Recent studies have suggested that n-3 PUFA are potentially useful agents for stimulating muscle growth. For example, feeding of diets enriched with n-3 PUFA results in a modest increase in muscle mass or muscle fibre diameter in pigs(Reference Huang, Zhan and Luo1, Reference Thivierge, Lévesque and Julien2). Gingras et al. (Reference Gingras, White and Chouinard3) showed that growing steers receiving an abomasal infusion of n-3 PUFA decrease whole-body phenylalanine flux and oxidative metabolism of amino acids in the fed state, indicating that the animals have increased protein anabolism. In healthy adult human subjects, n-3 PUFA supplementation (4 g Lovaza®/d) for 56 d significantly increases protein synthesis in skeletal muscle during hyperinsulinaemia–hyperaminoacidaemia. However, during the basal state, protein synthesis is not changed by n-3 PUFA supplementationReference Smith, Atherton and Reeds4. Those results indicated that the effect of dietary n-3 PUFA on protein synthesis in skeletal muscle is dependent on nutritional and/or hormonal stimuli.
The increased protein synthesis in skeletal muscle induced by n-3 PUFA supplementation is most probably due to increased activation of the mammalian target of rapamycin (mTOR) signalling pathway(Reference Smith, Atherton and Reeds4). Similarly, it has been shown that in growing steers, feeding a diet enriched with n-3 PUFA results in increased activation of the mTOR pathway in muscle during the fed state(Reference Gingras, White and Chouinard3). The mTOR pathway is critically involved in controlling cellular protein synthesis(Reference Wang and Proud5). It mediates feeding-induced protein synthesis in skeletal muscle(Reference Kimball, Jefferson and Nguyen6). Previous studies have shown that some essential amino acids, insulin and insulin-like growth factor 1 (IGF-1) are sufficient to activate the mTOR pathway(Reference Avruch, Hara and Lin7–Reference Proud9) and contribute to feeding-induced protein synthesis in skeletal muscle.
IGF-1 is critical for postnatal muscle growth. In chickens and rats, dietary n-3 PUFA have been demonstrated to increase muscle IGF-1 expression or circulating IGF-1 concentration(Reference Korotkova, Ohlsson and Hanson10, Reference Saprokina, Karus and Kuusik11). However, insulin has also been demonstrated to be an important regulator that stimulates muscle growth in growing animals(Reference Davis, Suryawan and Orellana12). In rats, dietary n-3 PUFA increase insulin binding or insulin signalling in skeletal muscle(Reference Le Foll, Corporeau and Le Guen13, Reference Liu, Baracos and Quinney14). Therefore, we hypothesise that dietary n-3 PUFA increase feeding-induced protein synthesis in skeletal muscle thorough increased IGF-1 production and/or insulin action.
In the present study, we evaluated the effect of feeding a DHA-enriched (DE) diet or soyabean oil (SO) diet on fractional protein synthesis rates (FSR) and the activation of anabolic signalling proteins in the skeletal muscle of growing pigs in the fed and feed-deprived states. To assess the involvement of IGF-1 and insulin action in muscle protein synthesis, we measured mRNA expression of IGF-1 in muscle and liver, and circulating IGF-1 concentration, the activation of insulin receptor (IR) and IR substrate 1 (IRS-1) in muscle. Another aim of the present study was to understand the mechanism underlying the regulation of mRNA expression of IGF-1 and insulin action by feeding the DE diet. Our previous microarray study showed that dietary n-3 PUFA increased the signal transducer and activator of transcription 5A (STAT5A) expression and decreased protein tyrosine phosphatase, non-receptor type 1 (PTPN1) and protein tyrosine phosphatase, non-receptor type 3 (PTPN3) expression in the skeletal muscle of pigs (H-K Wei, unpublished results). Thus, in the present study, mRNA expression of STAT5A and PTPN3 in skeletal muscle and liver, as well as the expression of PTPN1 and the activation of NF-κB in skeletal muscle were also measured.
Materials and methods
Animals and diets
A total of twelve Landrace barrows (Huazhong Agricultural University) weighing 29 (sem 1·4) kg were housed in individual cages with ad libitum access to clean fresh water. After a 7 d acclimatisation period, pigs were randomly assigned to two groups (n 6), fed diets enriched with either DHA (DE treatment) or SO (SO treatment) for 34 d. The protein and fat content and the digestible energy of the two diets were the same. The DE diet contained 7·5 % Trevera® (Novus International) and the SO diet contained 3·6 % SO. Ingredients of the diets are summarised in Table 1. Fatty acid composition of the two diets was analysed as described previously(Reference Luo, Wei and Huang15) and is listed in Table 2. Throughout the experimental period, pigs were housed individually and fed ad libitum during the trial period. The study was approved by the Huazhong Agricultural University Animal Care and Use Committee.
Table 1 Ingredients and nutrient content of the diets (as-fed basis)
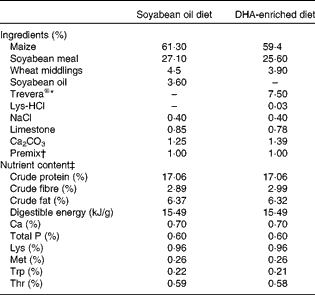
* Fatty acid profile of Trevera®: 42·3 % 22 : 6n-3, 17·7 % 22 : 5n-6, 1·9 % 20 : 5n-3, 8·5 % 14 : 0, 23·2 % 16 : 0, 0·4 % 18 : 3n-6, 0·8 % 20 : 4n-6, others 5·2 %.
† Per kg of premix provided: vitamin A, 3594 μg; vitamin D3, 62 μg; vitamin E, 200 mg; menadione, 2·5 mg; thiamin, 2·5 mg; riboflavin, 6·0 mg; niacin, 25 mg; d-pantothenic acid, 8 mg; vitamin B6, 3·0 mg; vitamin B12, 0·08 mg; d-biotin, 0·1 mg; folic acid, 12·5 mg; Cu, 20 mg; Fe, 50 mg; Mn, 30 mg; Zn, 80 mg; I, 0·8 mg.
‡ Nutrient content of the diets was calculated using published data for the individual ingredients.
Table 2 Fatty acid composition (g/100 g total fatty acids) of the diets*
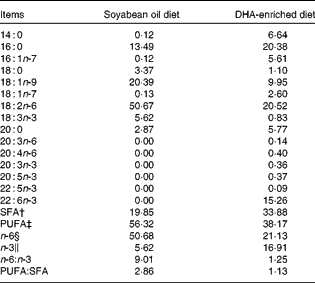
* Fatty acid percentage was analysed using GC.
† SFA percentage is the sum of 14 : 0, 16 : 0, 18 : 0, 20 : 0 and 22 : 0.
‡ PUFA percentage is the sum of 18 : 1n-9, 18 : 1n-7, 18 : 2n-6, 20 : 3n-6, 20 : 4n-6, 18 : 3n-3, 20 : 3n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
§ n-6 PUFA percentage is the sum of 18 : 2n-6, 20 : 3n-6 and 20 : 4n-6.
∥ n-3 PUFA percentage is the sum of 18 : 3n-3, 20 : 3n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
Stable isotope tracer infusion and sample collection
At 1 d before stable isotope tracer infusions, the ear vein of each pig was catheterised. At 20.00 hours, the pigs consumed their usual diets and then were food-deprived with ad libitum access to water until completion of the study the next day. At 08.00 hours the next day, primed constant infusions of [ring-2H5]phenylalanine (priming dose, 2 mol/kg body weight; infusion rate, 0·050 mol/kg body weight per min) were started. Muscle biopsy from the longissimus muscle was obtained (during local anaesthesia with 2 % lidocaine) at 09.00 hours using a 5 mm Bergström needle. At 13.00 hours, the second muscle biopsy was obtained to determine the FSR, the activation of signalling proteins, and the protein and mRNA expression of PTPN1 in the feed-deprived state.
For the feeding protocol, a meal providing a metabolisable energy of 50·2 kJ/kg body weight (15 % as protein, 55 % as carbohydrates and 30 % as fat) was partitioned into ten aliquots and provided at every 0·5 h, and the first meal was fed at 14.00 hours. The infusion rate was adjusted to 0·075 mol/kg body weight per min with a priming dose of 2 mol/kg body weight. The first muscle biopsy in the feeding state was obtained using the method described above (for determination of the protein synthesis rate, the activation of signalling proteins and the mRNA expression in the fed state) at 15.00 hours. At 19.00 hours, the second muscle biopsy in the fed state was obtained to assess phenylalanine labelling of muscle protein and muscle tissue fluid. All the pigs were humanely slaughtered after 12 h feed deprivation. Liver samples were rapidly removed and snap-frozen in liquid N2 before stored at − 80°C.
Pig plasma insulin was measured using a porcine insulin RIA kit (Linco), and IGF-1 concentration was measured using a commercial human ELISA kit (R&D Systems). Blood samples in the feed-deprived state were collected at 08.00 hours, and blood samples in the fed state were collected at 16.00 hours.
Analysis of phenylalanine tracer enrichment
Phenylalanine labelling in muscle proteins and in tissue fluid was determined using a modified version of the protocols outlined by Smith et al. (Reference Smith, Atherton and Villareal16). Samples (approximately 20 mg) were homogenised in 1 ml TCA solution (3 %, w/v). The homogenate was centrifuged at 14 000 g for 10 min. Both the pellet (consisting of muscle proteins) and the supernatant (consisting of free amino acids) were collected for further analysis. The pellet was washed and then hydrolysed in 6 m-HCl at 110°C for 24 h. Amino acids in the protein hydrolysate and supernatant samples were purified on cation exchange columns (Dowex 50 W-X8-200; Bio-Rad), and the t-butyldimethylsilyl derivative of phenylalanine was prepared to determine its tracer:trace ratio by GC–MS (QP2010 System; Shimadzu) as described by Gasier et al. (Reference Gasier, Riechman and Wiggs17). Selective ion monitoring of mass:charge (m/z) ratios 234 (m+0), 237 (m+3) and 239 (m+5) was conducted using a dwell time of 30 ms/ion. The extent of phenylalanine labelling in muscle tissue fluid and muscle protein was calculated based on the measured tracer:trace ratio of standards of known isotope labelling(Reference Patterson, Zhang and Chen18).
Calculation of fractional protein synthesis rates
The FSR of mixed muscle protein was calculated using a standard precursor-product model. The formula is as follows: ![]() $$FSR = (\Delta E _{p}/\Delta t )/( E _{M(1)} + E _{M(2)}/2)\times 60\times 100 $$, where ΔE p is the change between two consecutive biopsies in the extent of labelling (tracer:trace ratio) of protein-bound phenylalanine; E M(1) and E M(2) are phenylalanine enrichments in the free intracellular pool in the two sequential biopsies; Δt is the time between biopsies. Free phenylalanine labelling in muscle tissue fluid was chosen to represent the immediate precursor for muscle protein synthesis (i.e. aminoacyl-transfer RNA).
$$FSR = (\Delta E _{p}/\Delta t )/( E _{M(1)} + E _{M(2)}/2)\times 60\times 100 $$, where ΔE p is the change between two consecutive biopsies in the extent of labelling (tracer:trace ratio) of protein-bound phenylalanine; E M(1) and E M(2) are phenylalanine enrichments in the free intracellular pool in the two sequential biopsies; Δt is the time between biopsies. Free phenylalanine labelling in muscle tissue fluid was chosen to represent the immediate precursor for muscle protein synthesis (i.e. aminoacyl-transfer RNA).
Quantitative PCR
RNA was extracted from the longissimus muscle and liver using TRIzol (Invitrogen Corporation) as described by the manufacturer, followed by DNase digestion using a DNA-free kit (Applied Biosystems) according to the manufacturer's instructions. Synthesis of complementary DNA from 2 μg of total RNA was performed as described previously(Reference Luo, Wei and Huang15). Relative mRNA levels of genes were quantified using an iQ™127 5 Real Time PCR Detection System (Bio-Rad). 18S (for muscle sample) ribosomal RNA or β-actin (for liver sample) mRNA levels were similarly measured and served as the reference gene. Primers (Table 3) were optimised and tested for efficiency as recommended(Reference Bustin, Benes and Garson19). The PCR programme included forty cycles of denaturation at 94°C for 20 s, annealing at 60–62°C for 20 s, and extension and measuring signal at 72°C for 20 s. Results were expressed as fold changes of threshold cycle (C t) value relative to the reference gene using the ![]() $$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen20). All samples were measured in triplicate.
$$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen20). All samples were measured in triplicate.
Table 3 Primers used in quantitative PCR

IGF-1, insulin-like growth factor 1; STAT5A, signal transducer and activator of transcription 5A; PTPN1, protein tyrosine phosphatase, non-receptor type 1; PTPN3, protein tyrosine phosphatase, non-receptor type 3.
Preparation of total protein lysates and nuclear lysates
Total protein was extracted as described previously by Smith et al. (Reference Smith, Atherton and Villareal16). Muscle biopsy (approximately 20 mg) was rapidly homogenised in ice-cold buffer (50 mm-Tris–HCl (pH 7·5), 1 mm-EDTA, 1 mm-ethylene glycol tetraacetic acid, 10 mm-glycerophosphate, 50 mm-sodium flouride, 0·1 % Triton X-100 and 0·1 % 2-mercaptoethanol containing protease and phosphatase inhibitor tablet; Roche Diagnostics) at 5 μl/mg tissue. After 30 min extraction at 4°C, samples were then centrifuged at 14 000 g for 10 min at 4°C, and the supernatant containing the total protein was collected. Total protein was extracted as previously described by Syed et al. (Reference Syed, Afaq and Sarfaraz21). Fresh muscle biopsy (approximately 20 mg) was rapidly homogenised in 0·4 ml ice-cold lysis buffer (10 mm-HEPES (pH 7·9), 10 mm-KCl, 0·1 mm-EDTA, 0·1 mm-ethylene glycol tetraacetic acid, 1 mm-dithiothreitol and 1 mm-phenylmethanesulfonyl fluoride) with a protease inhibitor tablet (Roche Diagnostics) for 15 min, and then added 12·5 μl of 10 % nonyl phenoxypolyethoxylethanol (NP-40). The nuclear pellet was collected by centrifuging at 14 000 g for 1 min at 4°C and resuspended in 50 μl of ice-cold nuclear extraction buffer (20 mm-HEPES (pH 7·9), 0·4 m-NaCl, 1 mm-EDTA, 0·1 mm-ethylene glycol tetraacetic acid, 1 mm-dithiothreitol and 1 mm-phenylmethanesulfonyl fluoride) with freshly added protease inhibitor cocktail. The tubes were centrifuged at 14 000 g for 5 min at 4°C, and the supernatant (nuclear extract) was stored at − 80°C. The protein concentration in the supernatant was determined by the Bradford method with a commercial reagent (Sigma-Aldrich).
Immunoprecipitation and Western blotting
Equal amounts of protein (600 μg–1 mg) were immunoprecipitated at 4°C with antibodies against IRS-1 (Cell Signaling Technology) or IR β-chain (Santa Cruz Biotechnology). Immune complexes were collected on agarose beads and subjected to Western blot analysis. Western blot analysis was used to measure the phosphorylation of Akt, mTOR, p70-s6 kinase 1 (p70S6K1) and eIF4E-binding protein 1 (4E-BP1) and protein abundance of PTPN1 as described by Yao et al. (Reference Yao, Yin and Chu22). Briefly, the homogenate (50 g protein) from each sample was separated by SDS–PAGE and transferred on ice to methanol pre-wetted PVDF membranes. Blots were then incubated sequentially overnight at 4°C with 5 % (w/v) non-fat milk for 1 h, primary antibodies for phospho-specific mTOR (Ser2448), total mTOR, phospho-specific p70S6K1 (Thr389), total p70S6K1, phospho-specific 4E-BP1 (Thr37/46), total 4E-BP1, phospho-specific protein kinase B (PKB) (Thr308), total PKB (1:1000; all were from Cell Signaling Technology), total PTPN1 (Santa Cruz Biotechnology, 1:500) and tubulin (1:4000; Santa Cruz Biotechnology). After washing, blots were incubated with a secondary antibody conjugated with horseradish peroxidase (1:2000; New England Biolabs) for 1 h. To ensure uniformity in comparisons of samples from different blots, ratios of target protein:tubulin or phosphorylated protein:total protein were normalised relative to the mean ratio of the same two reference samples analysed in each blot. Detection was achieved using the enhanced chemiluminescence (ECL) chemiluminescence detection kit (Thermo Fisher Scientific). To measure the association of PTPN1 with IR, tyrosine phosphorylations of IR and tyrosine phosphorylations of IRS-1, equal amounts of the respective immunoprecipitants (IR and IRS-1) were subjected to SDS–PAGE followed by immunoblotting, as described above, with primary antibody for PTPN1 or phosphotyrosine (PY) (1:1000; Cell Signaling Technology).
Electrophoretic mobility shift assay
To detect DNA binding activation of NF-κB, electrophoretic mobility shift assay was performed with a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific) by following the manufacturer's protocol. The NF-κB 3′-end biotin-labelled DNA oligonucleotide (5′-TTGTTACAAGGGGACTTTCCGCTGGGGACTTTCCAGGGAGGC-3′) was used as a probe for the electrophoretic mobility shift assay. Specificity was determined by a competition assay with the addition of the unlabelled double-stranded NF-κB oligonucleotide. Binding reactions contained 10 μg nuclear extracts, 3·5 fmol of biotin-labelled probe, 2 μl of 10 × binding buffer (100 mm-Tris, 500 mm-KCl and 10 mm-dithiothreitol, pH 7·5), 1 μl of 50 % glycerol, 1 μl of 1 % NP-40, 1 μl of 100 mm-MgCl2, 0·5 μg of polydeoxyinosinic-deoxycytidylic (dI-dC), varied unlabelled concentrations and distilled water up to a total volume of 20 μl. Binding reactions were incubated at room temperature for 15 min and run on 6 % 0·5 × Tris/borate/EDTA (TBE) gels. The gels were transferred to a nylon membrane and cross-linked under UV light. Biotin was detected using streptavidin–horseradish peroxidase conjugate and the ECL chemiluminescence detection kit (Thermo Fisher Scientific).
Statistics
Effects of diet (DE diet v. SO diet), feeding (feed-deprived state v. fed state) and diet × feeding interaction on FSR in skeletal muscle, phosphorylation levels of mTOR, PKB, p70S6K1 and 4E-BP1 in skeletal muscle, IGF-1 and insulin plasma levels, and mRNA expression of IGF-1, STAT5A and PTPN were analysed by two-way ANOVA with a 2 × 2 factorial arrangement of treatments. Student's t test was used to test for the mRNA expression of IGF-1, STAT5A and PTPN3 in liver, the mRNA expression of PTPN1 and activation in skeletal muscle, and the muscle activation of NF-κB. The procedure was performed using SAS 8.0. Differences were considered significant at P< 0·05. Results are presented as mean values with their standard errors.
Results
Effects of diet and feeding on protein synthesis rate in skeletal muscle
In the feed-deprived pigs fed the SO diet, the FSR was 0·107 (sem 0·024) %/h (Fig. 1). This is similar to a previous study on pigs(Reference Watt, Corbett and Rennie23), although it is dramatically lower than that observed in rodents(Reference Baillie and Garlick24). The FSR was increased in the fed state in both treatments but reached a significantly higher level in pigs fed the DE diet than in pigs fed the SO diet as indicated by the positive interaction between diet and feeding (P< 0·05). There was no difference in skeletal muscle FSR between the diet treatments in the feed-deprived state (Fig. 1).

Fig. 1 Fractional synthesis rate (FSR) in the skeletal muscle of growing pigs fed a DHA-enriched (□) diet or soyabean oil (![]() ) diet in the fed and feed-deprived states. Values are means (n 6), with their standard errors represented by vertical bars. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). There was a significant effect for D, F or D × F interaction (P< 0·05). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc).
) diet in the fed and feed-deprived states. Values are means (n 6), with their standard errors represented by vertical bars. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). There was a significant effect for D, F or D × F interaction (P< 0·05). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc).
Effects of diet and feeding on phosphorylation levels of the mammalian target of rapamycin, protein kinase B, p70S6K1 and eIF4E-binding protein 1
For the phosphorylation levels of mTOR and PKB in muscle, there was a significant diet × feeding interaction; a higher increase in mTOR and PKB activation in the DE treatment after feeding was observed (Fig. 2(a) and (b)). In contrast, for the phosphorylation levels of p70S6K1, there was no diet × feeding interaction but only a main effect of feeding (P< 0·05; Fig. 2(c)). For the phosphorylation levels of 4E-BP1, the diet × feeding interaction tended to be significant (P< 0·10; Fig. 2(d)). Tukey's post hoc test revealed that, in the fed state, activation of 4E-BP1 in the DE treatment was significantly higher than that in the SO treatment. As expected, in the feed-deprived state, phosphorylation levels of mTOR, PKB, p70S6K1 and 4E-BP1 did not differ between the DE and SO treatments (Fig. 2(a)–(d)).

Fig. 2 (a) Phosphorylation (p) of the mammalian target of rapamycin (mTOR) at Ser2448, (b) protein kinase B (PKB) at Thr308, (c) p70S6K1 at Thr389 and (d) eIF4E-binding protein 1 (4E-BP1) at Thr37/46, in the skeletal muscle of growing pigs fed a DHA-enriched (□) diet or soyabean oil (![]() ) diet in the fed and feed-deprived states. Values are means (n 6), with their standard errors represented by vertical bars. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (a, b) There was a significant effect for D, F or D × F interaction (P< 0·05). (c) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). (d) There was a significant effect for F (P< 0·05), but no effect for D (P>0·10); however, the effect tended to be significant for D × F interaction (P< 0·10). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc).
) diet in the fed and feed-deprived states. Values are means (n 6), with their standard errors represented by vertical bars. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (a, b) There was a significant effect for D, F or D × F interaction (P< 0·05). (c) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). (d) There was a significant effect for F (P< 0·05), but no effect for D (P>0·10); however, the effect tended to be significant for D × F interaction (P< 0·10). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc).
Effects of diet and feeding on insulin-like growth factor 1 plasma levels and mRNA expression, plasma insulin concentration, and activation of insulin receptor and insulin receptor substrate 1 in skeletal muscle
For plasma IGF-1 level and IGF-1 mRNA expression in skeletal muscle, there was no diet × feeding interaction. ANOVA revealed only a main effect of feeding (P <0·05) for plasma IGF-1 level (Fig. 3(a)). In contrast, there was not only a significant effect of feeding but also a significant effect of diet for IGF-1 mRNA expression in skeletal muscle. In both feed-deprived and fed states, muscle IGF-1 mRNA expression levels in the DE treatment were significantly higher compared with the SO treatment (Fig. 3(c)). Liver IGF-1 mRNA expression did not differ between the DE and SO treatments (Fig. 3(b)).

Fig. 3 (a) Plasma insulin-like growth factor 1 (IGF-1) level, IGF-1 mRNA expression in (b) liver and (c) skeletal muscle, (d) plasma insulin level, and tyrosine (Tyr) phosphorylation of (e) insulin receptor (IR) and (f) IR substrate 1 (IRS-1) in the skeletal muscle of growing pigs fed a DHA-enriched (DE, □) diet or soyabean oil (SO, ![]() ) diet. Values are means (n 6), with their standard errors represented by vertical bars. Plasma IGF-1 level, IGF-1 mRNA expression in skeletal muscle and plasma insulin level were determined in both the fed and feed-deprived states. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (a) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). (c) There was significant F and D effects (P< 0·05), but no effect for D × F interaction (P>0·10). (d) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc). The mRNA expression of IGF-1 in liver was determined in the feed-deprived state. Tyr phosphorylation of IR and IRS-1 in skeletal muscle was determined in the fed state. † Mean value was significantly different from that of the SO treatment (P< 0·05; Student's t test).
) diet. Values are means (n 6), with their standard errors represented by vertical bars. Plasma IGF-1 level, IGF-1 mRNA expression in skeletal muscle and plasma insulin level were determined in both the fed and feed-deprived states. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (a) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). (c) There was significant F and D effects (P< 0·05), but no effect for D × F interaction (P>0·10). (d) There was a significant effect for F (P< 0·05), but no effect for D or D × F interaction (P>0·10). * Mean value was significantly different from that of the feed-deprived state (P< 0·05; Tukey's post hoc). The mRNA expression of IGF-1 in liver was determined in the feed-deprived state. Tyr phosphorylation of IR and IRS-1 in skeletal muscle was determined in the fed state. † Mean value was significantly different from that of the SO treatment (P< 0·05; Student's t test).
Similar to the plasma IGF-1 concentration, there was also no diet × feeding interaction but only a main effect of feeding (P <0·05) for plasma insulin level (Fig. 3(d)). However, in the fed state, tyrosine phosphorylation of IR in muscle was significantly higher in the DE treatment, suggesting that insulin action was increased in the muscle of pigs fed the diet enriched with DHA (Fig. 3(e)). Moreover, activation of IRS-1, a signalling protein that mediates IGF-1 and insulin-induced activation of the PKB–mTOR pathway, was also increased (Fig. 3(f)).
Effects of diet or feeding on mRNA expression of the signal transducer and activator of transcription 5A and protein tyrosine phosphatase, non-receptor type 3 in skeletal muscle and liver
The mRNA expression of STAT5A (Fig. 4(a)) and PTPN3 (Fig. 4(c)) in liver was not different between the DE and SO treatments. In skeletal muscle, there was only a main effect of diet (P <0·05) for mRNA expression of STAT5A (Fig. 4(b)) and PTPN3 (Fig. 4(d)). In both feed-deprived and fed states, Tukey's post hoc test revealed a significantly higher muscle mRNA expression of STAT5A in the DE treatment. In contrast, in both feed-deprived and fed states, a significant inhibition of the mRNA expression of PTPN3 in skeletal muscle was observed in the DE treatment.

Fig. 4 Signal transducer and activator of transcription 5A (STAT5A) mRNA expression in (a) liver and (b) skeletal muscle, and protein tyrosine phosphatase, non-receptor type 3 (PTPN3) mRNA expression in (c) the liver and (d) skeletal muscle of growing pigs fed a DHA-enriched (DE, □) diet or soyabean oil (SO, ![]() ) diet. Values are means (n 6), with their standard errors represented by vertical bars. The mRNA expression of STAT5A and PTPN3 in skeletal muscle was determined in both the fed and feed-deprived states. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (b, d) There was a significant effect for D (P< 0·05), but no effect for F or D × F interaction (P>0·10). * Mean values were significantly different between the feed-deprived and fed states (P< 0·05; Tukey's post hoc). Data of mRNA expression of STAT5A and PTPN3 in liver were determined in the fed state.
) diet. Values are means (n 6), with their standard errors represented by vertical bars. The mRNA expression of STAT5A and PTPN3 in skeletal muscle was determined in both the fed and feed-deprived states. Two-way ANOVA was used to analyse the effects of diet (D), feeding (F) and diet × feeding (D × F). (b, d) There was a significant effect for D (P< 0·05), but no effect for F or D × F interaction (P>0·10). * Mean values were significantly different between the feed-deprived and fed states (P< 0·05; Tukey's post hoc). Data of mRNA expression of STAT5A and PTPN3 in liver were determined in the fed state.
Effects of diet on the expression and activation of protein tyrosine phosphatase, non-receptor type 1 in skeletal muscle
PTPN1, a negative regulator of insulin signalling, mRNA expression was inhibited by about 2-fold in the DE treatment compared with the SO treatment (P <0·05; Fig. 5(a)), and there was a trend towards a lower protein expression of PTPN1 in the DE treatment (P< 0·1; Fig. 5(b)). The reduced expression of PTPN1 was associated with decreased formation of PTPN1–IR complexes (P <0·05; Fig. 5(c)). To evaluate whether NF-κB was involved in regulating PTPN1 expression, the activation of NF-κB in skeletal muscle was assessed. The results showed that activation of NF-κB in the DE treatment was significantly lower than that in the SO treatment (Fig. 5(d)).

Fig. 5 mRNA expression of (a) protein tyrosine phosphatase, non-receptor type 1 (PTPN1), (b) protein expression of PTPN1, (c) association of PTPN1 with insulin receptor (IR) substrate 1 and (d) activation of NF-B in the skeletal muscle of growing pigs fed a DHA-enriched (DE) diet or soyabean oil (SO) diet in the feed-deprived state. Values are means (n 6), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the DE treatment (P< 0·05; Student's t test). † Mean value tended to be significantly different from that of the DE treatment (P< 0·10; Student's t test).
Discussion
In the present study, growing pigs were fed diets enriched with either DHA or SO for 34 d. We showed that growing pigs fed the DE diet increased feeding-induced skeletal muscle FSR as indicated by the positive interaction between diet and feeding (Fig. 1). We also measured signalling intermediates of the insulin and IGF-1 pathways that control protein synthesis. From these results, we suggested that increased local mRNA expression of IGF-1 and insulin signalling contributed to the increased protein synthesis induced by the DE diet.
Both Smith et al. (Reference Smith, Atherton and Reeds4) and the present study showed that dietary n-3 PUFA did not affect skeletal muscle FSR in the feed-deprived state, suggesting that dietary n-3 PUFA alone is not sufficient to initiate muscle protein synthesis but enhance the muscle protein anabolic response to nutritional stimuli. Notably, feeding-induced muscle protein synthesis is mTOR dependent(Reference Kimball, Jefferson and Nguyen6). Rapamycin, a potent inhibitor of mTOR, prevents feeding-induced protein synthesis in skeletal muscle(Reference Kimball, Jefferson and Nguyen6, Reference Dickinson, Fry and Drummond25). Previous studies have suggested that mTOR activation partially mediates the stimulatory effect of dietary n-3 PUFA on muscle protein synthesis(Reference Smith, Atherton and Reeds4, Reference Smith, Atherton and Reeds26). We showed that dietary n-3 PUFA increased feeding-induced mTOR and 4E-BP1 activation (Fig. 2(a) and (d)), consistent with the hypothesis that mTOR activation might contribute to the enhanced feeding-induced muscle protein synthesis in pigs fed the DE diet.
In growing animals, feeding-induced muscle mTOR activation is caused by increased levels of IGF-1 and insulin. Insulin and IGF-1 stimulate mTOR activation by activating IRS-1, leading to the activation of PKB(Reference Rommel, Bodine and Clarke8). In the present study, increased mTOR activation was associated with increased PKB and IRS-1 activation in the DE treatment (Figs. 2(b) and 3(f)). Muscle mRNA expression of IGF-1 and tyrosine phosphorylation of IR in the DE treatment was also increased (Fig. 3(c) and (e)). These results suggested that insulin and IGF-1 were involved in the increased mTOR activation induced by the DE diet in the fed state.
For controlling muscle growth, local production of IGF-1 in muscle might be more important than circulating IGF-1(Reference Velloso27). Thus, it is not surprising that the increased local mRNA expression of IGF-1, but not liver mRNA expression of IGF-1 or circulating IGF-1, was associated with increased skeletal muscle FSR in the DE treatment. In fact, previous studies have also found that dietary n-3 PUFA increase IGF-1 expression in skeletal muscle(Reference Saprokina, Karus and Kuusik11) without altering hepatic IGF-1 expression or circulating IGF-1(Reference Saprokina, Karus and Kuusik11, Reference Dunn, Johnson and Kayser28–Reference Guernec, Chevalier and Duclos30). It is noteworthy that this tissue-dependent regulation of IGF-1 expression can now be explained from the present results. We showed that feeding the DE diet increased the mRNA expression of STAT5A (Fig. 4(b)), an essential mediator of growth hormone (GH)-induced IGF-1 expression(Reference Wang and Jiang31) in skeletal muscle but not in liver. Moreover, feeding the DE diet decreased muscle mRNA expression of PTPN3 (Fig. 4(d)), a negative regulator in GH-induced IGF-1 gene expression(Reference Pilecka, Patrignani and Pescini32), but did not affect hepatic PTPN3 expression. DHA is a ligand that binds to and activate porcine PPARγ(Reference Yu, Lin and Wu33), which can trigger STAT5A transcription(Reference Olsen and Haldosén34). Therefore, in the present study, PPARγ might mediate the increased mRNA expression of STAT5A in skeletal muscle induced by feeding the DE diet.
It was interesting to note that the effect of diet on the mRNA expression of IGF-1, STAT5A and PTPN3 in skeletal muscle was independent of the effect of feeding. However, the feeding-induced increase in muscle FSR and mTOR activation was higher in the DE treatment as indicated by the positive interaction between diet and feeding. These results suggested that, in pigs fed the DE diet, the increased feeding-induced protein synthesis and mTOR activation in skeletal muscle could not be totally explained only by the increased local mRNA expression of IGF-1. Indeed, we found that the increased response to insulin also contributed to the effect of dietary n-3 PUFA on feeding-induced muscle protein synthesis. PTPN1 is a negative regulator of insulin signalling. It interacts directly with IR to dephosphorylate tyrosine residues involved in receptor activation(Reference Goldstein, Bittner-Kowalczyk and White35). We showed that in pigs fed the DE diet, increased tyrosine phosphorylation of IR (Fig. 3(e)) in skeletal muscle was associated with decreased muscle PTPN1 expression (Fig. 5(a)) and the formation of PTPN1∙IR complexes (Fig. 5(c)). To our knowledge, this is the first study to determine the effect of n-3 PUFA on PTPN1 expression in skeletal muscle. The results from the present study provided novel and important evidence that decreased expression of PTPN1 in skeletal muscle contributed to the effect of dietary n-3 PUFA on insulin sensitivity in skeletal muscle.
NF-κB has been shown to induce the expression of PTPN1 in muscle(Reference Zabolotny, Kim and Welsh36), and n-3 PUFA can inhibit the activation of NF-κB(Reference Zhao, Joshi-Barve and Barve37). Consistent with these results, we observed lower NF-κB activation in the skeletal muscle of pigs fed the DE diet (Fig. 5(d)). These results suggested that the inhibited NF-κB activation correlated with decreased mRNA expression of PTPN1 in the skeletal muscle of pigs fed the DE diet. Because PPARγ and PPARδ activation have been demonstrated to inhibit NF-κB activation in skeletal muscle(Reference Coll, Alvarez-Guardia and Barroso38, Reference Remels, Langen and Gosker39), in the present study, the decreased NF-κB activation in pigs fed the DE diet might be PPAR dependent.
As mentioned earlier, healthy human adults taking n-3 PUFA supplementation for 56 d had increased protein synthesis during hyperinsulinaemia–hyperaminoacidaemia(Reference Smith, Atherton and Reeds4). In the present study, we showed that in growing pigs (considered as young animals), feeding the DE diet increased skeletal muscle FSR in the fed state (Fig. 1). However, in neonatal pigs, feeding of a diet enriched with n-3 PUFA has no effect on parenteral nutrition-induced protein synthesis rate in skeletal muscle(Reference Bergeron, Julien and Davis40). These data suggest that the effect of dietary n-3 PUFA on feeding-induced muscle protein synthesis was probably age dependent, but this remains to be explored in controlled studies.
Unfortunately, by using a frequent feeding model in the present study, the response of protein synthesis to insulin, IGF-1 and amino acids could not be determined individually. It will be of interest to know whether feeding an n-3 PUFA-enriched diet would increase the response of protein synthesis to IGF-1 and amino acids. Moreover, in the present study, we evaluated only the expression of genes and the activation of key signalling elements involved in protein synthesis in skeletal muscle at 1 h after the start of frequent feeding. We do not know whether the effect of diet was maintained throughout the whole feeding period.
In conclusion, the present study provided evidence that dietary n-3 PUFA increased feeding-induced muscle protein synthesis in growing pigs by promoting local mRNA expression of IGF-1 and/or insulin action in muscle. In future studies, it will be interesting to know whether the regulation of mRNA expression of IGF-1 in skeletal muscle by dietary n-3 PUFA is PPARγ dependent. Moreover, it will also be of interest to determine the interaction of PPARδ/γ activation induced by n-3 PUFA and NF-κB in skeletal muscle, and whether this interaction influences mRNA expression of PTPN1 and insulin-induced protein synthesis in skeletal muscle.
Acknowledgements
The present study was supported by the National Program on Key Basic Research Project (no. 2013CB12 7305), the National Natural Science Foundation of China (no. 30972107), the Special Fund for Agro-scientific Research in the Public Interest (no. 201003060), the Hubei Provincial Creative Team Project of Agricultural Science and Technology (no. 2007-620) and the Fundamental Research Funds for the Central Universities (2012MBDX010). We thank Novus International, Inc. for providing Treverra®. J. P., Si. J. and H.-K. W. designed the research; H.-K. W., Y. Z. and Sh. J. conducted the research; H.-K. W. analysed the data; Y.-X. T., H.-K. W. and J. P. wrote the paper. S. J. and J. P. had primary responsibility for the final content. All authors read and approved the final manuscript. All authors declare that there are no conflicts of interest.










