Obesity is a major risk factor for various diseases, and results from an imbalance between energy intake and expenditure. It is also known that altered lipid metabolism, including fat oxidation and fatty acid synthesis, is a related factor in obesity(Reference Pagliassotti, Gayles and Hill1, Reference Triscari, Nauss-Karol, Levin and Sullivan2). Furthermore, diet-induced obesity in man is often complicated by various lifestyle-related diseases, including diabetes, hyperlipaemia, hypertension and hyperinsulinaemia. Therefore, prevention of obesity is strongly encouraged in order to alleviate various lifestyle-related diseases.
Lignans are found in various foodstuffs, such as plant seeds, whole grains, legumes, vegetables and fruits(Reference Bhathena and Velasquez3). Flax (Linum usitatissimum) is one of the richest sources of plant lignans(Reference Thompson, Rickard, Cheung, Kenaschuk and Obermeyer4). The concentration of lignans in flaxseed was shown to be more than 100 times higher than that in most other foods(Reference Bhathena and Velasquez3). Most of the flaxseed lignan is secoisolariciresinol diglucoside (SDG) which is present in a much higher proportion in the seed than in other tissues(Reference Bhathena and Velasquez3). Flaxseeds are consumed daily as an ingredient in multigrain breads and topping for breads, bagels and muffins in Europe, Canada and the USA. They also contain 37 % of their mass as oil of which 50 % is α-linolenic acid (n-3 fatty acid) and have been the focus of interest in the field of food elements due to their potential health benefits(Reference Cunnane, Ganguli and Menard5, Reference Romans, Johnson, Wulf, Libal and Costello6). Flaxseed lignan SDG is converted by bacteria in the colon of man and other animals to the biologically active lignans, enterodiol (END) and enterolactone (ENL)(Reference Axelson, Sjovall, Gustafsson and Setchell7, Reference Nesbitt, Lam and Thompson8). The structures of END and ENL are similar to oestradiol, an endogenous oestrogen. This structural similarity accounts for the ability of these compounds to bind to oestrogen receptors and exert weak oestrogenic or anti-oestrogenic effects, depending on the presence of stronger oestrogen(Reference Bhathena and Velasquez3). They are suggested to protect and help against hormone-sensitive cancers such as those of the breast, endometrium and prostate(Reference Wang, Chen and Thompson9). Furthermore, SDG and the mammalian lignans END and ENL have anti-oxidant activity. The anti-oxidant activities of SDG, END and ENL were investigated by the ability to scavenge exogenously generated hydroxyl radical as measured by HPLC and the ability to prevent lipid peroxidation in liver homogenate(Reference Prasad10). In another work, SDG was claimed to be effective in reducing hypercholesterolaemic atherosclerosis by reducing oxidative stress(Reference Prasad11). Administration of SDG was reported to be effective in inhibiting the development of type 1 and type 2 diabetes and pulmonary metastasis of melanoma cells(Reference Li, Yee, Thompson and Yan12–Reference Prasad, Mantha, Muir and Westcott14). These reports suggest that flaxseed lignans could be a potentially useful dietary source for the prevention and improvement of lifestyle-related diseases.
In the present study, we have examined the effects of the flaxseed lignan SDG on high-fat diet-induced obesity in C57BL/6 mice. We show that purified SDG improves high-fat diet-induced visceral and liver fat accumulation, hypercholesterolaemia, hyperinsulinaemia and hyperleptinaemia.
Recent studies have shown that adipose tissue is not simply a store of excess energy, but also secretes a variety of hormones called adipocytokines, which are adipose-specific secretory factors(Reference Hu, Liang and Spiegelman15). Dysregulated secretion of adipocytokines also participates in the pathogenesis of lifestyle-related diseases(Reference Matsuzawa, Funahashi and Nakamura16). The adipocytokine adiponectin is believed to decrease TAG and to improve insulin resistance in obesity(Reference Yamauchi, Kamon and Waki17). Here we demonstrate that flaxseed lignans induce the expression of adiponectin in white adipose tissue in diet-induced obesity in mice. Flaxseed lignans are suggested to regulate adipogenesis-related gene expression through an increase in PPARγ DNA binding activity.
Materials and methods
Preparation of secoisolariciresinol diglucoside and enterodiol
Lignan SDG was isolated from flaxseeds (Linum usitatissimum) using a modification of the methods of Johnsson et al. (Reference Johnsson, Kamal-Eldin, Lundgren and Aman18). Briefly, flaxseeds were defatted with n-hexane. The defatted flaxseed flour was subjected to alkaline hydrolysis for 3 h under constant rotation using 0·4 mol/l sodium hydroxide in aqueous methanol at 70°C. Crude SDG was recovered after reversed-phase silica gel chromatography (DM1020T; Fuji Silysia Chemical Ltd, Kasugai, Aichi, Japan) of acidic extracts (pH 3·0 by the addition of phosphoric acid) by elution with 40 % methanol. The filtrate was evaporated, and pure SDG was obtained by passing crude SDG through silica gel (BW-200; Fuji Silysia Chemical Ltd) and eluting with chloroform containing increasing amounts of methanol (10–30 %). The purity of SDG was confirmed by HPLC(Reference Johnsson, Kamal-Eldin, Lundgren and Aman18) and time-of-flight MS(Reference Fritsche, Angoelal and Dachtler19). Isolated SDG was confirmed to have a purity of about 90 % or more by these methods. END was purchased from Sigma Aldrich Japan (Sinagawa, Tokyo, Japan).
Animals and diets
Male C57BL/6 mice were purchased at 7 weeks of age (Japan SLC Inc., Hamamatsu, Shizuoka, Japan) and fed commercial pellets (MR stock; Nosan Corp., Yokohama, Kanagawa, Japan) for 1 week to stabilize their metabolic condition. The mice were divided into four groups (n 5; two or three mice per cage) and were provided one of the four synthetic diets: a low-fat diet (containing 5 % maize oil (w/w) as fat, 20 % casein, 66·35 % maize starch, 4 % cellulose, 0·8 % vitamin mixture (AIN-76), 3·5 % mineral mixture (AIN-76), 0·2 % choline bitartrate and 0·15 % dl-methionine); a high-fat diet (containing 20 % maize oil and 10 % lard as fat, 20 % casein, 28·35 % maize starch, 13 % glucose, 4 % cellulose, 0·8 % vitamin mixture (AIN-76), 3·5 % mineral mixture (AIN-76), 0·2 % choline bitartrate and 0·15 % dl-methionine); or SDG diet consisting of the high-fat diet containing 0·5 or 1·0 % SDG. Mice were maintained on these diets for 4 weeks. This experimental protocol was approved by the Ethical Committee of Nippon Flour Mills Company.
Blood analysis
At the conclusion of this experiment, mice were killed under anaesthesia and blood was collected from the heart immediately. The serum concentration of TAG, total cholesterol, glucose, glutamic oxaloacetic transaminase and glutamic pyruvic transaminase was measured enzymatically with commercial kits (Wako Pure Chemical Industries, Osaka, Japan). Serum insulin and leptin concentrations were measured using ELISA kits (Sibayagi Co., Sibukawa, Gunma, Japan and Morinaga Institute of Biological Science Inc., Yokohama, Kanagawa, Japan, respectively).
Fat pad weights
Two fat pads (epididymal and perirenal fat) were dissected from each animal and weighed, and then separately stored at − 20°C in RNAlater RNA Stabilization reagent (Qiagen KK, Tokyo, Japan).
Measurement of faecal lipids
Faeces were collected twice on a per-cage basis for 2 d after 3–4 weeks of feeding. After drying, faecal lipids were extracted and measured following the method of Folch et al. (Reference Folch, Lees and Sloane Stanley20).
Measurement of liver TAG and total cholesterol
The liver TAG and total cholesterol contents were extracted following the method of Folch et al. (Reference Folch, Lees and Sloane Stanley20) and measured using commercial enzyme kits (Wako Pure Chemical Industries).
Quantitative real-time PCR analysis
Total RNA was extracted from liver, adipose tissue, skeletal muscle and differentiated 3T3-L1 cells using Isogen reagent (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. For quantitative real-time PCR, RNA was further purified using RNeasy (Qiagen). RNA (1 μg) was reverse-transcribed into cDNA using a ReverTra Ace α (Toyobo, Osaka, Japan). Then amplification was performed in a cDNA mixture on an ABI Prism 7000 Sequence Detector using SYBR Green Realtime PCR Master Mix (Toyobo), according to the manufacturer's protocol. Sequences of primers used for quantitative real-time PCR were as follows: sterol regulatory element binding protein 1c (SREBP-1c), 5′-atcggcggggaagctgtcggggtagcgtc-3′ and 5′-actgtcttggttgttgatgagctggagcat-3′; fatty acid synthase, 5′-ttccaagacgaaaatgatgc-3′ and 5′-aattgtgggatcaggagagc-3′; PPARα, 5′-aggcagatgacctggaaagtc-3′ and 5′-atgcgtgaactccgtagtgg-3′; carnitine palmitoyltransferase I (CPT-1), 5′-cgcacggaaggaaaatgg-3′ and 5′-tgtgcccaatattcctgg-3′; acyl-CoA oxidase, 5′-cttgttcgcgcaagtgagg-3′ and 5′-caggatccgactgtttacc-3′; adiponectin, 5′-gatggcagagatggcactcc-3′ and 5′-cttgccagtgctgccgtcat-3′; leptin, 5′-ccgccaagcagagggtcac-3′ and 5′-gcattcagggctaacatccaact-3′; β-actin, 5′-aagagaggtatcctgaccct-3′ and 5′-tacatggctggggtgttgaa-3′; adipose fatty acid binding protein, 5′-tgatgcctttgtgggaacct-3′ and 5′-gcttgtcaccatctcgttttctct-3′; GLUT4, 5′-tcgtcattggcattctggttg-3′ and 5′-agctcgttctactaagagcac-3′; PPARγ, 5′-ccagagtctgctgatctgcg-3′ and 5′-gccacctctttgctctgctc-3′; CCAAT/enhancer-binding protein α, 5′-caaagccaagaagtcggtggacaa-3′ and 5′-tcattgtgactggtcaactccagc-3′; glyceraldehyde-3-phosphate dehydrogenase, 5′-cctggagaaacctgccaagtatg-3′ and 5′-agagtgggagttgctgttgaagtc-3′. The relative amount of each transcript was normalized to the amount of β-actin and glyceraldehyde-3-phosphate dehydrogenase transcript in the same cDNA.
Cell culture and differentiation
3T3-L1 preadipocytes were cultured and differentiated as described previously(Reference Shoji, Kobori, Shinmoto, Yanagida, Kanda and Tsushida21). Briefly, cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal bovine serum until confluent; 2 d after reaching confluence (D0), they were stimulated to differentiate with DMEM containing 10 % fetal bovine serum, 0·25 μmol/l dexamethasone and 0·5 mmol/l isobutylmethylxanthine for 2 d (D2). Then, the cells were incubated with DMEM containing 10 % fetal bovine serum and 5 μg/ml insulin. After 2 d (D4), the medium was replaced with DMEM, and the medium was then changed every 2 d for 5 d (D9). After the cells had been differentiated into adipocytes, the cells were treated with END for 24 h.
Measurement of PPARγ DNA binding activity
Nuclear extracts from 3T3-L1 cells were prepared using Nuclear Extract Kit (Active Motif, Tokyo, Japan). PPARγ binding to the PPAR response element (PPRE) consensus sequence was detected using a PPARγ Transcription Factor Assay Kit (Cayman Chemical Co., Ann Arbor, MI, USA).
Statistical analysis
Statistical analysis was performed using SPSS software (Chicago, IL, USA). The data are presented as means and their standard errors. Statistical significance was evaluated with ANOVA. Homogeneity of variances among groups was tested using Bartlett's test. Differences between groups were examined for statistical significance using Dunnett's test. A P value of less than 0·05 was considered statistically significant.
Results
Effect of secoisolariciresinol diglucoside on body and visceral fat weight
Fig. 1 shows the body weight of mice for 4 weeks. A high-fat diet resulted in a significant increase in body weight compared with mice fed a low-fat diet. The average body weight of mice fed a high-fat diet containing 0·5 or 1·0 % SDG increased less than that of mice fed a high-fat diet. Although the differences were not significant, the result was reproducible (data not shown). During the test period, the food intake was higher in mice fed a low-fat diet than in mice fed a high-fat diet, but was not significantly different between the high-fat and a high-fat containing 0·5 or 1·0 % SDG diet groups (Table 1). The calculated energy intake was higher in mice fed a low-fat diet than in mice fed a high-fat diet (Table 1). A 1 % SGD diet reduced the energy intake in mice fed a high-fat diet. The visceral fat gain induced by a high-fat diet was significantly reduced in mice on the high-fat diet containing 0·5 or 1·0 % SDG (Table 1).
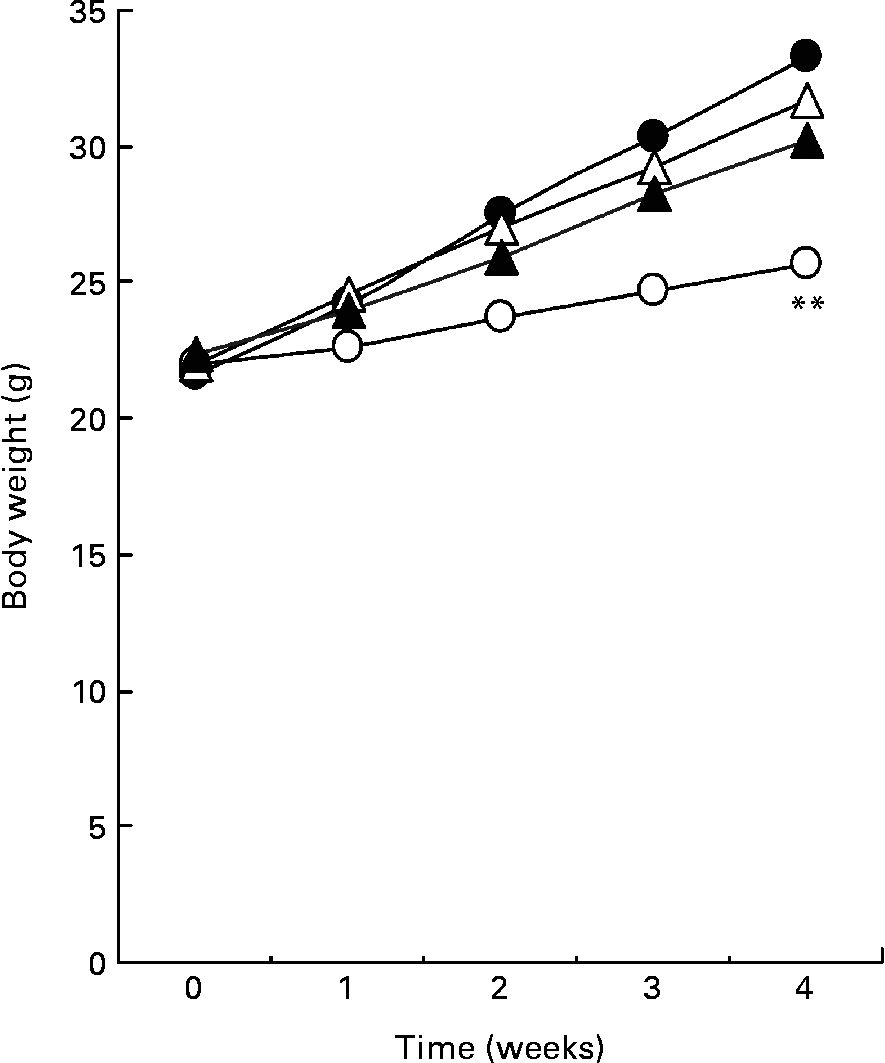
Fig. 1 Effect of secoisolariciresinol diglucoside (SDG) on body weight in mice fed different diets for 4 weeks (○, low-fat diet; ●, high-fat diet; △, high-fat diet+0·5 % SDG; ▲, high-fat diet+1·0 % SDG). Values are means (five mice per group). Mean value was significantly different from those of the high-fat diet groups at the end of the experiment: **P < 0·01.
Table 1 Effects of secoisolariciresinol diglucoside (SDG) on food and energy intake, liver lipid content and visceral fat weight in mice fed a high-fat diet†
(Mean values with their standard errors of five mice per group)

WAT, white adipose tissue.
Mean values were significantly different from those of the high-fat diet group: *P < 0·05, **P < 0·01.
† The energy intake of mice over 24 h was measured on 2 d/week during the experimental period. Liver weight, lipid content and visceral fat weight were measured after 4 weeks of feeding.
Effect of secoisolariciresinol diglucoside on liver lipid levels
As shown in Table 1, a high-fat diet significantly increased the liver TAG content as compared to a low-fat diet. A high-fat diet containing 1·0 % SDG significantly decreased the liver TAG content as compared to a high-fat diet (Table 1).
Effect of secoisolariciresinol diglucoside on serum biochemical levels
Table 2 shows the major serum biochemical parameters. A high-fat diet significantly increased serum TAG, total cholesterol, and insulin and leptin concentrations as compared to a low-fat diet. The result shows that a high-fat diet resulted in the development of hyperlipaemia, hypercholesterolaemia, hyperinsulinaemia and hyperleptinaemia. Administration of 1·0 % SDG significantly reduced serum TAG, total cholesterol, and insulin and leptin concentrations as compared to a high-fat diet (Table 2).
Table 2 Effects of secoisolariciresinol diglucoside (SDG) on blood parameters in mice fed a high-fat diet
(Mean values with their standard errors of five mice per group)
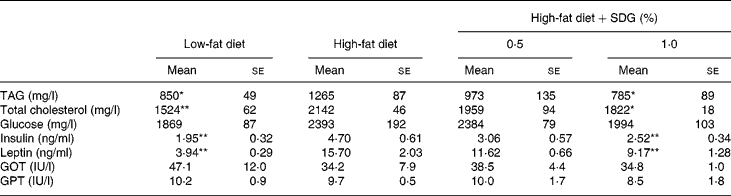
GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase.
Mean values were significantly different from those of the high-fat diet group: *P < 0·05, **P < 0·01.
Effect of secoisolariciresinol diglucoside on relative mRNA expressions of lipid metabolism-related genes in the liver
We analysed the mRNA levels of lipid metabolism-related genes in the liver by quantitative real-time PCR. When compared to a high-fat diet alone, a high-fat diet containing 0·5 and 1·0 % SDG significantly reduced the mRNA level of the transcription factor SREBP-1c, which regulates lipid synthetic genes in the liver (Fig. 2 (a)). Although the differences were not statistically significant, the mRNA level of fatty acid synthase, which is one of the target factors of SREBP-1c, also tended to be lower in a high-fat diet containing 0·5 and 1·0 % SDG than in a high-fat diet alone (Fig. 2 (a)). In contrast, differences in the mRNA expression levels of PPARα, CPT-1 and acyl-CoA oxidase, which are β-oxidation-related genes, were not significant between these experimental groups (Fig. 2 (b)).
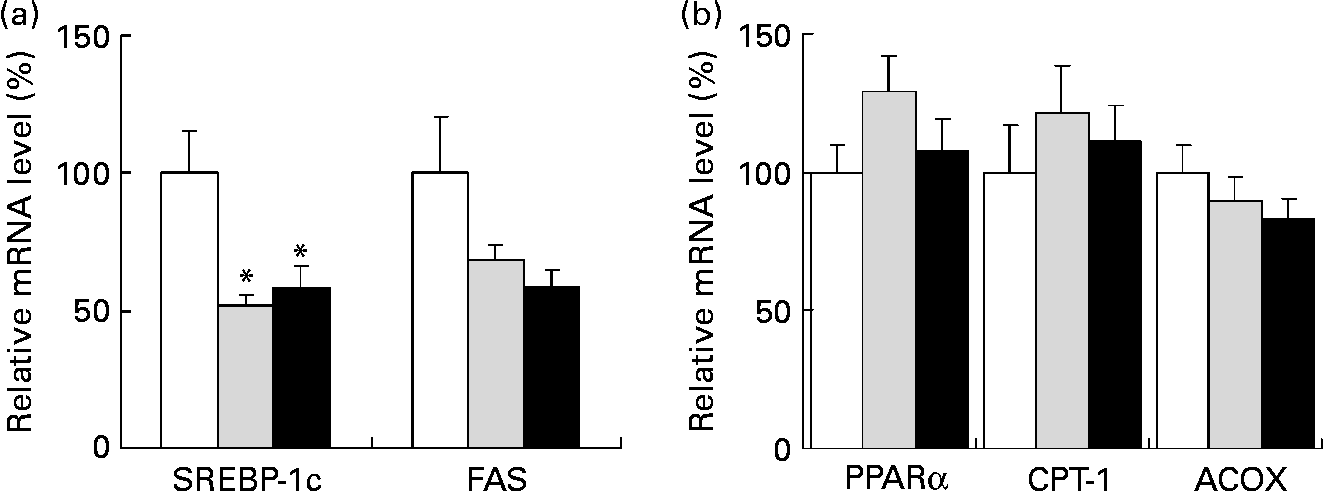
Fig. 2 Effect of secoisolariciresinol diglucoside (SDG) on mRNA levels of lipid metabolism-related genes in the liver of mice fed different diets for 4 weeks (□, high-fat diet; ![]() , high-fat diet+0·5 % SDG; ■, high-fat diet+1·0 % SDG). Shown are the amounts of mRNA of sterol regulatory element binding protein 1c (SREBP-1c) and fatty acid synthase (FAS) (fatty acid synthetic genes) (a) and PPARα, carnitine palmitoyltransferase I (CPT-1) and acyl-CoA oxidase (ACOX) (fatty acid oxidation genes) (b). Relative mRNA levels were quantified by real-time PCR as described in the Materials and methods. The mRNA values are normalized to β-actin content. Values are means with their standard errors depicted by vertical bars (five mice per group). Mean values were significantly different from those of the high-fat diet group: *P < 0·05.
, high-fat diet+0·5 % SDG; ■, high-fat diet+1·0 % SDG). Shown are the amounts of mRNA of sterol regulatory element binding protein 1c (SREBP-1c) and fatty acid synthase (FAS) (fatty acid synthetic genes) (a) and PPARα, carnitine palmitoyltransferase I (CPT-1) and acyl-CoA oxidase (ACOX) (fatty acid oxidation genes) (b). Relative mRNA levels were quantified by real-time PCR as described in the Materials and methods. The mRNA values are normalized to β-actin content. Values are means with their standard errors depicted by vertical bars (five mice per group). Mean values were significantly different from those of the high-fat diet group: *P < 0·05.
Effect of secoisolariciresinol diglucoside on adiponectin mRNA expression in white adipose tissue
We examined the mRNA levels of adiponectin, which are reduced by obesity, in white adipose tissue by quantitative real-time PCR. High-fat diets containing 0·5 and 1·0 % SDG increased the mRNA level of adiponectin from 100 (se11·3) to 166·5 (se15·9) and 204·7 (se31·8) %, respectively. Thus, a high-fat diet containing 1·0 % SDG significantly increased the mRNA level of adiponectin when compared to a high-fat diet.
Effect of secoisolariciresinol diglucoside on relative mRNA expressions of β-oxidation-related genes in skeletal muscle
Adiponectin shows acute and chronic effects on muscle metabolism by promoting β-oxidation(Reference Dyck, Heigenhauser and Bruce22). We therefore analysed the mRNA levels of β-oxidation-related genes in the skeletal muscle by quantitative real-time PCR. A high-fat diet containing 1·0 % SDG resulted in a significant increase in the mRNA level of CPT-1 compared to mice on a high-fat diet (Fig. 3). Although the differences in PPARα mRNA levels were not statistically significant between mice fed high-fat and SDG diets, the average level of PPARα mRNA was increased by the high-fat diet containing 0·5 or 1·0 % SDG compared to the high-fat diet alone (Fig. 3). In contrast, the mRNA levels of acyl-CoA oxidase were not changed in mice fed a high-fat diet and high-fat diets containing SDG (Fig. 3).

Fig. 3 Effect of secoisolariciresinol diglucoside (SDG) on mRNA levels of lipid metabolism-related genes in the skeletal muscle of mice fed different diets for 4 weeks (□, high-fat diet; ![]() , high-fat diet+0·5 % SDG; ■, high-fat diet+1·0 % SDG). Shown are the amounts of mRNA of PPARα, carnitine palmitoyltransferase I (CPT-1) and acyl-CoA oxidase (ACOX) (fatty acid oxidation genes). Relative mRNA levels were quantified by real-time PCR. The mRNA values are normalized to β-actin content. Values are means with their standard errors depicted by vertical bars (five mice per group). Mean values were significantly different from those of the high-fat diet group: *P < 0·05.
, high-fat diet+0·5 % SDG; ■, high-fat diet+1·0 % SDG). Shown are the amounts of mRNA of PPARα, carnitine palmitoyltransferase I (CPT-1) and acyl-CoA oxidase (ACOX) (fatty acid oxidation genes). Relative mRNA levels were quantified by real-time PCR. The mRNA values are normalized to β-actin content. Values are means with their standard errors depicted by vertical bars (five mice per group). Mean values were significantly different from those of the high-fat diet group: *P < 0·05.
Effects of enterodiol on adipogenesis in differentiated 3T3-L1 adipocytes
To understand the mechanism of up-regulation of adiponectin expression by SDG we examined the effect of the SDG metabolite END on adipogenesis-related gene expression in differentiated 3T3-L1 adipocytes. 3T3-L1 adipocytes were treated with different concentrations of END (0, 5, 10 and 20 μmol/l) for 24 h. As shown in Fig. 4, END significantly increased the levels of mRNA of the adipogenesis-related genes adiponectin, GLUT4, leptin and PPARγ.

Fig. 4 Effect of enterodiol (END) on adipogenesis in differentiated 3T3-L1 cells. Cells were incubated for 24 h with END at 5 (![]() ), 10 (
), 10 (![]() ) and 20 (■) μmol/l (
) and 20 (■) μmol/l (![]() , differentiated cells+dimethyl sulphoxide; □, undifferentiated cells+dimethyl sulphoxide). Relative mRNA levels were quantified by real-time PCR. The mRNA values are normalized to glyceraldehyde-3-phosphate dehydrogenase content. Values are means with their standard errors depicted by vertical bars of triplicate cultures. Mean values were significantly different from those of the differentiated cells group (no END): *P < 0·05, **P < 0·01. aP2, adipose fatty acid binding protein; C/EBPα, CCAAT/enhancer-binding protein α.
, differentiated cells+dimethyl sulphoxide; □, undifferentiated cells+dimethyl sulphoxide). Relative mRNA levels were quantified by real-time PCR. The mRNA values are normalized to glyceraldehyde-3-phosphate dehydrogenase content. Values are means with their standard errors depicted by vertical bars of triplicate cultures. Mean values were significantly different from those of the differentiated cells group (no END): *P < 0·05, **P < 0·01. aP2, adipose fatty acid binding protein; C/EBPα, CCAAT/enhancer-binding protein α.
Effects of enterodiol on PPARγ DNA binding activity in differentiated 3T3-L1 adipocytes
Activation of PPARγ involves ligand-specific conformational changes that recruit coactivators or corepressors. These protein–protein interactions lead to PPARγ heterodimerization with retinoid X receptor. This complex then binds to the specific PPRE within the promoters of PPARγ target genes, including adiponectin, leptin and GLUT4(Reference Armoni, Kritz, Harel, Bar-Yoseph, Chen, Quon and Karnieli23–Reference Marx, Duez, Fruchart and Staels27). We determined the effect of END on PPARγ DNA binding activity in differentiated 3T3-L1 adipocytes using a PPARγ Transcription Factor Assay Kit. As shown in Fig. 5, END significantly enhanced the PPARγ DNA binding activity in a dose-dependent manner.
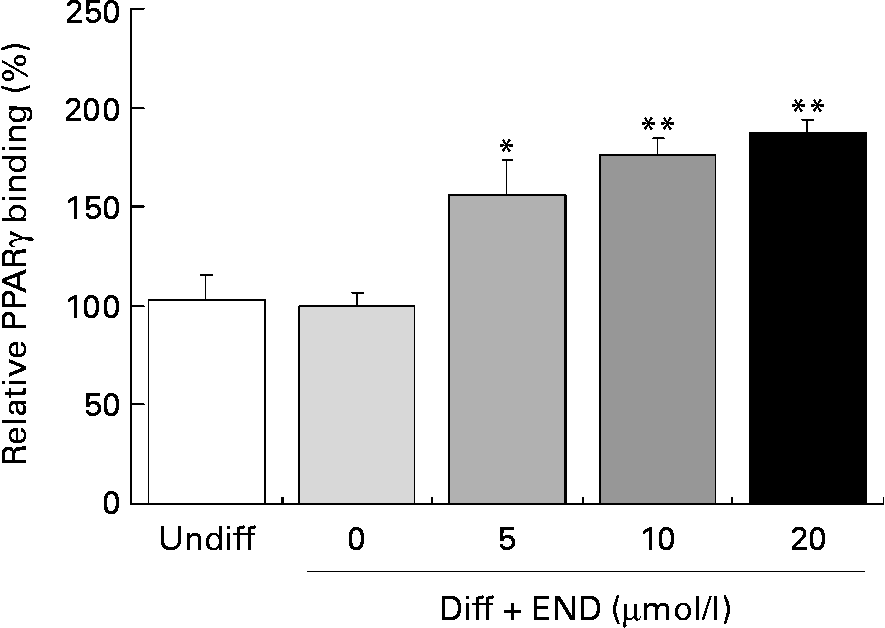
Fig. 5 Effect of enterodiol (END) on PPARγ binding activity in differentiated 3T3-L1 cells. Cells were incubated with END at 5, 10 and 20 μmol/l for 24 h. DNA binding activity was determined using a PPARγ Transcription Factor Assay Kit (ELISA) using nuclear extracts of 3T3-L1 cells. Values are means with their standard errors depicted by vertical bars of triplicate cultures. Mean values were significantly different from those of the differentiated cells group (no END): *P < 0·05, **P < 0·01. Diff, differentiated cells; Undiff, undifferentiated cells.
Discussion
In the present study, we measured the effects of dietary-supplied flaxseed lignan (SDG) on the development of diet-induced obesity in C57BL/6 mice. Flaxseed has been reported to lower human serum total cholesterol and TAG(Reference Plasad28, Reference Mandasescu, Mocanu, Dascalita, Haliga, Nestian, Stitt and Luca29). A flaxseed meal was reported to lower plasma TAG and total cholesterol and hepatic fat deposition in lean and obese rats(Reference Bhathena, Ali and Haudenschild30). The flaxseed lignan SDG was shown to reduce the serum cholesterol level and suppress hypercholesterolaemic atherosclerosis(Reference Prasad11). Here, we demonstrate that SDG reduced high-fat diet-induced visceral and liver fat accumulation, hyperlipaemia, hypercholesterolaemia, hyperinsulinaemia and hyperleptinaemia in mice. Although SDG did not significantly reduce the high-fat diet-induced body weight gain, the present results show that SDG is effective in reducing diet-induced obesity in mice. SGD at 1 % did not significantly reduce the food intake in mice fed a high-fat diet, but significantly reduced the calculated energy intake. The suppression of energy intake may partly contribute to the suppressive effect of SDG on high-fat diet-induced fat accumulation and hyperlipaemia.
Sterol regulatory element binding proteins are important transcription factors that regulate fatty acid and cholesterol metabolism in the liver. SREBP-1c in particular plays a crucial role in the dietary regulation of most hepatic lipogenic genes. A previous report demonstrated that genes involved in fatty acid and TAG synthesis that are regulated by sterol regulatory element binding proteins include fatty acid synthase(Reference Kim, Takahashi and Ezaki31). In the present study, 1·0 % SDG significantly reduced the high-fat diet-induced serum TAG, cholesterol and liver TAG levels. We also observed that a high-fat diet containing 0·5 or 1·0 % SDG resulted in a significant reduction in the mRNA levels of SREBP-1c in the liver compared to animals on a high-fat diet alone. The present findings suggest that the anti-obesity effects of dietary SDG are due to the suppression of genes involved in fatty acid and TAG synthesis through the regulatory activity of SREBP-1c.
The adipocytokine adiponectin has been shown to influence glucose and lipid homeostasis and insulin sensitivity. Adiponectin is expressed exclusively in the adipose tissue and is at lower levels in human and rodent models of obesity and type 2 diabetes(Reference Yamauchi, Kamon and Waki17). Furthermore, obesity-related decreases in plasma adiponectin levels have been reported in man and experimental animals and hypoadiponectinaemia is closely related to insulin resistance(Reference Fujita, Fujishima, Koshimura, Hosoba, Yoshioka, Shimotomai, Morii, Narita, Kakei and Ito32). Expression of adiponectin has been shown to improve insulin resistance by decreasing TAG content in muscle and liver in obese mice(Reference Yamauchi, Kamon and Waki17). Thus, increased adiponectin levels are strongly correlated with prevention of lifestyle-related diseases. In the present study, although significant differences were not detected in serum adiponectin levels in mice fed low-fat, high-fat and SDG diets, the mRNA level of adiponectin in white adipose tissue was significantly increased by SDG administration. Therefore, SDG in flaxseeds is likely to be useful as a food that can regulate adiponectin, and which can prevent or improve lifestyle-related diseases including obesity. In fact, feeding SDG has been reported to prevent lifestyle-related diseases including diabetes and arteriosclerosis(Reference Prasad11, Reference Prasad13).
CPT-1, located on the mitochondrial outer membrane, is a molecule involved in the initial, regulated step in β-oxidation of fatty acids. It was previously reported that CPT-1 mediates the transfer of the acyl-chain of the cytosolic long-chain acyl-CoA to carnitine(Reference Brown, Hill, Esser, Kirkland, Corkey, Foster and McGarry33). Adiponectin in white adipose tissue is known to promote β-oxidation in the skeletal muscle(Reference Dyck, Heigenhauser and Bruce22). In the present study, a high-fat diet containing 1·0 % SDG significantly increased the mRNA level of CPT-1 in the skeletal muscle compared to a high-fat diet alone. The result suggests that SDG increases fat oxidation in the skeletal muscle by induction of CPT-1 expression. SDG may contribute to the enhancement of fat oxidation in the skeletal muscle through the beneficial effects of adiponectin.
Adiponectin and leptin have been demonstrated to increase rates of fatty acid oxidation and are likely to be the mechanism underlying their insulin-sensitizing effects(Reference Dyck, Heigenhauser and Bruce22). GLUT4 is expressed in the insulin target tissue adipose, where it mediates an increase in glucose uptake and enhanced insulin sensitivity(Reference Armoni, Kritz, Harel, Bar-Yoseph, Chen, Quon and Karnieli23). In addition, PPARγ is one of the key transcription factors that regulate adipogenesis and glucose and lipid metabolism(Reference Rosen, Walkey, Puigserver and Spiegelman34). PPARγ regulates the expressions of adiponectin, leptin and GLUT4. SDG was reported to be metabolized to the mammalian lignans END and ENL by bacterial flora in the colon which show enhanced biological activities(Reference Axelson, Sjovall, Gustafsson and Setchell7, Reference Nesbitt, Lam and Thompson8, Reference Clavel, Henderson, Engst, Dore and Blaut35). Therefore, END and ENL but not SGD should mainly affect tissues after the consumption of SDG. We examined the effect of END and ENL on adipogenesis in differentiated 3T3-L1 adipocytes. In the present study, END significantly increased adiponectin, leptin, GLUT4 and PPARγ mRNA expression in differentiated 3T3-L1 adipocytes. END induced PPARγ binding to PPRE in a dose-dependent manner. In contrast, ENL significantly induced GLUT4 mRNA expression in differentiated 3T3-L1 cells at concentrations of 5, 10 and 20 μmol/l (data not shown). However, 5 μmol/l but not 10 or 20 μmol/l ENL significantly induced adiponectin, leptin, PPARγ and CCAAT/enhancer-binding protein α mRNA expression in 3T3-L1 adipocytes (data not shown). Moreover, 5 and 10 μmol/l but not 20 μmol/l ENL significantly induced PPARγ DNA binding activity in 3T3-L1 adipocytes (data not shown). The present results are reproducible and the disappearance of the effects of ENL at higher concentrations on adipogenesis-related gene expression and PPARγ DNA binding activity are under investigation. The present findings suggest that flaxseed lignans act as a PPARγ agonist and regulate the expression of adipogenesis-related genes, including adiponectin, leptin and GLUT4, through a PPRE-dependent mechanism in adipocytes. Previous studies demonstrated that the antidiabetic thiazolidinedione agents, troglitazone and rosiglitazone, were selective ligands for PPARγ and transcriptionally regulate adipogenesis-related genes containing the consensus PPRE(Reference Armoni, Kritz, Harel, Bar-Yoseph, Chen, Quon and Karnieli23–Reference Iwaki, Matsuda, Maeda, Funahashi, Matsuzawa, Makishima and Shimomura25, Reference Lehmann, Moore, Smith-Oliver, Wilkison, Willson and Kliewer36). Thus, flaxseed lignans as well as thiazolidinedione agents show an antidiabetic effect through PPARγ-induced gene expression.
In summary, we have shown that the flaxseed lignan SDG beneficially affected high-fat diet-induced obesity in mice. SDG reduced high-fat diet-induced visceral and liver fat accumulation, and improved hyperlipaemia, hypercholesterolaemia, hyperinsulinaemia and hyperleptinaemia. In the liver, the mRNA level of the fatty acid synthesis-related gene SREBP-1c was significantly reduced by high-fat diets containing SDG. Increases in mRNA levels of adiponectin in white adipose tissue and CPT-1 in the skeletal muscle were significantly induced by 1·0 % SDG. The present results suggest that SDG administration reduces fatty acid synthesis by suppressing SREBP-1c expression in liver and promotes β-oxidation in muscle by inducing adiponectin expression. Induction of adiponectin may also improve hyperinsulinaemia. Furthermore, we show that the SDG metabolite END induced the expression of adiponectin and other PPARγ-regulated genes in differentiated 3T3-L1 adipocytes. END is likely to transcriptionally regulate adipogenesis-related genes, including adiponectin, leptin and GLUT4 in vivo.
These effects may prevent or improve obesity and may reduce the risk of lifestyle-related diseases, including diabetes, atherosclerosis and hypertension. Flaxseeds, which also contain PUFA and dietary fibre, are therefore a promising food to help decrease the risk of lifestyle-related diseases.
Acknowledgements
We thank all members of the Kobori laboratory for helpful discussions and comments. S. F. conducted most of the experiments. K. A., N. U. and S. O. worked on the animal studies. Y. T. worked on the gene expression analysis. M. K. supervised the study and wrote the manuscript. The authors state no conflicts of interest.









