD. In contrast, Metwally et al. (Reference Metwally, Bayoumi and Romero-Gomez11) found that the FNDC5 rs3480 G variant was associated with more severe hepatic steatosis, but not with other histological features of NAFLD.
It is known that the interaction between metabolic risk factors and genetic background plays a key role in the progression of NAFLD(Reference Xia, Chen and Wu5,Reference Hu, Zhu and Liu12,Reference Zheng, Fan and Shi13) . However, it is currently uncertain whether skeletal muscle-related gene polymorphisms influence the association between low skeletal muscle mass and NAFLD. Thus, the major aim of our cross-sectional study was to investigate the influence of the FNDC5 rs3480 polymorphism on the association between sarcopenia and the histological severity of NAFLD. In addition, since sex plays an important role in the disease progression in NAFLD and may also affect muscle mass(Reference Lonardo and Suzuki14,Reference Capozza, Cointry and Cure-Ramírez15) , we have further investigated the association between FNDC5, sarcopenia and the severity of NAFLD in both men and women, separately.
Materials and methods
Study population and design
We consecutively enrolled a total of 638 adults with suspected NAFLD (based on the presence of hepatic steatosis on imaging methods and/or elevated serum liver enzymes), who consecutively attended the First Affiliated Hospital of Wenzhou Medical University (China) from December 2016 to November 2018. As detailed in Fig. 1, 268 subjects were excluded for the following reasons: (1) excessive alcohol consumption (≥140 g/week in men or ≥70 g/week in women); (2) presence of viral hepatitis, autoimmune hepatitis, drug-induced liver injury or other known chronic liver diseases; (3) incomplete clinical/biochemical or genetic data and (4) fatty liver infiltration <5 % on liver histology. As a consequence of these exclusion criteria, a sample of 370 adults with biopsy-proven NAFLD was included in the final analysis. All these patients did not have any prior history of cancer.

Fig. 1. Flow chart for the study. NAFLD, non-alcoholic fatty liver disease.
The study was approved by the internal review board for ethics of the First Affiliated Hospital of Wenzhou Medical University (2016-246, 1 December 2016). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration. The study protocol was registered in the Chinese Clinical Trial Registry (ChiCTR-EOC-17013562). Written informed consent was obtained from each participant enrolled in the study.
Laboratory and clinical data
Venous fasted blood samples were taken after a 12-h fast in all participants. Laboratory biochemical parameters were centrally analysed by using an automated analyzer (Abbott AxSYM). BMI was calculated using the formula: weight (kg) divided by height (m) squared. Obesity was defined as BMI ≥ 25 kg/m2 or waist circumference ≥90 cm in men or ≥80 cm in women(Reference Goda and Masuyama16,Reference Alberti, Eckel and Grundy17) . Hypertension was diagnosed as blood pressure ≥130/85 mmHg or the use of any anti-hypertensive drugs. Diabetes was diagnosed as either self-reported history of disease, fasting plasma glucose level ≥ 7·0 mmol/l, HbA1c ≥ 6·5 % (≥48 mmol/mol) or treatment with hypoglycaemic drugs. Atherogenic dyslipidaemia was defined as any of the following criteria: TAG > 1·70 mmol/l; HDL-cholesterol < 1·03 mmol/l in men and <1·29 mmol/l in women or use of any lipid-lowering drugs(Reference Alberti, Eckel and Grundy17).
All the aforementioned anthropometric and laboratory data were obtained from participants within 24 h of liver biopsy examinations.
Definition of sarcopenia
Body weight and composition were measured using the segmental multi-frequency bioelectrical impedance analyser (BIA, InBody720; InBody Japan Inc.). BIA measured impedance in each segment, including the bilateral upper and lower limbs, providing an estimate of the appendicular skeletal muscle mass (ASM). Specifically, the ASM was calculated as the sum of skeletal muscle mass in the four limbs divided by body weight and expressed as a percentage (ASM/weight × 100, skeletal muscle index%). The ASM:BMI ratio was also calculated. According to previous studies conducted in East Asian people(Reference Koo, Kim and Joo18,Reference Kim, Lee and Chung19) , relevant to the ethnicity of our participants, sarcopenia was defined as a skeletal muscle index <29·0 % in men and <22·9 % in women, or ASM:BMI ratio <0·789 in men and <0·512 in women, respectively(Reference Koo, Kim and Joo18).
Liver histology
Liver histology assessment was undertaken by an experienced liver histopathologist according to the non-alcoholic steatohepatitis (NASH)-Clinical Research Network Scoring System(Reference Kleiner, Brunt and Van Natta20). The liver histopathologist was blinded to the research measurements related to sarcopenia. NAFLD was diagnosed by the presence of hepatic steatosis in more than 5 % of hepatocytes. Based on the NASH-Clinical Research Network(Reference Kleiner, Brunt and Van Natta20), the NAFLD activity score was calculated as the sum of the three histological components including steatosis (0–3), ballooning (0–2) and lobular inflammation (0–3). Liver fibrosis was not included as a component of NAFLD activity score. Individuals with NAFLD activity score of 1–2 were defined as simple steatosis (NAFL), 3–4 was defined as borderline NASH and 5 or greater was defined as definite NASH. Liver fibrosis was staged as 0–4 according to Brunt’s criteria(Reference Brunt, Janney and Di Bisceglie21). Fibrosis with 2 or greater on liver histology indicated significant fibrosis.
FNDC5 genotype
Genotyping assays for FNDC5 rs3480 A>G, patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 C>G and transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 C>T, on human peripheral blood leucocytes, were carried out using the MassARRAY platform (Agena Bioscience). Locus-specific PCR and detection primers were designed using Assay Design Suite version 3.1. After the DNA samples were amplified via multiplex PCR, allele detection was performed using MALDI-TOF MS.
Statistical analysis
Continuous variables were expressed as means and standard deviations or medians with interquartile ranges, according to whether their distribution was normal or skewed. Categorical variables were expressed as percentages. Comparisons between the patient groups were made by the χ 2 test or the Fisher exact test for categorical variables, and by the unpaired Student’s t test, the Mann–Whitney U test, one-way ANOVA or the Kruskal–Wallis test for normally and non-normally distributed continuous variables as appropriate. The χ 2 test was also used to assess whether the genotypes were in Hardy–Weinberg equilibrium. FNDC5 rs3480 associations were assessed using a dominant genetic model. The association between sarcopenia and presence of either definite NASH or significant fibrosis was assessed by binary logistic regression, and the models were adjusted for potential risk factors of NASH and fibrosis (as specified in the ‘Results’ section below). Stratified and interaction analyses were used to examine the impact of FNDC5 rs3480 polymorphism on the association of sarcopenia with the histological severity. Statistical analyses were two-sided, and statistical significance was set at P < 0·05. All statistical tests were performed using SPSS version 23.0 (SPSS Inc.).
Results
Baseline characteristics of the study population
Among the 370 adults with biopsy-proven NAFLD, sixty-one adults (16·5 %) were diagnosed as having sarcopenia. The mean age of the whole cohort was 41 years, and 75·7 % were male. The mean values of waist circumference and BMI were 92·1 cm and 26·8 kg/m2, respectively. A total of 117 subjects (31·6 %) had established type 2 diabetes, 139 (37·6 %) subjects had hypertension and 314 (84·9 %) subjects had atherogenic dyslipidaemia. Definite NASH was diagnosed in 131 subjects (35·4 %) and significant fibrosis in fifty-eight subjects (15·7 %). Compared with those without sarcopenia, sarcopenic patients were more likely to be male and had higher levels of BMI, waist circumference, alanine aminotransferase, aspartate transaminase, γ-glutamyltranspeptidase, homoeostasis model assessment-insulin resistance, more severe histological grades of hepatic steatosis and fibrosis, and higher proportions of definite NASH and significant fibrosis (Table 1).
Table 1. Baseline characteristics of study participants, stratified by sarcopenia status
(Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)

ASM, appendicular skeletal muscle mass; ALT, alanine aminotransferase; AST, aspartate transaminase; GGT, γ-glutamyltranspeptidase; HOMA-IR, homoeostasis model assessment-insulin resistance; FNDC5, fibronectin type III domain-containing protein 5; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2; NAS, NAFLD activity score; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
* Skeletal muscle index was defined as ASM divided by body weight.
The frequency distributions of FNDC5 rs3480, PNPLA3 rs738409 and TM6SF2 rs58542926 genotypes were in Hardy–Weinberg equilibrium (P = 0·587, 0·143 and 0·609, respectively). Stratifying by the FNDC5 rs3480 polymorphism, 188 (50·8 %) subjects had A/A genotype, 155 (41·9 %) had A/G genotype and 27 (7·3 %) had G/G genotype. No significant differences in clinical characteristics and liver histological severity were observed among the groups of patients with different FNDC5 genotypes, except for hypertension (online Supplementary Table S1). The G variant carriers had a lower percentage of hypertension (A/A 45·5 % v. A/G 29·7 % v. G/G 29·6 %, P = 0·009).
Sarcopenia is associated with histological severity of non-alcoholic fatty liver disease
As shown in Table 3, in the unadjusted logistic regression model, patients with sarcopenia exhibited a 2-fold increase in the risk of having definite NASH (OR 1·91, 95 % CI 1·06, 3·43, P = 0·031) and a 4-fold increase in the risk of having significant fibrosis (OR 3·87, 95 % CI 1·94, 7·69, P < 0·001), compared with those patients without sarcopenia. In a logistic regression model with NASH/no NASH as the outcome, the association between sarcopenia and definite NASH was significant even after adjusting for age, sex, obesity, type 2 diabetes, hypertension, dyslipidaemia and smoking (model 2: OR 1·91, 95 % CI 1·02, 3·55, P = 0·042). However, this association was no longer significant after further adjustment for serum liver enzymes and homoeostasis model assessment-insulin resistance values (model 3: OR 1·65, 95 % CI 0·86, 3·18, P = 0·130). Notably, the association between sarcopenia and significant fibrosis was slightly attenuated but remained significant in the fully adjusted regression model (model 4: OR 2·79, 95 % CI 1·31, 5·95, P = 0·008).
Association between sarcopenia and the histological severity of non-alcoholic fatty liver disease stratified by FNDC5 genotypes
Stratified analyses were conducted to evaluate the effect of FNDC5 rs3480 A>G variant on the severity of NAFLD histology. We observed a strong relationship between the presence of sarcopenia and more severe histological grades of ballooning and fibrosis, and a higher prevalence of both definite NASH and significant fibrosis in patients carrying the rs3480 AA genotype, but not in those carrying the rs3480 AG or GG genotypes (Fig. 2 and Table 2). Moreover, sarcopenic patients had higher serum aspartate transaminase and γ-glutamyltranspeptidase levels in those carrying the rs3480 AA genotype but not in those carrying the AG or GG genotypes (Table 2).
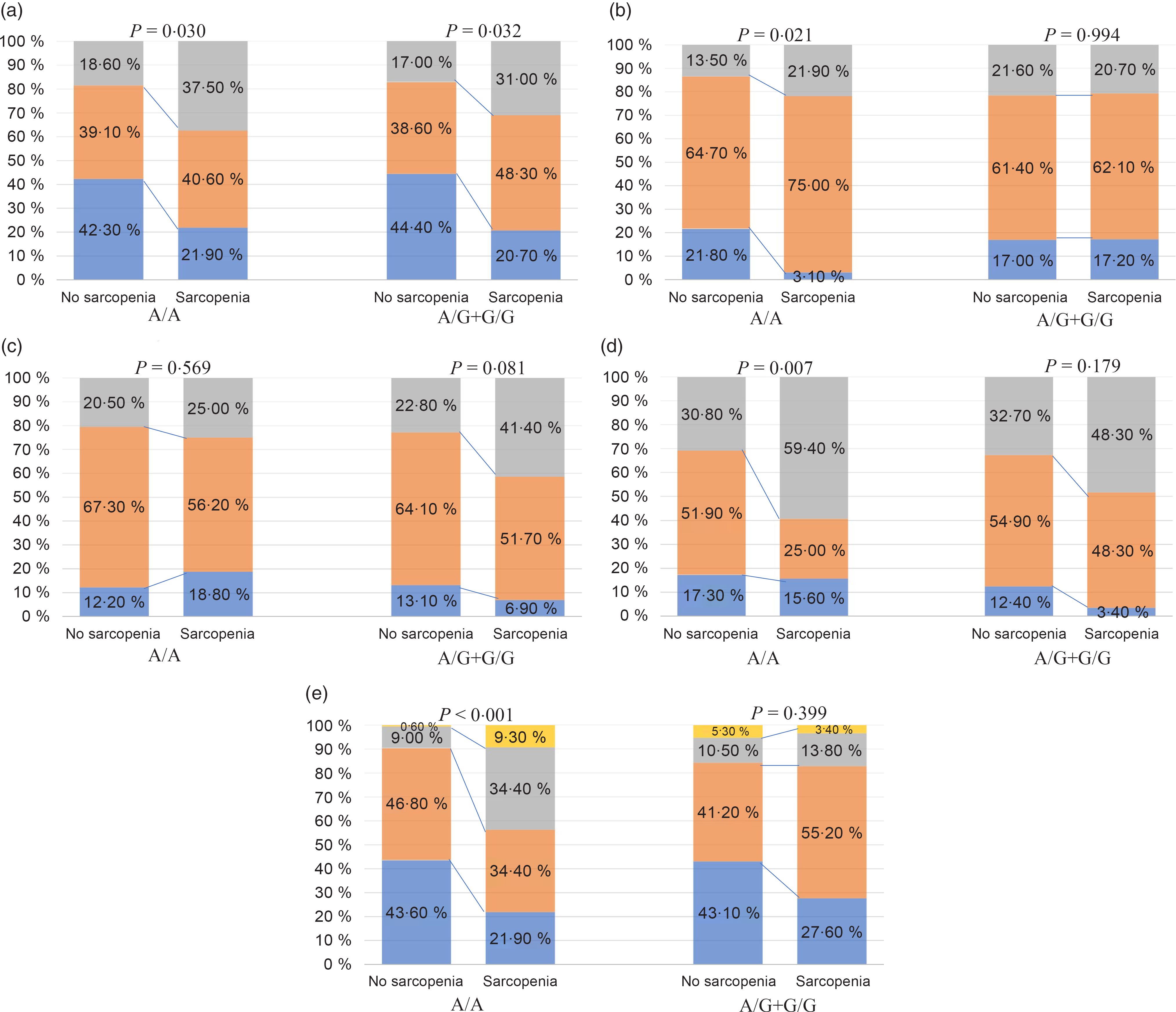
Fig. 2. Association between sarcopenia and histological features of non-alcoholic fatty liver disease (NAFLD), stratified by fibronectin type III domain-containing protein 5 (FNDC5) rs3480 genotypes. (a) ![]() , Steatosis 1;
, Steatosis 1; ![]() , steatosis 2;
, steatosis 2; ![]() , steatosis 3; (b)
, steatosis 3; (b) ![]() , ballooning 0;
, ballooning 0; ![]() , ballooning 1;
, ballooning 1; ![]() , ballooning 2; (c)
, ballooning 2; (c) ![]() , inflammation 0;
, inflammation 0; ![]() , inflammation 1;
, inflammation 1; ![]() , inflammation ≥2; (d)
, inflammation ≥2; (d) ![]() , NAFLD;
, NAFLD; ![]() , borderline non-alcoholic steatohepatitis (NASH);
, borderline non-alcoholic steatohepatitis (NASH); ![]() , definite NASH; (e)
, definite NASH; (e) ![]() , fibrosis 0;
, fibrosis 0; ![]() , fibrosis 1;
, fibrosis 1; ![]() , fibrosis 2;
, fibrosis 2; ![]() , fibrosis ≥3.
, fibrosis ≥3.
Table 2. Baseline characteristics of study participants stratified by both fibronectin type III domain-containing protein 5 (FNDC5) rs3480 genotypes and sarcopenia status
(Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)

ASM, appendicular skeletal muscle mass; ALT, alanine aminotransferase; AST, aspartate transaminase; GGT, γ-glutamyltranspeptidase; HOMA-IR, homoeostasis model assessment-insulin resistance; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2; NAS, NAFLD activity score; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Table 3. Associations between presence of sarcopenia (as the exposure variable) and definite non-alcoholic steatohepatitis (NASH) or significant fibrosis (as the outcome measures) in participants with different fibronectin type III domain-containing protein 5 (FNDC5) genotypes* †
(Odds ratios and 95 % confidence intervals)

ALT, alanine aminotransferase; AST, aspartate transaminase; GGT, γ-glutamyltranspeptidase; HOMA-IR, homoeostasis model assessment-insulin resistance; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2.
* Data are expressed as OR and 95 % CI tested by logistic regression analysis.
† Model 1: adjusted for age and sex; model 2: adjusted for age, sex, obesity, type 2 diabetes, hypertension, dyslipidaemia and smoking history; model 3: adjusted for covariates included in model 2 plus serum ALT, AST, GGT and HOMA-IR levels; model 4: adjusted for covariates included in model 3 plus the PNPLA3 rs738409 and TM6SF2 rs58542926 variants.
As shown in Table 3, in the unadjusted logistic regression model, the presence of sarcopenia was associated with a 3·3-fold increase in the risk of definite NASH (OR 3·29, 95 % CI 1·50, 7·20, P = 0·003) and nearly a 7-fold increase in the risk of significant fibrosis (OR 7·31, 95 % CI 3·04, 17·59; P < 0·001) in individuals with rs3480 AA genotype. However, these significant associations were not found among the rs3480 AG or GG genotype carriers.
To further explore the independent effect of sarcopenia on liver disease severity in individuals with different FNDC5 genotypes, we performed multivariable logistic regression models with potential risk factors as covariates (Table 3). The association between sarcopenia and the histological severity of NAFLD remained significant after adjusting for age, sex, smoking, obesity, diabetes, hypertension, dyslipidaemia status, serum liver enzymes (alanine aminotransferase, aspartate transaminase and γ-glutamyltranspeptidase), homoeostasis model assessment-insulin resistance, PNPLA3 rs738409 and TM6SF2 rs58542926 variants in individuals carrying the rs3480 AA genotype (adjusted OR 3·27, 95 % CI 1·15, 9·31, P = 0·026 for definite NASH; adjusted OR 7·19, 95 % CI 2·40, 21·55, P < 0·001 for significant fibrosis), but not in those carrying the AG or GG genotypes (adjusted OR 1·17, 95 % CI 0·43, 3·13, P = 0·759 for definite NASH; OR 1·01, 95 % CI 0·29, 3·57, P = 0·983 for significant fibrosis).
The FNDC5 rs3480 G variant provided a protective effect for significant fibrosis in patients with sarcopenia
The aforementioned analyses suggested that the FNDC5 rs3480 variant might have a protective effect on the severity of NAFLD histology. However, there were no significant differences in the histological severity of NAFLD among the groups of patients with different FNDC5 genotypes (online Supplementary Table S1). As reported in Fig. 3(a), patients with sarcopenia carrying the G variant genotype appeared to have a lower proportion of definite NASH than those carrying the A/A genotype, but the effect was not statistically significant (48·3 v. 59·4 %, P = 0·385). Among patients without sarcopenia, there was no difference in the percentage of definite NASH between patients with the A/A genotype and those with the G variant genotype (30·8 v. 32·7 %, P = 0·718). As shown in Fig. 3(b), there was a significantly lower proportion of patients with significant fibrosis in sarcopenic patients with the G variant genotype, than in those with the A/A genotype (17·2 v. 43·8 %, P = 0·031). However, among patients without sarcopenia, there was no significant difference in the percentage of significant fibrosis between the two genotypes (15·7 v. 9·6 %, P = 0·108). Notably, there was a significant interaction between FNDC5 genotype and sarcopenia, with significant fibrosis (P value for interaction = 0·006).
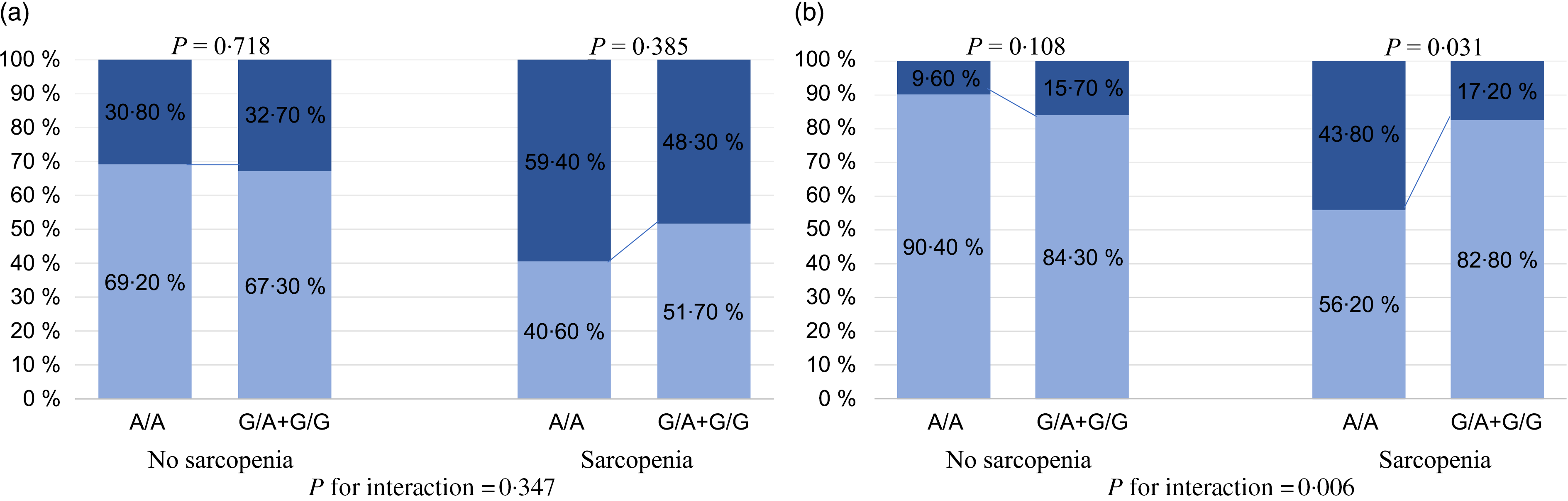
Fig. 3. Association between fibronectin type III domain-containing protein 5 (FNDC5) rs3480 genotypes and the histological severity of non-alcoholic fatty liver disease (NAFLD), stratified by sarcopenia. (a) ![]() , NAFLD + borderline non-alcoholic steatohepatitis (NASH);
, NAFLD + borderline non-alcoholic steatohepatitis (NASH); ![]() , definite NASH; (b)
, definite NASH; (b) ![]() , no significant fibrosis;
, no significant fibrosis; ![]() , significant fibrosis.
, significant fibrosis.
Stratified analyses according to sex differences
We further explored the association between sarcopenia, FNDC5 genotypes and liver fibrosis both in men and in women. As shown in Table 4, in the unadjusted model, the presence of sarcopenia was associated with an increased risk of significant fibrosis in both sexes (men: OR 3·85, 95 % CI 1·82, 8·14; women: OR 8·82, 95 % CI 1·41, 55·35). After adjustment for potential confounding factors (fully adjusted model 3), the association between sarcopenia and significant fibrosis remained statistically significant in men (adjusted OR 2·57, 95 % CI 1·10, 5·98, P = 0·029). We also observed a significant association between sarcopenia and significant fibrosis in women, even after adjustment for age, obesity, diabetes, hypertension and dyslipidaemia (adjusted OR 11·74, 95 % CI 1·43, 96·11, P = 0·022). Further adjustment for other potential confounding variables was not feasible due to the relatively small number of women included in the study.
Table 4. Associations between presence of sarcopenia (as the exposure variable) and significant fibrosis (as the outcome measure) in participants with different fibronectin type III domain-containing protein 5 (FNDC5) genotypes, stratified by sex* †
(Odds ratios and 95 % confidence intervals)

ALT, alanine aminotransferase; AST, aspartate transaminase; GGT, γ-glutamyltranspeptidase; HOMA-IR, homoeostasis model assessment-insulin resistance; PNPLA3, patatin-like phospholipase domain-containing protein 3; TM6SF2, transmembrane 6 superfamily member 2.
* Data are expressed as OR and 95 % CI tested by logistic regression analysis.
† Model 1: adjusted for age, obesity, type 2 diabetes, hypertension, dyslipidaemia and smoking history; model 2: adjusted for covariates included in model 2 plus serum ALT, AST, GGT and HOMA-IR levels; model 3: adjusted for covariates included in model 3 plus the PNPLA3 rs738409 and TM6SF2 rs58542926 variants.
‡ The adjusted model failed due to the small sample size.
As shown in Fig. 4, in NAFLD patients carrying the rs3480 AA genotype, both men and women with sarcopenia had a higher proportion of significant fibrosis than those without sarcopenia. However, in those carrying the AG or GG genotype carriers, the proportion with significant fibrosis was not significantly different between patients with and without sarcopenia irrespective of sex. In addition, as shown in Table 4, the association between sarcopenia and significant fibrosis remained significant after full adjustment for potential confounders only in the men with rs3480 AA genotype (adjusted OR 4·47, 95 % CI 1·26, 15·79, P = 0·020), but not in men with AG or GG genotypes (OR 1·05, 95 % CI 0·24, 4·57, P = 0·950). This fully adjusted regression model was not feasible in women due to their small sample size.
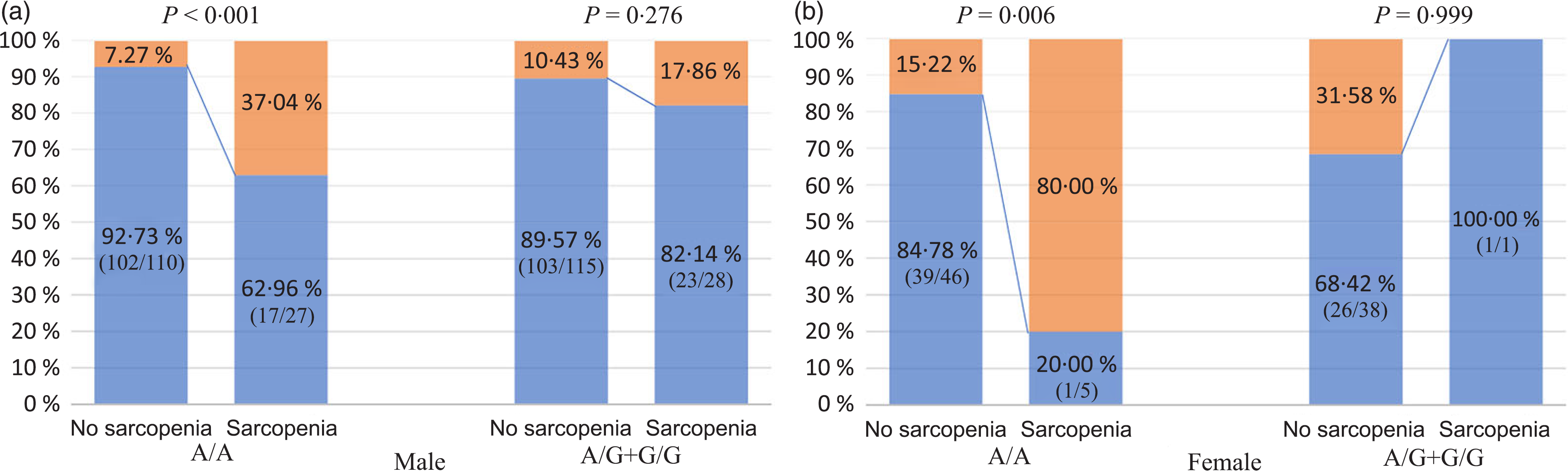
Fig. 4. Association between sarcopenia, fibronectin type III domain-containing protein 5 (FNDC5) genotypes and significant fibrosis, stratified by sex. (a and b) ![]() , No significant fibrosis;
, No significant fibrosis; ![]() , significant fibrosis.
, significant fibrosis.
Discussion
Our novel results show that in patients with biopsy-confirmed NAFLD, sarcopenia is independently associated with significant liver fibrosis and, in this association, there is a significant interaction between FNDC5 genotype and sarcopenia status. In addition, when our NAFLD patients were stratified by sex, both men and women carrying the rs3480 AA genotype who had sarcopenia exhibited a significantly higher proportion of significant fibrosis than those without sarcopenia. However, in NAFLD patients carrying the rs3480 AG or GG genotypes, neither female nor male patients with sarcopenia had a higher proportion of significant fibrosis than their counterparts without sarcopenia. These results remained essentially unchanged in men even after adjustment for potential confounding variables.
FNDC5 has effects in several organs, including a role in the liver and muscle(Reference Canivet, Bonnafous and Rousseau22). A recent study found that the hepatic expression of FNDC5 in NAFLD could dampen hepatocyte fat accumulation, insulin resistance and liver injury(Reference Canivet, Bonnafous and Rousseau22). These beneficial effects could occur in both liver and muscle as irisin mediates adipose tissue thermogenesis and may regulate carbohydrate and lipid metabolism. To our knowledge, no previous studies have explored the potential influence of skeletal muscle-related gene FNDC5 polymorphisms on the relationship between sarcopenia and NAFLD. Therefore, we analysed the association between sarcopenia and the severity of NAFLD histology in subjects stratified by FNDC5 rs3480 polymorphism. Our stratified analyses revealed that these associations became statistically not significant among individuals with the FNDC5 rs3480 G variant genotype.
In recent years, the relationship between sarcopenia and NAFLD has attracted attention(Reference Cai, Song and Chen23). However, most published studies have used non-invasive tests to evaluate the severity of liver steatosis and fibrosis(Reference Lee, Kim and Song2–Reference Xia, Chen and Wu5), and importantly, the use of non-invasive tests may result in misclassification of disease status(Reference Castera, Friedrich-Rust and Loomba24). There is still a lack of reliable non-invasive tests for the assessment of NAFLD severity, and liver biopsy remains, to date, the ‘gold standard’ for diagnosing NASH and fibrosis. In this study, we showed that patients with NAFLD and sarcopenia have higher levels of serum liver enzymes and more severe liver histological features compared with patients without sarcopenia. Even after adjusting for age, sex, anthropometric, biochemical and genetic risk factors, the presence of sarcopenia remained significantly associated with approximately a two-fold increased risk of significant fibrosis. This association is in agreement with the results from other Asian and European studies(Reference Koo, Kim and Joo18,Reference Petta, Ciminnisi and Di Marco25) . Moreover, we also showed a strong relationship between sarcopenia and both obesity and insulin resistance, which is consistent with the findings reported in other clinical settings(Reference Koo, Kim and Joo18,Reference Petta, Ciminnisi and Di Marco25) .
Contrary to our expectation, patients with sarcopenia were younger than those without sarcopenia in the present study. This unexpected finding might in part be related to the associated metabolic factors that were more adversely affected in patients with sarcopenia than in those without sarcopenia (e.g. increased central obesity and insulin resistance). Moreover, the diagnosis of sarcopenia was not only affected by the muscle mass but also by body weight and BMI. Using the age cut-off of 60 years to define the older and the younger patients, we observed that the former not only had lower muscle mass (20·6 (sd 3·8) v. 23·5 (sd 4·3) kg) but also lower body weight (70·2 (sd 9·3) v. 77·7 (sd 13·8) kg) and lower BMI (25·8 (sd 2·4) v. 27·1 (sd 3·5) kg/m2) compared with the younger patients. The observation that the younger patients were heavier and have a higher BMI than the older patients may influence the association between sarcopenia and age in our cohort of NAFLD patients. The occurrence of NAFLD parallels the high rates of obesity in young individuals(Reference Doycheva, Watt and Alkhouri26). Moreover, the participants in our cohort were selected from patients who were referred with suspected NAFLD rather than the general population. Thus, our findings are applicable only to patients with NAFLD.
Low muscle mass has a strong negative prognostic impact in obese individuals and may lead to increased morbidity and mortality(Reference Barazzoni, Bischoff and Boirie27). Maintaining skeletal muscle mass in obesity is important, and therefore, the term ‘sarcopenic obesity’ has been proposed. Our study also demonstrated a high proportion of sarcopenic obesity in NAFLD. When obesity was defined by a BMI ≥ 25 kg/m2, 16·2 % (60/370) of our NAFLD patients met the criteria for sarcopenic obesity. When obesity was defined by a BMI ≥ 30 kg/m2, 5·7 % (21/370) of our NAFLD patients met the criteria for sarcopenic obesity.
The role of FNDC5 on the progression of NAFLD is controversial. Our results showed that there were no significant differences in the prevalence of sarcopenia and severity of liver histology among individuals with different genotypes. One recent study has found that the FNDC5 rs3480 G variant was associated with lower levels of significant fibrosis(Reference Petta, Valenti and Svegliati-Baroni10), although another study has found that the G variant was only associated with more severe steatosis(Reference Metwally, Bayoumi and Romero-Gomez11). Differences in baseline characteristics might, at least in part, explain these conflicting results. As our study found, the presence of sarcopenia influenced the effect of the FNDC5 gene on NAFLD and the G variant only provided a protective effect on patients with sarcopenia. The underlying mechanism is uncertain. However, what is certain is that FNDC5 rs3480 G variant may affect the stability and expression of FNDC5 (Reference Metwally, Bayoumi and Romero-Gomez11), which is cleaved as irisin. Irisin has been shown to have favourable metabolic effects on metabolic diseases, including NAFLD(Reference Polyzos, Anastasilakis and Efstathiadou28,Reference Shanaki, Moradi and Emamgholipour29) . Zhang et al. (Reference Zhang, Zhang and Ma30) also reported that increased serum irisin levels were associated with lower serum liver enzymes and decreased hepatic TAG content in obese Chinese adults.
The underlying mechanisms explaining the association between sarcopenia and significant fibrosis are not fully understood. The widely accepted mechanism is that loss of muscle mass reduces a key cellular target for insulin, contributing to systemic insulin resistance and insulin resistance is very strongly associated with NAFLD(Reference Cleasby, Jamieson and Atherton31). Skeletal muscle is responsible for the majority of the body’s postprandial glucose disposal, and insulin mediates GLUT-4 glucose uptake in skeletal muscle. Therefore, the potential mechanisms linking sarcopenia to NAFLD may involve skeletal muscle insulin resistance(Reference Montalcini, Pujia and Donini32). The findings of our research also suggest another possible mechanism. Sarcopenia might contribute to liver damage via a reduced production of myokines, for example, IL-6 and irisin, and the expression of FNDC5 directly affects irisin levels. This suggests that irisin may play an important role in the association between FNDC5 variants, sarcopenia with liver fibrosis.
There are some important limitations to our study. First, owing to the cross-sectional design of the study, it is not possible to draw any conclusion about causality. However, the genetic variant is inherited, and therefore, reverse causation does not apply. Second, skeletal muscle mass was measured by BIA. BIA is an instrument for screening low skeletal muscle mass in NAFLD(Reference Kim, Lee and Lee33). Previous studies have confirmed that the use of BIA (instead of MRI or dual-energy X-ray absorptiometry) for estimating ASM is appropriate(Reference Bosy-Westphal, Jensen and Braun34). Third, all participants in our study are of Asian ethnicity, and therefore, our findings need to be verified in other ethnic groups. Finally, it is well known that muscle mass is affected by sex and reproductive status. Unfortunately, no detailed information was available on pre-menopausal and post-menopausal status in our cohort. Future larger cohorts of NAFLD patients with available data on reproductive status are needed to better examine the association between FNDC5, sarcopenia and liver fibrosis in NAFLD.
In conclusion, the results of our study show that sarcopenia is independently associated with significant fibrosis and there is a significant interaction between FNDC5 genotype and sarcopenia status with significant fibrosis in a well-characterised cohort of patients with biopsy-proven NAFLD.
Acknowledgements
We thank Professor Ji-Min Liu, a pathologist from McMaster University, Canada, who conducted quality control of pathology data.
This work was supported by grants from the National Natural Science Foundation of China (81500665, 82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032) and Project of New Century 551 Talent Nurturing in Wenzhou. GT is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy. CDB is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK.
The guarantor of the article is M.-H. Z.
Study concept and design: F. G. and M.-H. Z.; acquisition of data: H.-L. M., G. L., L.-J. T., R. S. R., W.-Y. L. and X.-Y. P.; pathology analysis: Y.-Y. L.; drafting of the manuscript: F. G. and K. I. Z.; critical revision: G. T. and C. D. B.; statistical analysis: F. G. and P.-W. Z.; study supervision: M.-H. Z. and Y.-P. C.; all authors contributed to the manuscript for important intellectual content and approved the submission.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520004559











