Fish has been acknowledged as an integral component of a well-balanced diet, providing a healthy source of energy, high-quality proteins, vitamins (D, A, E and B12), essential metals (Se, Mn and Cu) and especially n-3 long-chain PUFA (n-3 LC-PUFA), mainly EPA and DHA, whose pleiotropic effects in health promotion and disease prevention are increasingly known.
Hence, n-3 LC-PUFA play a vital role in human health from conception through every stage of human development, maturation and ageing(Reference Pieniak, Verbeke and Scholderer1–Reference Tur, Bibiloni and Sureda5). Reported health benefits of these fatty acids include lowering the risk of CHD(6–Reference Lopez-Huertas9) and contributing to normal neurodevelopment in children(Reference Escolano-Margarit, Ramos and Beyer10–Reference Larqué, Gil-Sánchez and Prieto-Sánchez12).
In contrast to the potential health benefits of dietary fish intake, certain chemical pollutants (e.g. heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls (PCB), polybrominated diphenyl ethers, dioxins, furans and chlorinated pesticides) contained in seafood have emerged as an issue of concern, particularly for frequent fish consumers and sensitive groups of populations(6, Reference Domingo13–Reference Olmedo, Pla and Hernández17). These chemicals have adverse effects on nervous system function, modulate the immune system and are associated with elevation in the risk of CVD. Therefore, the question of benefits and risk from fish consumption is very complex and relevant(6, Reference Bushkin-Bedient and Carpenter18).
The Codex Committee on Food Additives and Contaminants requested the Codex Alimentarius Commission to seek scientific advice from the FAO of the UN and the WHO on the health risks and health benefits of fish consumption, particularly the health risks associated with the contaminants methylmercury and dioxins (including polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) as well as dioxin-like PCB) that may be present in fish. That request was based on the growing public concern in recent years regarding the presence of chemical contaminants in fish v. the multiple nutritional benefits of including fish in the diet have become increasingly scientifically documented and proven. Indeed, the FAO and WHO held an Expert Consultation on the Risks and Benefits of Fish Consumption in 2010(6). This review should be considered as a master document to assess the risk–benefit for specific end points, including those for sensitive groups of population. In fact, the Expert Consultation drew a number of conclusions regarding the health benefits and health risks associated with fish consumption and recommended a series of steps that Member States should take to better assess and manage the risks and benefits of fish consumption and more effectively communicate these risks and benefits to their citizens.
The present review summarises the work on the benefits and risks of fish consumption presented during the World Nutrition Research Conference on Mediterranean Foods in Heath and Disease, held in Reus, Spain in May 2013, as a Satellite Conference of the 20th International Congress of Nutrition.
Nutrient composition of fish and seafood products
Fish and other seafood products, namely molluscs, crustaceans and echinoderms, consumed directly or processed provide a huge variety of products of interest for nutritional benefits in human. The detailed composition of finfish, crustacean, mollusc and echinoderm species can be found in a selected number of international databases (United States Department of Agriculture National Nutrient Database for Standard Reference Nutrient Data Laboratory (NDL)/Food and Nutrition Information Center (FNIC) Food Composition Database, http://ndb.nal.usda.gov; SELFNutritionData, http://nutritiondata.self.com; International Network of Food Data Systems Standards and Guidelines, http://www.fao.org/infoods/infoods/standards-guidelines/en/as well as in some national databases, e.g. Base española de datos de composición de alimentos, http://www.bedca.net/
Depending on the species, fish has a water content ranging 60–80 % weight and the marine invertebrates 53–96 %. The N fraction is composed of proteins (12–20 %) and of non-protein N compounds (1–2 %). Seafood proteins have a high digestibility and biological value because muscles mainly constitute sarcoplasmic (myoalbumin, globulins and enzymes) and myofibrillar proteins (actin, myosin and tropomyosin) with a very low content of connective proteins (collagen ranging 3–10 %, compared with 17 % of mammals). All essential amino acids are present in fish protein in adequate amounts compared with milk, eggs and meat. Free amino acids, namely histidine and taurine, some peptides, such as anserine and carnosine, as well as other non-protein compounds, such as free nucleotides and creatine, are also present in comparatively high amounts(Reference Oehlenschläger3, Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19).
Lipids are important nutrients for fish. Depending on its lipid content, fish is classified into lean fish ( < 2·5 % fat, mainly Gadidae and Pleuronectidae, e.g. cod, haddock, saithe and sole), medium fatty fish (2·5–6 % fat, mainly Merlucciidae and Phycidae, e.g. hake, sea bass and ocean perch) and fatty fish (>6–25 % fat, mainly Cupleidae, Engraulidae, Scombridae and Salmonidae, e.g. anchovy, herring, sardine, mackerel, tunas, bonitos and salmon). The quantitative and qualitative lipid contents vary according to the species, age, sex, period of the year, etc. n-3 LC-PUFA are key compounds abundant in sea fish, ranging from about 0·2 % weight in lean fish to about 3 % weight in fat fish. Crustaceans, shellfish and cephalopods are lean species with lipid content ranging 0·9–2·2 %(Reference Oehlenschläger3, Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19).
Fish have on average 35 mg cholesterol/100 g and contribute little to the dietary cholesterol intake. However, most of crustaceans, e.g. shrimps and prawns, show high contents of cholesterol (about 100–150 mg/100 g). Even higher levels are found in cephalopods (>200 mg/100 g) and the richest content is found in fish eggs and derived by-products such as caviar (about 500 mg/100 g)(Reference Oehlenschläger3, Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19).
The carbohydrate content of fish and other seafood products is usually lower than 0·5 %(Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19).
The amount of vitamins and minerals is species-specific and varies according to the diet and season of the year. Fish is considered a good source of Ca (about 10–100 mg/100 g), Mg (10–170 mg/100 g) and P (200–300 mg/100 g), as well as F (300–400 μg/100 g), I (10–300 μg/100 g), Se (35–45 μg/100 g), Fe (0·3–2·8 mg/100 g), Zn (0·3–1·3 mg/100 g) and Cu (0·1–0·2 mg/100 g). However, fish is a poor source of Na (20–140 mg/100 g) but rich in K (200–400 mg/100 g). Seafood products are one of the few natural sources of I and Se(Reference Oehlenschläger3, Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19). The highest Se levels are usually present in tuna, swordfish and scad(Reference Olmedo, Hernández and Pla16). Mussels, scad and sardines are the fresh species with the highest Zn levels. Molluscs and crustaceans are the major contributors of Cu and Fe; their remarkable concentration of Cu could be accounted for the presence of haemocyanin, a Cu-containing respiratory protein found in the blood of those species(Reference Olmedo, Hernández and Pla16, Reference Sirot, Dumas and Leblanc20).
Fish is rich in vitamins, namely thiamin (vitamin B1) (40–210 μg/100 g), riboflavin (vitamin B2) (50–360 μg/100 g), niacin (vitamin B3) (2–10 mg/100 g), pyridoxine (200–980 μg/100 g) and specially cobalamin (vitamin B12) (1–9 μg/100 g). Clupeidae and Engraulidae exhibit the highest content of vitamin B12. Liposoluble vitamins, mainly vitamins A and D, are mostly accumulated in the liver, although some species also exhibit a high content in the muscle mass. It is well known that high content of vitamins A and D is present in the liver of codfish species. Vitamin A in fish fillet ranges 3–180 mcg (micrograms)/100 g. The vitamin D content of fish may vary enormously and it is not well correlated with the fat content, values range 3–20 μg/100 g(Reference Oehlenschläger3, Reference Ros, Martinez-Graciá, Santaella-Pascual and Gil19, Reference Lund21).
Health outcomes related to fish and other seafood consumption
The epidemiological evidence to support the consumption of seafood is mainly derived from cohort studies. However, it is difficult to identify which nutrient is important due to the presence of other covariants in the diet(Reference Lund21).
Moderate-to-high intake of fish has been associated with a decrease in the prevalence of chronic diseases associated with obesity, namely CVD, diabetes and some cancers(Reference Oehlenschläger3, Reference Lund21). Although n-3 LC-PUFA, derived mainly from fatty fish, have been the most important focus of research related to benefits of fish consumption and because of their relevant benefits will be considered separately in the present report, protein, some non-protein N compounds, namely taurine and choline, some minerals, particularly Se, and vitamins B12 and D have been reported to be associated in protection against CHD. Seafood consumption is associated with reduced markers of inflammation(Reference Zampelas, Panagiotakos and Pitsavos22), which can explain, at least in part, its effect of protection against CVD and diabetes. It is also associated to reduced blood pressure and less vascular damage(Reference Panagiotakos, Zeimbekis and Boutziouka23). Meta-analyses of cohort studies also suggest a strong protective effect of stroke(Reference Bouzan, Cohen and Connor24, Reference He, Song and Daviglus25). This effect cannot be exclusively attributed to EPA and DHA, but also to other seafood components. Thus, taurine in experimental animals have positive effects on the cardiovascular system and diabetes. In addition, it has been associated with a number of health effects such as better fat digestion and visual acuity improvement in infants(Reference Manna, Das and Sil26). Vitamin B12 can also be important in relation to both CVD and stroke through reduction of plasma homocysteine(Reference Ryan-Harshman and Aldoori27). Mineral elements are among the most important nutrients provided by fish because they participate in many biological processes. In fact, seafood consumption may contribute to the reduction in the prevalence of mineral inadequacies and as a consequence seafood consumption could be promoted(Reference Sirot, Dumas and Leblanc20). Zn is a cofactor to more than 300 enzymes involved in important functions such as RNA and DNA metabolism and plays a major role in the stabilisation of the structure of a large number of proteins, including signalling enzymes at all levels of cellular signal transduction(Reference Chasapis, Loutsidou and Spiliopoulou28). Through its ability to change oxidation state, Cu is a well-established essential element that is required as a catalytic cofactor in numerous critical enzyme reactions(Reference Stern29). Se is a critical component of numerous selenoproteins in humans, some of which are important antioxidant systems (e.g. glutathione peroxidase) that actively protect against damage from free radicals and reactive oxygen species, which in turn could protect against cancer or CVD(Reference Flores-Mateo, Navas-Acien and Pastor-Barriuso30, Reference Greenwald, Anderson and Nelson31). Fish intervention studies, e.g. SEAFOOD Plus and AQUAMAX, funded by the European Union, have focused on biomarkers of effect. Indeed, the intake of 300–450 g/week of both cod and salmon improves the Se status and contributes to the reduction of inflammatory and cardiovascular biomarkers and lead to some benefit in weight control and blood pressure(Reference Parra, Bandarra and Kiely32). Similarly, in pregnant women, the weekly intake of two portions of salmon is associated to a better status of Se in mothers and their newborns, as well as to a higher activity of glutathione peroxidase(Reference García-Rodríguez, Mesa and Olza33). Several studies also showed that Se may protect against the toxic effects of Hg, particularly organic methylmercury(Reference Bates, Prentice and Birch34, Reference Park and Mozaffarian35). Accordingly, the Hg:Se ratio could be a useful tool to better assess the risk associated with fish intake, especially in predatory species such as tuna(Reference Olmedo, Hernández and Pla16, Reference Burger and Gochfeld36).
Large cohort studies show that fish intake is not associated with either increase or decreases in the risk of cancer overall. However, there is significant evidence of protection for colorectal cancer(Reference Geelen, Schouten and Kamphuis37) and some evidence in relation to prostate cancer(Reference Linseisen, Rohrmann and Bueno-de-Mesquita38). Not only n-3 LC-PUFA but also vitamin D and Se can have a role in this protective effect(Reference Lund21).
Health benefits of fish consumption related to n-3 long-chain PUFA
n-3 LC-PUFA are conditionally essential nutrients for adequate growth, development and function in humans. The effects of DHA and EPA are mediated by modulation of membrane biophysical properties but also by effects on cell growth, differentiation and functional maturation, and by modulating gene expression during development and at all subsequent stages of human life(Reference Gil, Serra-Majem and Calder2, Reference Corella and Ordovás39). Additionally, fish consumption and hence n-3 LC-PUFA has been associated with a reduced risk for a number of chronic diseases (Table 1).
Table 1 Levels of evidence of effects of fish and n-3 long-chain (LC) PUFA consumption on disease prevention

BC, breast cancer; CHD, coronary heart disease; CRC, colorectal cancer; PC, prostate cancer.
Neurodevelopment in pregnancy and lactation
n-3 LC-PUFA supplementation during pregnancy has been reported to moderately increase duration of gestation and birth weight(Reference Larqué, Gil-Sánchez and Prieto-Sánchez12). Most of the studies found significant differences in some visual or cognitive tests in the offspring or, at least, positive associations between DHA status in the neonate and pregnant mother(Reference García-Rodríguez, Mesa and Olza33) and neurodevelopmental outcomes(Reference Campoy, Escolano-Margarit and Anjos11). Although some inflammatory and vascular homeostasis biomarkers change during pregnancy, they are not affected by the increased intake of farmed salmon(Reference García-Rodríguez, Olza and Aguilera40). In addition, consumption of two portions of salmon per week enhances the antioxidant defence system in pregnant women. Furthermore, the effects of n-3 LC-PUFA in reducing allergic biomarkers in children seem very promising(Reference Kremmyda, Vlachava and Noakes41, Reference Miles and Calder42).
The effects of n-3 LC-PUFA supplementation in pregnant and lactating women and infants during postnatal life on neurodevelopment of children, namely the visual acuity, psychomotor development, mental performance and growth, have been evaluated(Reference Campoy, Escolano-Margarit and Anjos11). Some of these studies have reported beneficial effects of DHA supplementation during pregnancy and/or lactation especially on visual acuity outcomes and some on long-term neurodevelopment, but only a few showed positive effects on growth. In particular, some beneficial effects of perinatal DHA supply on later neurological development have been established. In a European multicentre study, neurological development was assessed in children at the age of 4 and 5·5 years after n-3 LC-PUFA supplementation using maternal supplementation with fish oil and the results suggested that higher DHA levels in cord blood may be related to a better neurological outcome at 5·5 years of age(Reference Escolano-Margarit, Ramos and Beyer10).
Some studies also evidenced that most children with inborn errors are deficient in n-3 LC-PUFA and demonstrated that supplementation might improve their neural function or prevent the progression of neurological impairment although further investigations are needed on this issue(Reference Gil-Campos and Sanjurjo Crespo43).
Dementia and age related cognitive impairment
Observational epidemiological and case–control studies largely support a protective role of fish and other sources of n-3 LC-PUFA consumption on cognitive function with advancing age, albeit with important unexplained heterogeneity in findings(Reference Dangour, Andreeva and Sydenham44). Indeed, there is not enough scientific evidence to support the routine use of n-3 LC-PUFA supplements for the prevention, or amelioration, of cognitive decline in later life.
Cardiovascular and other inflammatory diseases
Dietary n-3 LC-PUFA are associated with plasma biomarker, reflecting lower levels of inflammation and endothelial activation in CVD and other chronic and acute diseases, including chronic renal disease, sepsis and acute pancreatitis(Reference Rangel-Huerta, Aguilera and Mesa45). In fact, EPA gives rise to eicosanoid mediators that are less inflammatory than those produced from arachidonic acid and both EPA and DHA give rise to lipoxins, resolvins, protectins and other mediators that are anti-inflammatory and inflammation resolving(Reference Serhan, Krishnamoorthy and Recchiuti46).
n-3 LC-PUFA are effective in preventing cardiovascular events, especially in persons with high cardiovascular risk. The accumulated evidence indicates that marine n-3 LC-PUFA, when administered as food or in supplements for at least 6 months, reduces cardiovascular events by 10 %, cardiac death by 9 % and coronary events by 18 %, while showing a trend for a lower total mortality. These results are based on the evaluation of studies that included mainly persons with high cardiovascular risk and on studies that are highly heterogenic in the dose administered, although there is no evidence of dose-dependent protection(Reference Delgado-Lista, Perez-Martinez and Lopez-Miranda8, 47).
High doses of n-3 LC-PUFA (>3 g/d) produce a small but significant decrease in blood pressure, especially systolic blood pressure, an important risk factor for cardiac and brain events, in older and hypertensive subjects(Reference Cabo, Alonso and Mata48). In addition, the well-known hypotriglyceridaemic effect of n-3 LC-PUFA may produce further benefits by reducing the percentage of pro-atherogenic small dense LDL particles and also perhaps by ameliorating the inflammatory process associated with metabolic syndrome, which in turn is associated with diabetes mellitus and CVD(Reference Lopez-Huertas9). However, based on all available evidence from prospective studies, neither EPA or DHA nor fish/seafood intake have significant associations with the risk of diabetes mellitus overall(Reference Wu, Micha and Imamura49).
Marine n-3 PUFA have been shown to have a fairly consistent, but modest, benefit of lowering inflammatory symptoms in rheumatoid arthritis, namely joint swelling and pain, duration of morning stiffness, global assessments of pain and disease activity, and use of non-steroidal anti-inflammatory drugs(Reference Miles and Calder42). However, available data do not allow supporting the use of n-3 LC-PUFA supplementation for the treatment of both active and inactive inflammatory bowel disease(Reference Cabré, Mañosa and Gassull50).
Cancer
Observational studies on colorectal, prostate and breast cancers only provide limited evidence suggesting a possible role of n-3 LC-PUFA in cancer prevention. Knowing the anti-inflammatory activity of these fatty acids, they could play a role as adjuvant in view of the latest randomised controlled trials on lung cancers even if randomised controlled trials on other cancers still need to be undertaken(47, Reference Gerber51).
Health risks of fish consumption
Marine toxins and infectious agents including parasites (nematodes, cestodes and trematodes) and bacteria have been found in seafood products. For example, bivalve molluscs feed by filtering large volumes of seawater. During this process, they can accumulate and concentrate pathogenic micro-organisms. The illnesses caused by these agents range from gastrointestinal diseases to severe poisonings (paralytic, amnesic, neurotoxic and diarrhoeic syndromes). Allergens have also been found in seafood products and are usually associated with allergy processes in sensitive subjects.
However, the most concerning problem from a public health point of view is the exposure to low doses of chemical pollutant mixtures (heavy metals and organic compounds such as organochlorine pesticides, polycyclic aromatic hydrocarbons, PCB, dioxins and dibenzofurans) among populations in non-occupational settings, especially women of reproductive age, pregnant or nursing women; breast-fed infants; and young children living in industrial areas. They are exposed by inhaling pollutants from industrial emissions and also by eating and drinking polluted food (including seafood) and water(Reference Rodríguez-Barranco, Lacasaña and Aguilar-Garduño52, Reference Hernández, Gil, Tsatsakis and Gupta53). Food Safety and Nutrition Agencies have raised public concern as it claimed that some pollutants (e.g. methylmercury) in certain fish species make them unsuitable for consumption by children and pregnant women, so that it is important to provide information in order to achieve a better risk assessment from seafood consumption. For example, European regulations limit the amount and type of certain contaminants that can appear in foodstuffs(54).
More than 1000 chemical substances are known to have neurotoxic effects in experimental animals. Of these, Pb, methylmercury and As are three relevant substances that have been shown to cause neurodevelopmental disorders in humans and subclinical brain dysfunction(Reference Rodríguez-Barranco, Lacasaña and Aguilar-Garduño52). A number of epidemiological studies on neurobehavioral development in children have been conducted in populations consuming seafood products(Reference Grandjean, Weihe and White55–Reference Davidson, Strain and Myers57) and there is convincing evidence of adverse neurological/neurodevelopmental outcomes in infants and young children associated with methylmercury exposure during fetal development due to maternal fish consumption during pregnancy(6, Reference Axelrad, Bellinger and Ryan58). Other important conclusions from FAO and WHO Expert Committee were as follows: (1) the absence of probable or convincing evidence of risk of CHD associated with methylmercury; (2) when comparing the benefits of n-3 LC-PUFA with the risks of methylmercury among women of childbearing age, maternal fish consumption lowers the risk of suboptimal neurodevelopment in their offspring compared with the offspring of women not eating fish in most circumstances evaluated.
Also several epidemiological studies provide limited evidence of an association between methylmercury body burden, primarily from fish consumption and CVD(Reference Hallgren, Hallmans and Jansson59, Reference Yoshizawa, Rimm and Morris60).
More recently, our research team(Reference Olmedo, Pla and Hernández17) has published the levels of Hg, Cd, Pb, Sn and As in fresh, canned and frozen fish and shellfish products from a total of 485 samples of the forty-three most frequently consumed species in Andalusia (Southern Spain). High Hg concentrations were found in some predatory species (blue shark, cat shark, swordfish and tuna), although they were below the regulatory maximum levels (Table 2 and Fig. 1). In the case of Cd, bivalve molluscs such as canned clams and mussels presented higher concentrations than fish, but almost none of the samples analysed exceeded the maximum levels regulated. Pb concentrations were almost negligible with the exception of frozen common sole, which showed median levels above the legal limit. Sn levels in canned products were far below the maximum regulatory limit, indicating that no significant Sn was transferred from the can. As concentrations were higher in crustaceans such as fresh and frozen shrimps. Storelli et al. (Reference Storelli, Normanno and Barone61) found similar Hg levels in fresh tuna (0·530 mg/kg ww) and swordfish (0·800 mg/kg ww). However, in our samples analysed, Cd concentrations were rather low, which is in contrast to Storelli et al. (Reference Storelli, Normanno and Barone61) who found remarkable Cd levels in cuttlefish and swordfish (0·85 and 0·25 mg/kg ww, respectively) caught in Italy. The risk assessment performed indicated that fish and shellfish products were safe for the average consumer, although a potential risk cannot be dismissed for regular or excessive consumers of particular fish species, such as tuna, swordfish, blue shark and cat shark (for Hg) and common sole (for Pb).
Table 2 Estimated amounts of toxic elements ingested by fish in Andalusia, Spain and their respective provisional tolerable weekly intake percentages
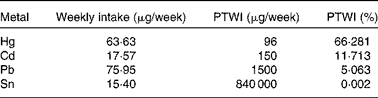
PTWI, provisional tolerable weekly intake.
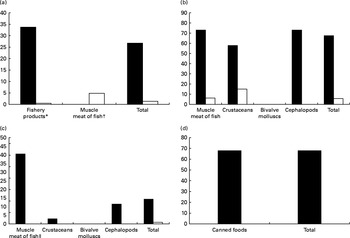
Fig. 1 Comparison between metal levels ((a) mercury, (b) lead, (c) cadmium and (d) tin) found in fish and shellfish Spanish samples analysed and the legal categories for each metal according to the European Commission (Regulation EC no. 1881/2006 amended by EC no. 629/2008 and EC no. 420/2011). * Includes muscle meat fish excluding 3.3.2 category. † Muscle meat of fish for category 3.3.2. ‡ Muscle meat of fish (all categories). ■, Number of samples and percentage under limit of detection. □, Number of samples and percentage over the maximum legal limit.
We have also published(Reference Olmedo, Hernández and Pla16) the levels of four essential elements (Cu, Mn, Se and Zn) in fish and shellfish products mentioned earlier, including the risk and nutritional assessment and Hg:Se ratios as well as Se health benefit value. Concerning Se, probably the most important of them, two fresh predatory fish species (tuna and swordfish) presented the most remarkable concentrations of this element. All the species analysed showed beneficial Hg:Se ratios and Se health benefit values, except for the shark species (blue shark and cat shark) and gilt-head bream because of their high Hg levels and low Se content, respectively. Nevertheless, the biomagnification usually observed in hazardous metals such as Hg would not occur for the essential elements measured in predatory species. The contribution of seafood products to the recommended daily allowances and adequate intakes of these mineral elements ranges from 2·5 % (Mn) to 25·4 % (Se). The species more advisable according to Se health benefit values were sardine and anchovy.
Moreover, PCDD and PCDF, PCB including dioxin-like PCB and non-dioxin-like PCB, organochlorine pesticides, and polybrominated diphenyl ethers are lipophillic organic compounds whose origin comes from many different sources. PCDD/PCDF and PCB are ubiquitous and persistent environmental pollutants with a well-known potential toxicity(Reference Domingo and Bocio62, Reference Yu, Guo and Zeng63). Dioxin and PCB levels in fish are usually low and potential carcinogenic and other effects are outweighed by potential benefits of fish intake and should have little impact on choices or consumption of seafood(Reference Mozaffarian and Rimm14). Although dioxins can cause a variety of adverse health effects, including cancer, effects on the immune system, reproductive system, nervous system and endocrine system, it has been concluded that at levels of maternal exposure to dioxins (from fish and other dietary sources) that do not exceed the provisional tolerable monthly intake of 70 pg/kg body weight established by Joint FAO/WHO Expert Committee on Food Additives (JECFA) (for PCDD, PCDF and coplanar PCB), neurodevelopmental risk for the fetus is negligible. At levels of maternal exposure to dioxins (from fish and other dietary sources) that exceed the provisional tolerable monthly intake, neurodevelopmental risk for the fetus may no longer be negligible. Finally, they observed that there was insufficient evidence for adverse health effects (e.g. endocrine disruption, immunological and neurodevelopmental effects, and cancer) associated with exposure to dioxins from fish consumption(6). Only a specific fish preparation (Cantonese salted fermented fish) has been identified as being convincingly associated with nasopharyngeal cancer(64). For example, in France, PCB and dioxins were far below the regulatory thresholds in oysters ( < 0·6 pg/g), mussels ( < 0·6 pg/g) and king scallops ( < 0·4 pg/g), despite of species that filter large volumes of water to extract their food and thus could be excellent bioaccumulators of marine environmental pollutants(Reference Guéguen, Amiard and Arnich65). However, it is always necessary to consider the exception of certain products from specific regions located around known heavy-point source. Generally, European consumers have higher exposure levels of PCDD/PCDF and dioxin-like-PCB, while American and Asians have relatively higher exposure levels of organochlorine pesticides and PCB. By contrast, all global populations are found to have lower exposure levels of polybrominated diphenyl ethers, which may be attributed to its relatively shorter history of use compared with PCB and organochlorine pesticides(Reference Yu, Guo and Zeng63).
Total dietary intake of PCDD/PCDF for the general population of Tarragona County was estimated to be 27·81 pg WHO-toxic equivalents/d established by the WHO in 1998, value notably lower than that found in the 2002 study (63·80 pg WHO-toxic equivalents/d). Fish and seafood were the most important contributors (28 %) to this intake, although it seems quite evident that in recent years the dietary intake of PCDD/PCDF has considerably diminished as a direct consequence of the reduction in the atmospheric emissions of these environmental pollutants(Reference Martí-Cid, Bocio and Domingo66).
Guéguen et al. (Reference Guéguen, Amiard and Arnich65) have also published the benzo(a)pyrene concentration in shellfish (marketed mussels and farmed shellfish) but does not exceed the regulatory European threshold. Martorell et al. (Reference Martorell, Perelló and Martí-Cid15) have shown an important decreasing trend in the dietary exposure to polycyclic aromatic hydrocarbons (PAH) for the population living in Catalonia and they found that fish and seafood products contributed with 3·6 % to the total PAH food intake in contrast to meat and meat products (49·2 %).
Several international projects have been developed to approach risk–benefit assessment from fish consumption. Among them, Benefit–Risk Analysis of Foods(Reference Hoekstra, Hart and Boobis67), Benefit–Risk Assessment for Food and Quality of Life-Integrated Benefit and Risk Analysis should be highlighted(6). Other specific tools have been performed for risk–benefit assessment such as European Food Safety Agency (EFSA) guidelines(68), disability-adjusted life-year(Reference Hoekstra, Verkaik-Kloosterman and Rompelberg69), quality-adjusted life-year(Reference Ponce, Bartell and Wong70), fish risk–benefit assessment by the Institute of Medicine in the USA and quantitative risk–benefit assessment of fish consumption by the USFDA(71). In general, the aim has been to establish diagrams for exposure and dose–response modelling and to compare/estimate following a matrix combining the positive effects or benefits from fish intake (e.g. from n-3 LC-PUFA) and negative effects or risks (e.g. from environmental pollutant) on the common health end points (e.g. child intelligence quotient or mortality), depending on the number of servings per week. Thereby, these matrices allow estimating changes in these end points resulting from the child's mother having consumed fish with different contaminants and essential elements including EPA and DHA contents at different servings per week.
Finally, Table 3 summarises the main health outcomes associated with seafood product consumption including the benefits and risks(Reference Oehlenschläger3, Reference Lund21–Reference Ryan-Harshman and Aldoori27).
Table 3 Health benefits v. risks derived from regular fish consumption*
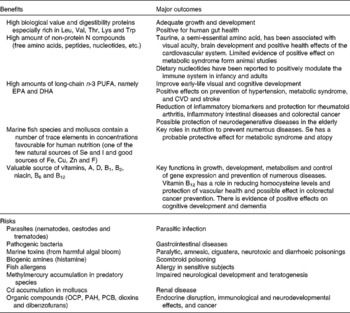
OCP, organochlorine pesticides; PAH, polycyclic aromatic hydrocarbons; PCB, polychlorinated biphenyls.
* Based on Oehlenschläger(Reference Oehlenschläger3), Lund(Reference Lund21), Zampelas et al. (Reference Zampelas, Panagiotakos and Pitsavos22), Panagiotakos et al. (Reference Panagiotakos, Zeimbekis and Boutziouka23), Bouzan et al. (Reference Bouzan, Cohen and Connor24), He et al. (Reference He, Song and Daviglus25), Manna et al. (Reference Manna, Das and Sil26), Ryan-Harshman & Aldoori(Reference Ryan-Harshman and Aldoori27).
Conclusions
Fish consumption and hence n-3 LC-PUFA have key roles in neurodevelopment and in the prevention of chronic diseases, particularly cardiovascular pathologies. Consumer vigilance is necessary among regular fish consumers, and especially for those residing in fishing areas, for pregnant and breast-feeding women, and for very young children. In addition, general recommendations about fish consumption should be done taking into account the data concerning levels of environmental pollutants in the most consumed marine species in each specific region or country. According to the FAO/WHO Expert Committee, the fish species, the frequency of consumption and the meal size are essential issues for an adequate balance of the health benefits and risks of a regular fish intake. In conclusion, for major health outcomes among adults, the vast majority of epidemiological studies have proven that the benefits of fish intake exceed the potential risks excepting a few selected species in sensitive populations. However, in order to minimise the risk in these specific populations, it would be necessary to develop and improve existing databases on specific contaminants in seafood products. Therefore, fish consumption is beneficial to health although seafood products must be controlled, especially on environmental pollutants.
Acknowledgements
This work was financially supported by the Redes Temáticas de Investigación Cooperativa (A. G., Red no. RD08/0072/0028 and SAMID RD12/0026/0015) and the Instituto de Salud Carlos III-Fondo de Investigación Sanitaria (F. G., Project no. PI10/00527), Ministry of Health, Spain.
The authors' contributions are as follows: A. G. and F. G. have contributed equally to the conception, design and discussion of the revision article and to the writing of the manuscript; A. G. (a nutritionist and biochemistry scientist) has contributed especially to the benefits section; F. G. (a toxicologist scientist) has mainly carried out the risk section of seafood product consumption.
None of the authors has no conflict of interest.






