Fatty acids are hydrocarbon chains of varying length with one end of the chain terminated by a methyl group and the other end by a reactive carboxyl groupReference Calder, Burdge, Nicolaou and Kokotos1. The hydrocarbon chain can be saturated, monounsaturated or polyunsaturated. Unsaturated fatty acids contain double bonds between pairs of adjacent carbon atoms; monounsaturated fatty acids contain one double bond, whereas polyunsaturated fatty acids (PUFAs) contain more than one double bond. There are only two classically essential fatty acids. These are linoleic and α-linolenic acid, which cannot be synthesised de novo in animal cells and must therefore be obtained from the diet. Linoleic acid is an n-6 PUFA, described by its shorthand notation of 18 : 2n-6, which refers to an 18-carbon fatty acid with two double bonds, the first of which is on carbon atom 6 from the methyl end. α-Linolenic acid is an n-3 PUFA with a shorthand notation of 18 : 3n-3, describing an 18-carbon fatty acid with three double bonds, the first being positioned at carbon atom 3 from the methyl end (Fig. 1). Dietary sources of linoleic and α-linolenic acids include plant seeds and nuts, plant oils and margarinesReference Calder, Burdge, Nicolaou and Kokotos1. In most diets intake of linoleic acid is much greater (5 to 20-fold) than that of α-linolenic acidReference Burdge and Calder2. Both essential fatty acids can be further elongated and desaturated in animal cells forming longer chain and more unsaturated members of the n-6 and n-3 families of PUFAs (Fig. 2). The metabolism of the n-6 and n-3 fatty acids is competitive, since both pathways employ the same set of enzymes. The major end-product of the n-6 pathway is arachidonic acid (20 : 4n-6). This pathway is quantitatively the most important pathway of PUFA metabolism in humans, because linoleic acid is consumed in greater quantities than α-linolenic acid. The major end-products of the n-3 pathway are eicosapentaenoic acid (EPA; 20 : 5n-3) and docosapentaenoic acid (DPA; 22 : 5n-3); apparently relatively little α-linolenic acid proceeds along the entire metabolic pathway to give rise to docosahexaenoic acid (DHA; 22 : 6n-3)Reference Burdge and Calder2. Oily fish and fish oils contain a high proportion of the very long chain n-3 PUFAs, EPA, DPA and DHA3.
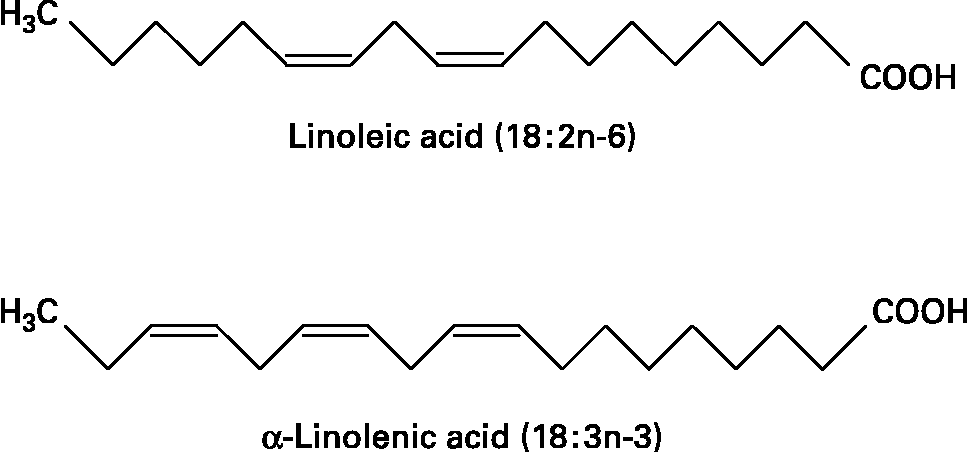
Fig. 1 The structure of the two essential fatty acids.
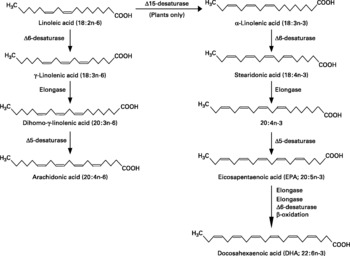
Fig. 2 The metabolic pathways by which essential fatty acids are converted to longer chain, more unsaturated derivatives.
Fatty acids play diverse roles in all cells. They are important as a source of energy, as structural components of cell membranes so influencing the physical and functional properties of membranes, and as signaling molecules and regulators of gene expressionReference Calder, Burdge, Nicolaou and Kokotos1. In addition, some PUFAs including dihomo-γ-linolenic acid (20 : 3n-6), arachidonic acid, EPA and DHA can serve as precursors for the synthesis of bioactive lipid mediators including prostaglandins, leukotrienes, lipoxins, and resolvinsReference Lewis, Austen and Soberman4–Reference Calder8. Within the immune system, different fatty acids act by differentially influencing cell membrane structure and function, cell signaling and gene expression, and patterns of lipid mediator productionReference Calder8–Reference Yaqoob11. Through this variety of mechanisms fatty acids can modify the functional activities of cells of the immune system and the immune response as a whole. There are many reviews of this areaReference Calder8–Reference Yaqoob19, and comprehensive coverage of all aspects is beyond the scope of this article. Instead this article will focus upon three specific areas in which significant new findings have been made in recent years. These are lipid rafts and T-cell signaling, antigen presentation and lipid body formation.
Lipid rafts, T cell signaling and polyunsaturated fatty acids
Lipid rafts are dynamic microenvironments in the exoplasmic leaflets of the phospholipid bilayer of plasma membranes. They are rich in saturated fatty acids, sphingolipids, cholesterol and glycosylphosphatidylinositol (GPI)-anchored proteinsReference Pike20, Reference Simons and Toomre21. Rafts serve as platforms to facilitate the association of signaling molecules and interactions and crosstalk between cell typesReference Pike20, Reference Simons and Toomre21. Activation of the proteins within rafts by an extracellular ligand can result in rapid clustering, which appears to be important for signal transduction in both T and B lymphocytesReference Pierce22–Reference Harder25. The T-cell receptor (TCR) clusters within lipid rafts upon contact with an antigen presenting cell, forming an ‘immunological synapse’, or contact zone, where intracellular signaling is thought to be initiated. Interestingly, the role of membrane rafts in Th1 and Th2 cells appears to be markedly different, with TCR activation in Th1 cells being dependent on rafts, while that in Th2 cells is notReference Balamuth, Leitenberg, Unternaehrer, Mellman and Bottomly26. The reason for this difference is not clear, but has been suggested to be due to differences in the composition, distribution or quantity of lipid raftsReference Horejsi27.
Some key signaling proteins, such as the tyrosine kinase lck and the signaling molecule linker of activated T cells (LAT), are retained in T cell rafts via acylation, while other important signaling proteins, including CD4, ZAP70 and phospholipase C-γ1, translocate to rafts upon stimulation of the TCR. Acylated proteins that are anchored to the inner lipid leaflet are displaced from rafts when T cells are treated with long chain n-3 PUFAs and to a somewhat lesser extent also with n-6 PUFAsReference Stulnig, Berger, Sigmund, Raderstorff, Stockinger and Waldhausl28. In contrast, GPI-anchored proteins remain located in detergent-resistant membranes with PUFA treatmentReference Stulnig, Berger, Sigmund, Raderstorff, Stockinger and Waldhausl28. Thus, PUFAs appear to selectively alter the protein composition of the inner membrane lipid leaflet of T cells. Notably the extent of displacement of acylated proteins from detergent-resistant membranes correlates with impairment of calcium signaling indicating a functional impact of these alterationsReference Stulnig, Berger, Sigmund, Raderstorff, Stockinger and Waldhausl28. PUFAs are readily esterified to the sn-2 position of membrane phospholipids of both rafts and the bulk plasma membrane thereby altering raft protein localizationReference Stulnig, Huber, Leitinger, Imre, Angelisoval, Nowotny and Waldhausl29. Feeding mice on an n-3 PUFA-rich diet resulted in incorporation of EPA and DHA into the lipids of the rafts of spleen T cells and this was associated with a decreased sphingomyelin contentReference Fan, McMurray, Ly and Chapkin30. Hence, incorporation of PUFAs into membrane lipids is a likely mechanism for protein displacement from rafts. Phosphorylation of LAT is the most upstream step that is inhibited by long chain n-3 PUFA treatment of T cellsReference Zeyda, Szekeres, Saemann, Geyregger, Stockinger, Zlabinger, Waldhausl and Stulnig31 and it appears that LAT displacement from rafts is a molecular mechanism mediating inhibition of T cell responses by n-3 PUFAs, at least in vitro Reference Zeyda, Staffler, Horejsi and Waldhausl32. Importantly, animal studies have shown that dietary fish oil affects early signaling events in T cells such as phosphorylation of phospholipase C-γ1Reference Sanderson and Calder33 and have linked alterations of T cell rafts by dietary n-3 PUFAs with functional changes such as decreased proliferation and interleukin-2 productionReference Fan, Ly, Barhoumi, McMurray and Chapkin34. There is no information regarding the extent to which lipid rafts in human lymphocytes could be modulated by dietary PUFAs, although human lymphocyte lipids are readily modified by fish oil supplementationReference Gibney and Hunter35–Reference Rees, Miles, Banerjee, Wells, Roynette, Wahle and Calder38.
Polyunsaturated fatty acids and antigen presentation
Studies conducted in the early to mid 1990 s found that cell surface expression of major histocompatibility complex (MHC) II and antigen presentation via MHC II are decreased following in vitro exposure of antigen presenting cells to EPA or DHAReference Fujikawa, Yamashita, Yamazaki, Sugiyama, Suzuki and Hamazaki39–Reference Hughes, Southon and Pinder43. There are limited studies investigating the effect of dietary PUFAs on MHC II expressionReference Huang, Misfeldt and Fritsche44–Reference Sanderson, MacPherson, Jenkins and Calder46. The most thorough study of this type is that of Sanderson et al. Reference Sanderson, MacPherson, Jenkins and Calder46 who showed that feeding a fish oil-rich diet to rats resulted in decreased expression of MHC II on dendritic cells which was associated with a decreased capacity to present antigen to antigen-sensitised spleen T cells. The reduction in antigen presentation was probably much greater than could be explained by the reduction in MHC II expression, suggesting that other interactions between antigen presenting cells and T lymphocytes were affected by dietary n-3 PUFAs. Indeed, levels of CD2, CD11a and CD18 were also decreased on dendritic cells from fish oil-fed ratsReference Sanderson, MacPherson, Jenkins and Calder46.
Recently the effect of PUFAs on MHC I expression and on MHC I-mediated antigen presentation were reported for the first timeReference Shaikh and Edidin47. Expression of MHC I was decreased with in vitro treatment of B lymphocytes with arachidonic acid or DHA. The effect was fatty acid concentration dependent, with arachidonic acid being slightly more effective than DHA. There was a functional effect of the reduced MHC I expression: cytotoxic T lymphocyte mediated lysis of target cells enriched with either arachidonic acid or DHA was decreased in a fatty acid concentration dependent manner. By blocking of resident MHC I molecules on the cell surface, it was identified that arachidonic acid and DHA decreased the surface appearance of new MHC I. Arachidonic acid and DHA were shown to decrease cell surface MHC I expression by slowing flow of new class I molecules from the endoplasmic reticulum to the Golgi. The lower forward trafficking rate could account for the lower level of MHC I surface expression. The finding of arachidonic acid- and DHA-induced inhibition of MHC I traffickingReference Shaikh and Edidin47 highlights a novel mechanism by which fatty acids affect the biology of antigen presentation. EPA was not examined in this work. Furthermore the work was restricted to in vitro investigations and there is an obvious need to examine these effects and mechanisms in a dietary setting.
Lipid bodies as structures for generation of fatty acid-derived mediators
Eicosanoids and other fatty acid-derived lipid mediators have numerous roles in the regulation of immune and inflammatory responsesReference Lewis, Austen and Soberman4–Reference Serhan, Arita, Hong and Gotlinger7, the true extent of which has probably not yet been realised. It is becoming clear that the regulation of eicosanoid formation involves activation of enzymes at specific intracellular sites and that this local generation of eicosanoids might be facilitated by the presence of lipid bodies present within many (if not all) cell types. Lipid bodies within eosinophils increase in number following an inflammatory stimulus and appear to contain all of the enzymes necessary for eicosanoid synthesisReference Bandeira-Melo, Bozza and Weller48, Reference Bozza, Melo and Bandeira-Melo49. Unlike lipid rafts, these distinct intracellular domains are not resistant to detergent solubilization and there are consequently some methodological limitations to their study. Since it is not yet possible to isolate lipid bodies, their structure and composition have not been elucidated.
Novel techniques have been used to cross-link newly-synthesized leukotriene (LT) C4 at sites of synthesis within eosinophils and to follow its fate upon stimulationReference Bandeira-Melo, Bozza and Weller48. This approach demonstrated that LTC4 formation does indeed occur in lipid bodies and that, depending on the nature of the stimulus, LTC4 can be either targeted towards the perinuclear membrane or released into the extracellular milieuReference Bandeira-Melo, Bozza and Weller48, Reference Bandeira-Melo, Phoofolo and Weller50. Like lipid rafts, the distribution of lipid bodies can be polarized, but it is not clear whether those producing eicosanoids destined to be secreted are located close to the plasma membrane, while those that are perinuclear produce eicosanoids solely for autotropic effects. Recent studies have identified roles for lipid bodies in inflammatory mediator synthesis in a range of situations. A potential role for lipid bodies in sepsis is suggested by the observation that their numbers are higher in leukocytes from septic patients compared with healthy patients and that they are inducible by lipopolysaccharide in murine macrophagesReference Pacheco, Bozza, Gomes, Bozza, Weller, Castro-Faria-Neto and Bozza51. Administration of lipopolysaccharide to mice resulted in increased lipid body content of peritoneal cells and this was associated with increased prostaglandin (PG) E2 and LTB4 in peritoneal lavage fluidReference Leite, Pacheco, Gomes, Guedes, Castro-Faria-Neto, Bozza and Koatz52. Lipid body formation and presence of inflammatory mediators was examined in mice fed different oils prior to lipopolysaccharide exposure: olive oil was found to decrease peritoneal leukocyte infiltration, lipid body formation and PGE2 and LTB4 levelsReference Leite, Pacheco, Gomes, Guedes, Castro-Faria-Neto, Bozza and Koatz52. This was associated with a significant reduction in mortalityReference Leite, Pacheco, Gomes, Guedes, Castro-Faria-Neto, Bozza and Koatz52. Fish oil was not examined in that study but it might be expected to have similar effects since it is known that dietary fish oil decreases in vivo and ex vivo inflammatory mediator responses to lipopolysaccharideReference Yaqoob and Calder53, Reference Sadeghi, Wallace and Calder54, and improves survivalReference Mascioli, Leader, Flores, Trimbo, Bistrian and Blackburn55, Reference Mascioli, Iwasa, Trimbo, Leader, Bistrian and Blackburn56. Other recent studies have attempted to address the mechanism of lipid body formation in the different contexts. In murine models of allergy, lung eosinophil influx and lipid body formation occurred in parallel in response to an allergic challengeReference Vieira-de-Abreu, Assis, Gomes, Castro-Faria-Neto, Weller, Bandeira-Melo and Bozza57. 5-Lipoxygenase expression and LTC4 production were associated with lipid bodies. The induction of lipid body formation appeared to involve either eotaxin or RANTES acting via the CCR3 receptorReference Vieira-de-Abreu, Assis, Gomes, Castro-Faria-Neto, Weller, Bandeira-Melo and Bozza57. Infection of macrophages with Mycobacterium bovis bacillus induced lipid body formation which was associated with increased PGE2 production and localization of cyclooxygenase-2 to the lipid bodiesReference D'Avila, Melo, Parreira, Werneck-Barroso, Castro-Faria-Neto and Bozza58. The authors confirmed that lipid bodies were the main site of new PGE2 synthesis. The effect of infection on induction of lipid bodies was inhibited in toll-like receptor 2, but not toll-like receptor 4, deficient miceReference D'Avila, Melo, Parreira, Werneck-Barroso, Castro-Faria-Neto and Bozza58, suggesting a role for the former in lipid body formation in macrophages in response to infection. It is clear that more needs to be understood about lipid bodies in the contexts of immune activation and inflammation in humans and of formation of different families of lipid mediators from different substrate fatty acids.
Conclusion
Recent studies have focused attention on the central role of fatty acids in immune cell regulation, highlighting that their location and organization within cellular lipids has a direct influence on the behaviour of a number of proteins involved in immune cell activation, including those associated with T cell responses, antigen presentation and fatty acid-derived inflammatory mediator production. Studies have tended to focus upon PUFAs per se or on PUFAs of different types (e.g. n-6 vs. n-3). While these studies clearly suggest that the fatty acid composition of lipid domains can strongly influence immune cell activation and functional responses, they have been mainly conducted in model systems and the relevance to the human setting requires urgent investigation. Nevertheless, the studies described herein indicate several novel mechanisms by which altered fatty acid availability can modulate immune responses and impact upon clinical outcomes relevant to infection and to inflammatory conditions.
Conflict of interest statement
PCC has research funding from B. Braun Melsungen, receives consultancy fees from Equazen Ltd. and Royal Dutch Numico and speaking fees from Solvay Healthcare, B. Braun Melsungen and Fresenius Kabi. PY has no conflicts of interest to declare. PCC and PY co-wrote the manuscript.




