Soya milk, as a protein-rich drink containing isoflavones, is increasingly consumed in developed countries. Potential benefits include relief from hot flushes, improved lipid profiles, protection against oxidative damage to DNA and, in particular, maintenance of bone health(Reference Lydeking-Olsen, Beck-Jensen and Setchell1–Reference Atkinson, Compston and Day3). Long-term consumption of isoflavones can have bone-sparing effects due to attenuation of bone loss(Reference Atkinson, Compston and Day3, Reference Chen, Ho and Lam4). Little is known on whether fermentation of soya milk will also affect Ca absorption from the small intestine.
Natural soya milk contains approximately 20 mg Ca/100 ml compared with cows' milk which contains approximately 120 mg Ca/100 ml. Commercially available soya milk is now fortified to the same level as cows' milk by adding fortificants such as calcium phosphate or carbonate. Not all Ca fortificants, however, are equivalent(Reference Heaney, Rafferty and Bierman5). The bioavailability rather than the total content of Ca in soya milk is thus an important issue. Ca bioavailability is improved by the presence of high amounts of soluble Ca in food(Reference Schroder, Griffin and Specker6, Reference Theobald7) and by facilitating ionisation of Ca in the digestive system.
One way to potentially enhance the biological activity and nutritional value of soya milk is through fermentation with probiotics. The fermentation of soya milk in vitro with β-glucosidase-producing probiotic bacterial strains allows acetyl-glucoside and β-glucoside isoflavones to undergo enzymatic hydrolysis into biologically available aglycone structures and also increases Ca solubility(Reference Tang, Shah and Wilcox8). Aglycones are absorbed faster and in greater amounts than their corresponding glucosides(Reference Izumi, Piskula and Osawa9, Reference Kano, Takayanagi and Harada10).
In addition, probiotics are a living microbial food supplement which may have beneficial effects on symptoms of lactose intolerance, atopic disorders and coeliac disease, and they are useful in the treatment of diarrhoea, ulcerative colitis and irritable bowel syndrome(Reference Gibson, Rastall, Fiuller, Fuller and Perdigon11). Claims are also made for cholesterol-lowering effects, anti-carcinogenic actions and improved immune function(Reference Tannock12).
Our hypothesis is that fermented soya milk may have greater Ca bioavailability as measured by fractional Ca absorption than an otherwise equivalent non-fermented soya milk.
Post-menopausal women are at high risk of osteoporosis following increased bone loss. In the present study, we have investigated whether fermentation of fortified soya milk improves hourly fractional Ca absorption. A well-established crossover radioisotope method was used to compare Ca absorption from Ca-fortified soya milk v. fermented Ca-fortified soya milk in osteopenic but otherwise healthy Australian post-menopausal women.
Experimental methods
Calcium fortification of soya milk
The Ca-fortified soya milk (CFSM) used in the present study is widely sold throughout Australia (So Good; Sanitarium Health Foods, NSW, Australia). It is made from soya protein isolate (4 %) and has been fortified with a ‘proprietary’ phosphate of Ca to achieve Ca content similar to that of cows' milk (120 mg/100 ml).
Labelling of the fortificant with 45Ca after soya milk manufacture
The fortificant present in the CFSM was labelled by adding 1 μg of high-specific activity 45CaCl2 to a 20 ml amount of soya milk, yielding a tracer concentration of approximately 185 kBq. Labelled CFSM was vortexed continuously for 1 min and then heat-treated (90°C for 30 min) before storing at 4°C for 24 h to allow for Ca exchange. The labelled CFSM was then either given after the 24 h exchange as the test drink or fermented before consumption with Lactobacillus acidophilus American Type Culture Collection (ATCC) 4962.
Bacteria
A pure culture of L. acidophilus ATCC 4962 was obtained from the Victoria University Culture Collection (Werribee, VIC, Australia). Purity was checked by Gram staining before storage at − 80°C in 40 % glycerol.
Fermentation of calcium-fortified soya milk
The probiotic culture L. acidophilus ATCC 4962 was activated through three successive transfers in de Man Rogosa Sharpe broth(Reference De Mann, Rogosa and Sharpe13) at 37°C for 20 h using a 2 % inoculum. The labelled CFSM was aseptically inoculated with a 1 % (v/v) inoculum, incubated at 37°C for 24 h and stored for a maximum period of 48 h at 4°C before consumption.
Human study protocol
Twelve osteopenic but otherwise healthy post-menopausal women aged 50–68 years were recruited by advertisement and screened by telephone interview. Women were included if they were post-menopausal non-smokers diagnosed as osteopenic (i.e. with bone mineral density T-score − 1 to − 2·5 as measured by dual-energy X-ray absorptiometry); were otherwise healthy (no chronic disease by self-report; including gastrointestinal, kidney, liver, parathyroid or CVD); were not taking medications or antibiotics affecting Ca absorption; and had not taken hormone replacement therapy during the preceding 12 months. The participants were required to be lactose tolerant and not allergic to soya. Each completed an eating habit questionnaire (extracted from a FFQ; Australian Cancer Council, VIC, Australia) to assess dietary Ca intake. They were asked to avoid any Ca supplements for at least 4 weeks before and during the study. The present study was conducted according to the guidelines of the 1995 Declaration of Helsinki as revised in Edinburgh, 2000, and all procedures were approved by the Southern Health Human Research and Ethics Committee (project number 07013A). Written informed consent was obtained from all the participants.
Test milk drinks for the acute pilot study
Soya milk for the acute study had a tracer concentration of 185 kBq/dose. Each dose comprises 20 ml of the test drink containing a microgram amount of 45CaCl2 (Amersham Biosciences, Rydalmere, NSW, Australia) in a total 44 mg of Ca carrier (20 mg as 45Ca and 24 mg as 40Ca present in the CFSM). Immediately after ingestion of the test soya milk, 200 ml of distilled water was consumed.
Study design
The participants arrived at Monash Medical Centre after an overnight fast and were tested for Ca absorption on two separate occasions. Treatments were randomised and given in a crossover design study with a minimum washout between tests of 3 weeks. At each test, the subjects consumed either radiolabelled CFSM or radiolabelled and fermented CFSM. At each visit, bioelectrical impedance was determined (SFB7; Impedimed, Brisbane, QLD, Australia). A 10 ml venous blood sample was collected from an antecubital vein for a baseline sample and for measurement of serum 25-hydroxyvitamin D. Participants then consumed the 20 ml test drink immediately followed by the consumption of 200 ml distilled water. Blood samples were collected after 60 min as described by Nordin et al. (Reference Nordin, Morris and Wishart14). These were centrifuged at 3500 g for 10 min, and the activity of 45Ca in 1 ml plasma aliquots was measured using a liquid scintillation counter (Wallac 1410; Perkin Elmer Life Sciences, MA, USA). Fractional Ca (α) was then calculated(Reference Marshall and Nordin15).
Calculation and statistical analysis
The sample of twelve women recruited into this crossover design pilot study provides a probability of 90 % that a treatment difference can be detected at the 5 % level of significance (two sided), if the true difference between the treatments is 15 %. This calculation is based on the assumption that the within-patient sd of the response variable is 10 %. Data from the present study were compared by Student's paired t test. SPSS for Windows (version 11.5; SPSS Australasia Limited, Melbourne, VIC, Australia) was used for statistical analyses. A P value of < 0·05 was considered as significant.
Results
Comparison of calcium absorption from calcium-fortified soya milk v. fermented calcium-fortified soya milk
Women in the study had a mean age of 54·8 (sd 12·3) years and were overweight (mean BMI 26·5 (sd 5·5) kg/m2). Mean serum 25-hydroxyvitamin D was 62·6 (sd 19·1) nmol/l (range 31–96 nmol/l). Most women had normal levels, but 38·5 % women had vitamin D insufficiency (serum 25-hydroxyvitamin D < 50 nmol/l).
The mean fractional Ca absorption (α) values for fermented CFSM compared to CFSM were 0·71 (sd 0·29) and 0·64 (sd 0·23), respectively. The mean fractional Ca absorption (α) of the fermented CFSM was approximately 10 % higher compared with that of CFSM, a difference not of statistical significance (P = 0·122). The individual differences in fractional Ca absorption between fortified soya milk and fermented fortified soya milk in the participants are shown in Fig. 1.
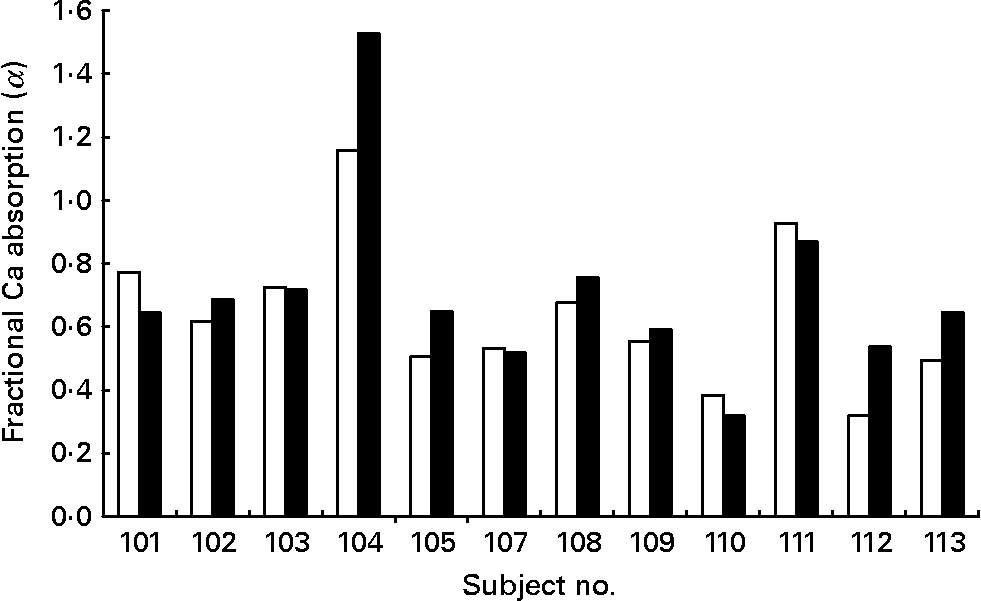
Fig. 1 Fractional Ca absorption from calcium-fortified soya milk (□) and fermented Ca-fortified soya milk (■) in twelve individual osteopenic post-menopausal women.
Discussion
In vitro studies indicate that fermentation of soya milk with some probiotics may enhance Ca solubility and bioavailability(Reference Tang, Shah and Wilcox8, Reference Tsangalis, Ashton and Stojanovska16). To date, no other studies have examined the effects of fermenting CFSM on Ca absorption in human subjects. In the present study, the participants were not vegetarian, and most of them (77 %) rarely consumed soya milk or soya products. Around one-third (38·5 %) of them regularly had Ca and vitamin D supplements. All performed only low to moderate physical activity. Habitual intake of Ca (by self-report) was moderate, and Ca supplementation was avoided during the study. Ca intake by the participants is, thus, unlikely to have affected the present results; moreover, from the crossover design of the study, each participant acted as their own control.
The study was based on the single isotope radiocalcium absorption test, a robust, well-validated measure of Ca absorption(Reference Nordin, Morris and Wishart14). This method would be applicable to other drinks fortified with Ca. The test can be completed over a short-time period, allowing absorption from a segment of small intestine to be followed via a sharp peak of radioactivity(Reference Nordin17). The rate of Ca absorption measured by this method correlates strongly with that measured in balance studies and correlates very highly with double isotope Ca absorption tests(Reference Nordin, Morris and Horowitz18). It is important, however, when employing this method, to use a small Ca load (e.g. 44 mg as here). Higher loads increase absorption time and will reduce test sensitivity. The larger the carrier dose, the more interference during the Ca absorption diffusion process and the less valid the single-isotope procedure(Reference Nordin, Morris and Wishart14). The labelling of the fortificant with 45Ca after soya milk manufacture was shown to have a tracer distribution pattern very similar to that when the fortificant was labelled before the soya milk manufacture, provided a heat treatment was applied(Reference Tang, Walker and Wilcox19). In the present study, the soya milk fortificant was also labelled before the fermentation. No studies have indicated negative effects of probiotics on the availability of the 45Ca radioisotope during Ca absorption, although a recent study found that Ca2+ plays a positive catalytic role for human gut colonic bacteria(Reference Zhu, Suits and Thompson20).
We have shown that fermenting CFSM with L. acidophilus ATCC 4962 did not improve fractional Ca absorption in twelve osteopenic post-menopausal women. The insignificant effect of fermentation on Ca bioavailability observed may in part reflect our choice of CFSM for fermentation. We have previously demonstrated that the fractional Ca absorption (α) from the CFSM used in the present study is comparable to that of cows' milk(Reference Tang, Walker and Wilcox19). The fortificant present in this CFSM may already be in its most absorbable form so that fermentation in this case does not significantly improve acute Ca absorption. Optimum Ca absorption (α) was observed 1 h after ingestion of the unfermented soya milk. It remains possible that fermentation will improve Ca absorption in other soya milk drinks where other methods of Ca fortification have been used. It would thus be valuable to repeat the present study with other types of commercially available fortified soya milk.
Even without change in Ca bioavailability, fermentation may have health benefits as it significantly increases aglycone content (increasing daidzein, glycitein and genistein)(Reference Tang, Shah and Wilcox8). Fermentation also increases the solubility of Ca by decreasing soya milk pH. Moreover, the phytase enzyme produced by some probiotics will hydrolyse phytic acid and IP6-generating myo-inositols with reduced numbers of phosphate groups (IP3–IP5)(Reference Haros, Bielecka and Sanz21, Reference Haros, Carlsson and Almgren22) causing a beneficial effect on Ca bioavailability. In the present study, the CFSM was made from soya protein isolate rather than from a whole soya bean, and even before fermentation, it had minimal phytic acid content.
Fig. 1 shows the individual fractional Ca absorption from CFSM to fermented CFSM. Four of the twelve post-menopausal women absorbed Ca from the fermented CFSM better than from the non-fermented CFSM (32, 28, 69 and 31 %, respectively). These women may have come from the approximate one-third of the population who are ‘equol producers’, a mechanism known to facilitate Ca absorption(Reference Setchell and Cole23). In further studies, it would be advised to assess equol-producing status via 24 h urine excretion(Reference Setchell and Cole23). Although our acute study indicates that fermentation of CFSM has no effect on Ca bioavailability under conditions of acute absorption from the small intestine, it cannot rule out the possibility that fermented CFSM facil1itates slower Ca absorption from the large intestine. Post hoc analysis suggests that for adequate statistical power, 174 subjects would be needed for the present study per treatment group. Our pilot study may thus have been underpowered to detect any small difference in bioavailability between the two test drinks. It does not exclude a greater difference with fermentation in different soya milk drinks fortified by other methods. Our findings do not therefore preclude possible benefits on long-term consumption of fermented CFSM on bone health and Ca balance.
In summary, during this acute pilot study, there was no significant improvement on fractional Ca absorption from the ingestion of CFSM fermented with L. acidophilus ATCC 4962 in osteopenic post-menopausal women. Limitations of this randomised crossover pilot study include the sample size of twelve, the use of a test soya milk with relatively high Ca bioavailability in its non-fermented state and the comparison of small-intestinal Ca absorption in the acute setting only, therefore looking at neither differences in colonic Ca absorption nor longer-term Ca accretion with the two test soya milk drinks. To observe a significant improvement in fractional Ca absorption with this particular brand of soya milk, a much larger sample size study would be required. The effects of fermentation might be observed more readily with soya milk of different composition (e.g. whole bean rather than soya protein isolate based) and Ca fortification system. Fermentation of CFSM may also contribute to the potential cumulative long-term benefits on Ca bioavailability and bone health. A longer-term study, over at least 6–12 months, looking at markers of bone turnover and bone mineral densitometry, may be needed to test this hypothesis further.
Acknowledgements
We thank members of the Department of Nuclear Medicine, the Body Composition Laboratory from the Monash Medical Centre and the Department of Medicine, Monash University, Victoria, Australia, for their kind support. The present study was supported by the Sanitarium Health Food Company from the Australian Research Council Sanitarium linkage grant funding. A. L. T. F. C. contributed to the overall study from experimental design, collection and analysing of data to writing of the manuscript; G. W., K. Z. W., N. P. S., J. F. A., B. S. and L. S. conceived the idea, designed the experiment and helped with data interpretation and editing of the manuscript; J. F. A. is currently employed by the Sanitarium Health Food Company, Australia. The sponsors of the study and the authors had no conflict of interest.



