The concentration of HDL is inversely associated with the incidence of CHD. HDL are thus considered anti-atherogenic lipoproteins(Reference Barter, Caulfield and Eriksson1, Reference Wilson, Abbott and Castelli2). One of the major anti-atherogenic activities of HDL is the regulation of cholesterol homeostasis through the reverse cholesterol transport (RCT) process. RCT involves the transport of excess cholesterol from peripheral tissues back to the liver for elimination in the bile and faeces. The initial step in RCT is thought to be the transfer of cholesterol from cell membranes to acceptor particles and then to apoA-I and HDL(Reference Glomset3–Reference Bortnick, Rothblat and Stoudt5).
HDL-mediated cholesterol efflux (CE) is the natural rate-limiting step of RCT(Reference Ruano, Lopez-Miranda and Fuentes6) and occurs via three pathways. The first is aqueous diffusion by which free cholesterol molecules spontaneously desorb from the plasma membrane, diffuse through the aqueous phase and become adsorbed on acceptor particles by collision(Reference Tall, Costet and Wang7). The second involves scavenger receptor class B type I (SR-BI)-mediated bidirectional free cholesterol exchanges that depend on the cholesterol gradient. This pathway mediates CE to a wide variety of cholesterol acceptors(Reference Yancey, Bortnick and Kellner-Weibel8). The third involves the ATP-binding cassette receptors ABCA1 and ABCG1, which mediate CE in a unidirectional manner to lipid-poor apoA-I and to other subfamily members of HDL, respectively(Reference Smith9–Reference Baldan, Bojanic and Edwards11). The capacity of HDL to mediate CE depends on their biophysical structure and biochemical composition. For example, phosphatidylcholine-enriched HDL increases CE, whereas sphingomyelin-enriched HDL decreases cholesterol influx to macrophages(Reference Yancey, de la Llera-Moya and Swarnakar12).
A large body of literature has indicated that diet is one of the most important modifiable determinants of the risk of developing CVD(Reference Brunner and Iso13, Reference Ordovas, Kaput and Corella14). Replacement of dietary SFA with higher intakes of MUFA from vegetable oils has been reported to be inversely associated with the risk of CHD(Reference Hu, Stampfer and Manson15).
Olive oil is the main source of fat in Mediterranean regions. The alleged beneficial effects of extra-virgin olive oil (EVOO) have been linked to both its MUFA (mainly oleic acid) and its antioxidant components (e.g. hydroxytyrosol and oleuropein). Olive oil phenolics exert their antioxidant effect both in vitro and in vivo and have potential cardioprotective activities (reviewed in Visioli et al. (Reference Visioli, Poli and Gall16)). Considerable attention is being paid to the potential health benefits of olive oil. Human consumption of olive oil decreases the major risk factors associated with atherosclerosis by improving the lipoprotein profile, blood pressure, glucose metabolism, oxidative stress and thrombotic profiles (reviewed in Lopez-Miranda et al. (Reference Lopez-Miranda, Perez-Jimenez and Ros17)). Olive oil supplementation also protects against LDL and HDL oxidation(Reference Castaner, Fito and Lopez-Sabater18–Reference Aviram and Eias20) and reduces total cholesterol and LDL-cholesterol levels(Reference Nicolaiew, Lemort and Adorni21). Several studies have investigated the effect of olive oil consumption on plasma HDL levels and have led to controversial results(Reference Aguilera, Mesa and Ramirez-Tortosa22–Reference Perona, Canizares and Montero25). However, no studies have investigated the effect of olive oil consumption on the functionality of HDL and, particularly, on their capacity to mediate CE. However, it is becoming increasingly apparent that the anti-atherogenic effects of HDL not only depend on their concentration and cholesterol content in the circulatory system, but also, and more importantly, on their biological functionalities(Reference Movva and Rader26). Furthermore, studies have reported that diet, such as supplementation with MUFA in human subjects, influences lipoprotein functionality by enhancing HDL-mediated CE(Reference Sola, Motta and Maille27, Reference Rosenblat, Volkova and Coleman28) and that EVOO consumption increases the phenolic compound content of LDL and HDL, which prevents lipid peroxidation and promotes the anti-atherogenic effect of HDL(Reference Rosenblat, Volkova and Coleman28).
The goals of the present study were to investigate the effect of 12 weeks of EVOO consumption on the capacity of HDL to promote CE and to determine which CE pathways are modulated by EVOO consumption.
Materials and methods
Chemicals
SDS, EDTA and bovine serum albumin were from Sigma. Dialysis membranes were from Spectrum Medical Industries, Inc. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Wisent, Inc. All other chemicals were from Sigma-Aldrich. THP-1 cells and J774 macrophages were from the American Type Culture Collection. Fu5AH rat hepatoma cells were provided by Dr J. Genest (McGill University). Roswell Park Memorial Institute (RPMI) 1640 medium was from Invitrogen Canada, Inc. EVOO was from Atlas Olive Oil sarl.
Subjects
A total of twenty-six healthy volunteers aged between 25 and 83 years (eight men and eighteen women, mean age 53·28 (sem 20·66) years) were recruited. They were all non-smokers with a normal serum lipid profile and blood pressure and were not taking medication, including lipid-lowering drugs or oral antioxidants. Both pre- and postmenopausal women were included in the study. However, none of the women was taking oestrogen replacement therapy for menopause. They were all non-obese and none showed clinical signs of inflammation or diabetes. The clinical and biochemical parameters of the volunteers are presented in Table 1.
Table 1 Clinical and biochemical parameters of the participants at baseline and after 12 weeks of extra-virgin olive oil consumption (Mean values with their standard errors or standard deviations)
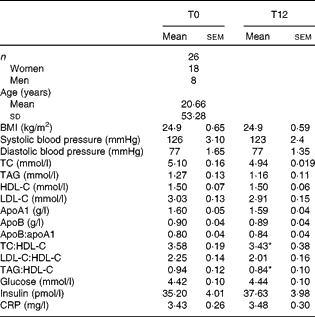
TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; CRP, C-reactive protein.
* Mean values were significantly different from those at T0 (P< 0·05; Wilcoxon matched-pairs signed-rank test).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethics Committee of the University Institute of Geriatrics of Sherbrooke (no. 2009/19). Written informed consent was obtained from all subjects.
Study design and procedure
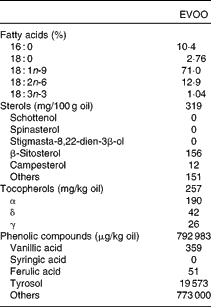
The volunteers were asked to consume 25 ml/d of raw EVOO for 12 weeks. The chemical composition of EVOO used in the study is described in Table 2(Reference Khallouki, Younos and Soulimani29). Blood tests were performed at recruitment (T0) and after 12 weeks of EVOO consumption (T12). Plasma tyrosol and hydroxytyrosol levels were measured by HPLC, using the diode-array UV detection method as developed by Ruiz-Gutierrez et al. (Reference Ruiz-Gutierrez, Juan and Cert30), to evaluate compliance.
Table 2 Chemical composition of extra-virgin olive oil (EVOO) used in the study(Reference Khallouki, Younos and Soulimani29)
Lipoprotein isolation
Fasting human plasma was collected in heparin-containing tubes. Whole HDL and HDL2/HDL3 subclasses were isolated within 2 and 4 h, respectively, using the method of Sattler et al. (Reference Sattler, Mohr and Stocker31). HDL were stored in phosphate buffer containing 10 % sucrose at − 80°C until used. Before the analysis, frozen HDL were dialysed overnight at 4°C in 10 mm-sodium phosphate buffer (pH 7·0) to remove sucrose, and protein concentrations were measured using commercial assay kits (Bio-Rad) according to the manufacturer's instructions.
Cell cultures
Human THP-1 monocytes and J774 macrophages were grown in RPMI-1640 medium and DMEM, respectively. The media were supplemented with 10 % heat-inactivated FBS, 50 nm-β-mercaptoethanol (only for THP-1), 2 mm-l-glutamine, 5 mg/ml of glucose and 100 U/ml of penicillin. THP-1 monocytes were differentiated into macrophages by cultivating the cells in the presence of 100 μm-phorbol myristate acetate for 96 h. Fu5AH hepatoma cells were grown in DMEM supplemented with 5 % heat-inactivated FBS and 100 U/ml of penicillin–streptomycin.
Human monocyte isolation and differentiation into macrophages
Human monocytes were isolated as described previously(Reference Chinetti, Griglio and Antonucci32) and were differentiated into macrophages (human monocyte-derived macrophages (HMDM)). Briefly, peripheral blood mononuclear cells were isolated by density gradient centrifugation on Ficoll-Paque™ PLUS columns according to the manufacturer's instructions (GE Healthcare). Peripheral blood mononuclear cells were resuspended in 10 % FBS-RPMI medium, plated in twelve-well plates (0·5 × 105 cells per well) pre-coated with 20 % autologous serum, and were allowed to attach for 20 min. Unattached cells were then removed from the medium. Attached monocytes were incubated with 10 % FBS-RPMI containing 100 μm-phorbol myristate acetate to differentiate them into HMDM. The medium was changed every 2–3 d. The monocytes differentiated into macrophages after 7 d. The macrophages were used to assess ABCA1, ABCG1 and SR-BI gene and protein expression at baseline (T0) and after 12 weeks of EVOO consumption (T12).
Cholesterol efflux measurements
THP-1, J774 and Fu5AH cells were incubated in fresh culture medium containing 0·2 μCi/ml of [3H]cholesterol for 24 h. The cells were then washed and incubated in serum-free medium containing 1 % bovine serum albumin for 12 h for equilibration following which they were washed and suspended in fresh medium without HDL (control), fresh medium containing 5 % serum, fresh medium containing 50 μg/ml of whole HDL, or fresh medium containing 50 μg/ml of HDL3 or HDL2 obtained from each volunteer.
In another series of experiments, J774 macrophages were incubated for 12 h with 0·3 mm-cyclic AMP to yield ABCA1-enriched macrophages. The cells were then washed and incubated with 5 % serum, HDL2 or HDL3 for 24 h. ABCG1 overexpression on THP-1 macrophages was induced by incubating phorbol myristate acetate-pretreated THP-1 macrophages with 5 μm-22(R)-hydroxy-cholesterol for 24 h(Reference Kaplan, Gan and Menke33). RT-PCR and Western blot analyses were performed to confirm the overexpression of ABCG1 mRNA and protein, respectively.
HMDM were loaded with [3H]cholesterol (1 μCi/ml) for 48 h. Labelled HMDM were washed and incubated in serum-free medium containing 1 % bovine serum albumin for 12 h for equilibration following which they were incubated for 4 h with 25 μg/ml of human apoA-I in order to measure CE.
CE was measured by liquid scintillation counting. The percentage of radiolabelled CE was calculated using the following formula:
where cpm is counts per min.
Agarose gel electrophoresis and Western blotting
HDL subclasses were analysed by agarose gel electrophoresis. Aliquots of plasma and HDL2 and HDL3 particles obtained from the volunteers at T0 and T12 were separated on 0·75 % agarose gels and transferred to a nitrocellulose membrane. ApoA-I was identified using an anti-apoA-I monoclonal primary antibody and an IgG-horseradish peroxidase secondary antibody. Immunocomplexes were visualised using an enhanced chemiluminescence Western blotting detection system followed by autoradiography.
HDL particle sizes were also measured by linear PAGE (Quantimetrix Lipoprint System; Quantimetrix). Overall, twelve plasma samples were kindly analysed by Quantimetrix to compare the effect of 12 weeks of EVOO consumption on the distribution of the HDL subclasses. The analysis was performed using polyacrylamide gel tubes and the Lipoprint LDL system (Quantimetrix)(Reference Apostolou, Gazi and Kostoula34).
Quantitative PCR analyses
RNA (2 μg) extracted from HMDM (TRIzol; Invitrogen) were transcribed using Reverse Transcriptase Superscript II (Invitrogen). Quantitative PCR assays were performed using 25 ng of template complementary DNA. The conditions for all the reactions were as follows: an initial 10 min denaturation step at 95°C, followed by 40 s cycles at 95, 56 and 72°C. Quantitative PCR assays were performed using an Mx3005P® QPCR system (Agilent Technologies) and a Brilliant II SYBR Green QPCR Master Mix (Agilent Technologies). Results were calculated using the 2−ΔΔC t relative quantification method normalised to β-actin. The primer sets are listed in Table 3. The comparative threshold cycle (C t) method was used to quantify transcript levels and to normalise β-actin expression.
Table 3 Sequences of primers for real-time quantitative PCR
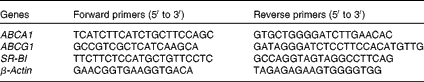
ABCA1, ATP-binding cassette A1; ABCG1, ATP-binding cassette G1; SR-BI, scavenger receptor class B type I.
Western blotting analyses
To gain more insight into the effect of EVOO consumption on the modulation of CE, ABCA1, ABCG1 and SR-B1 protein expression in HMDM were quantified by Western blot analysis at T0 and T12. Moreover, the expression of these proteins was also measured in J774 macrophages after incubation of these cells, for 12 h, with EVOO total phenolic extract or with the major phenolic compounds of EVOO such as tyrosol and hydroxytyrosol at concentrations of 5 and 10 μm, respectively. In both experiments, lysate proteins were loaded on 10 % acrylamide gels and separated by SDS–PAGE. The bands were transferred to nitrocellulose membranes, which were blocked with 5 % skimmed milk in PBS/Tween-20. The blots were incubated with primary antibodies and then with specific IgG-horseradish peroxidase secondary antibodies. β-Actin was used as a control of protein loading. Protein bands were detected using an enhanced chemiluminescence reagent(Reference Berrougui, Isabelle and Cloutier35).
HDL fluidity measurements
Lipoprotein fluidity was measured based on the steady-state anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH) dissolved in tetrahydrofuran, as described previously(Reference Beck, Bertolino and Abbot36). Briefly, HDL were incubated with 1 μm-DPH for 15 min at 37°C in the dark with constant stirring. Steady-state fluorescent polarisation of DPH was measured at 37°C using a Hitachi spectrofluorometer model F-4500 (Hitachi, Limited). DPH was excited using a vertically polarised light at 360 nm, and emission intensities were detected at 430 nm through a polariser orientated parallel and perpendicular to the direction of polarisation of the excitation beam. Steady-state fluorescence anisotropy (r) was calculated using the following equation from the FL solution program (Hitachi):
where I v and I p are the parallel and perpendicular polarised fluorescence intensities, and G is the monochromator grating correction factor. Fluidity is the inverse value of anisotropy and is expressed as 1/r for steady-state fluorescence anisotropy.
Statistical analysis
Values are expressed as means with their standard errors. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, Inc.).
Results
Table 1 presents the clinical and biochemical parameters of the volunteers at baseline (T0) and after 12 weeks of EVOO consumption (T12). The volunteers (mean age 53·28 (sem 20·66) years) were healthy and non-obese (BMI 24·9 (sem 0·65) kg/m2) with lipid profiles and systolic and diastolic blood pressure in normal ranges. There were no significant differences in serum TAG, total cholesterol, HDL-cholesterol and LDL-cholesterol, and other cardiovascular risk markers between T0 and T12. The TAG:HDL and total cholesterol:HDL ratios, which are atherogenic indices, decreased respectively by 10·1 % (P< 0·05) and 4·2 % (P< 0·05) at T12. Plasma tyrosol and hydroxytyrosol contents showed a small (not statistically significant) increasing trend after 12 weeks of EVOO consumption (results not shown).
Effect of extra-virgin olive oil consumption on serum-mediated cholesterol efflux
CE is the first rate-limiting step of RCT and is influenced by the capacity of HDL to act as a cholesterol acceptor and of peripheral cells to release excess cholesterol.
We first investigated the effect of 12 weeks of EVOO consumption on the capacity of serum to mediate CE. As shown in Fig. 1(a), 12 weeks of EVOO consumption resulted in a 9·8 % (P< 0·01) increase in the capacity of serum to mediate CE from THP-1 macrophages.

Fig. 1 Extra-virgin olive oil (EVOO) consumption increases serum-mediated cholesterol efflux. [3H]Cholesterol-loaded (a) THP-1 macrophages, (b) ATP-binding cassette (ABC) A1-non-enriched (control) and enriched J774 macrophages and (c) Fu5AH cells were incubated for 24 h (4 h for J774 macrophages) with 5 % serum. Sera were obtained from volunteers at baseline (T0) and after 12 weeks of EVOO consumption (T12). Cholesterol efflux was determined by liquid scintillation counting, and the percentage of radiolabelled cholesterol efflux was calculated using the following formula: (cpm in medium/cpm in the cell+medium) × 100, where cpm is counts per min. For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those at T0: ** P< 0·01, *** P< 0·001.
We then investigated which CE pathway was stimulated the most after 12 weeks of EVOO consumption. We used J774 macrophages to investigate the involvement of ABCA1. J774 macrophages have a low basal expression of ABCA1, which is overexpressed following a pretreatment with cyclic AMP(Reference Yancey, Bortnick and Kellner-Weibel8). It was found that 12 weeks of EVOO consumption increased the capacity of serum to mediate CE from ABCA1-enriched J774 macrophages by 18·85 % (P< 0·001) while no effect was observed with ABCA1-non-enriched macrophages (Fig. 1(b)).
We used Fu5AH cells to investigate the involvement of the SR-BI pathway in this process. Fu5AH cells express high levels of SR-BI and lack functional ABCA1(Reference Bortnick, Rothblat and Stoudt5). We found that 12 weeks of EVOO consumption enhanced the capacity of serum to mediate CE from Fu5AH cells via the SR-BI pathway by 14·81 % (P< 0·001; Fig. 1(c)).
Effect of extra-virgin olive oil consumption on HDL-mediated cholesterol efflux
In light of the results obtained with serum and to better understand the beneficial effect of EVOO on cholesterol homeostasis, we investigated the effect of 12 weeks of EVOO consumption on the capacity of HDL to mediate CE using THP-1 macrophages and Fu5AH cells incubated with whole HDL. We did not use ABCA1-enriched J774 cells because they do not interact with whole HDL or with the HDL2 subfraction(Reference Tall, Costet and Wang7, Reference Shao, Tang and Heinecke37–Reference Wang, Silver and Costet39). Fig. 2(a) shows that 12 weeks of EVOO consumption enhanced the capacity of whole HDL to mediate CE from THP-1 macrophages by 11·93 % (P< 0·01).

Fig. 2 Extra-virgin olive oil (EVOO) consumption stimulates HDL-mediated cholesterol efflux. (a) THP-1 macrophages were loaded with [3H]cholesterol and were then incubated for 24 h with HDL. (b) [3H]Cholesterol-loaded THP-1 macrophages were incubated overnight at 37°C without (control) or with 5 μm-22(R)-hydroxy-cholesterol (22(R)-OH-Chol) to yield ATP-binding cassette G1-enriched macrophages. After washing, the macrophages were incubated for 24 h with HDL to measure cholesterol efflux. (c) Scavenger receptor class B type I-rich Fu5AH cells were loaded with [3H]cholesterol and were then incubated with HDL for 24 h to measure cholesterol efflux. All experiments were carried out with 50 μg/ml of HDL isolated from the plasma of the volunteers at baseline (T0) and after 12 weeks of EVOO consumption (T12). For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those at T0: * P< 0·05, ** P< 0·01.
We then pretreated THP-1 macrophages with 22(R)-hydroxy-cholesterol for 24 h to induce overexpression of ABCG1(Reference Kaplan, Gan and Menke33). mRNA and protein expression measurements confirmed that ABCG1 was up-regulated in THP-1 macrophages (results not shown). While the up-regulation of ABCG1 increased HDL-mediated CE at T0, the increase was significantly higher at T12 (24·57 (sem 4·89) % at T12 v. 11·77 (sem 4·33) % at T0; Fig. 2(b)).
The effect of 12 weeks of EVOO consumption on the capacity of HDL to mediate CE via the SR-BI pathway was also investigated. Fu5AH cells were used as a cholesterol acceptor. As shown in Fig. 2(c), the capacity of HDL to mediate CE was 16·4 % higher at T12 than at T0 (P< 0·05).
To determine which HDL subclass was involved in the increase in CE, HDL2 and HDL3 isolated from the volunteers at T0 and T12 were incubated separately with [3H]cholesterol-loaded Fu5AH cells. HDL2-mediated CE from Fu5AH cells was 24·1 % (P< 0·05) higher at T12 than at T0, while HDL3 had no effect (Fig. 3(a)). In contrast, HDL3-mediated CE from ABCA1-enriched J774 macrophages was 15·2 % (P< 0·05) higher than from control J774 macrophages (Fig. 3(b)).

Fig. 3 Extra-virgin olive oil (EVOO) stimulates HDL2- and HDL3-mediated cholesterol efflux via the scavenger receptor class B type I and ATP-binding cassette (ABC) A1 pathways, respectively. (a) Fu5AH cells were loaded with [3H]cholesterol and were then incubated for 24 h with 50 μg/ml of HDL2 or HDL3. (b) ABCA1-enriched and non-enriched (control) J774 macrophages were incubated with 50 μg/ml of HDL3 for 4 h. HDL2 and HDL3 were isolated from the plasma of the volunteers at baseline (T0) and after 12 weeks of EVOO consumption (T12). For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those at T0: * P< 0·05. cAMP, cyclic AMP.
The percentage increase in HDL-mediated CE at T12 was comparable to that observed when 5 % serum was used as the cholesterol acceptor.
Effect of extra-virgin olive oil consumption on the distribution of HDL subclasses and their biophysical properties
We investigated whether the increase in CE through the ABCA1/ABCG1 and SR-BI pathways induced by EVOO consumption might be related to a difference in HDL distribution, particularly the levels of pre-β-HDL3 and α-HDL3 levels. We found that 12 weeks of EVOO consumption did not induce a significant change in either pre-β-HDL or α-HDL levels (results not shown). We also assessed the distribution of all HDL particles (small v. intermediate v. large HDL) in an attempt to explain the increase in the capacity of HDL to mediate CE through the ABCG1 and SR-BI pathways. No significant changes were observed between T0 and T12 in the distribution of HDL particles (Table 4).
Table 4 HDL distribution at baseline (T0) and after 12 weeks of extra-virgin olive oil (EVOO) consumption (T12)* (Mean values with their standard errors)
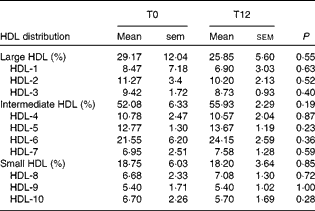
* A Wilcoxon matched-pairs signed-rank test was used to compare the differences between T0 and T12.
In addition to the interactions with the ABCA1 transporter and SR-BI receptor, the capacity of HDL to mediate CE also depends on their physico-chemical properties(Reference Sola, Motta and Maille27), including an increase in the fluidity of the phospholipidic layer of HDL that, in turn, increases the capacity of HDL to mediate CE. Fluidity is an indirect measure of the fatty acid composition of HDL and is determined by anisotropy fluorescence measurements. Due to its high MUFA content, olive oil can induce physico-chemical changes in the lipid composition of HDL and thus in their biophysical structure. The results confirmed that 12 weeks of EVOO consumption increased the fluidity of the phospholipidic layer of HDL by 13 % (P< 0·001) as measured by fluorescence anisotropy (Fig. 4).

Fig. 4 Extra-virgin olive oil (EVOO) improves HDL phospholipidic layer fluidity. The fluidity of the phospholipidic layer of HDL was measured at baseline (T0) and after 12 weeks of EVOO consumption (T12). Fluidity is the inverse value of anisotropy and is expressed as 1/r (for steady-state fluorescence anisotropy). r was calculated using the following formula: I v− GI p/(I v− 2GI p), where I v and I p are the parallel and perpendicular polarised fluorescence intensities, and G is the monochromator grating correction factor. The results were obtained using a 1,6-diphenyl-1,3,5-hexatriene probe and polarisation fluorescence. For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. *** Mean values were significantly different from those at T0 (P< 0·001).
Effect of extra-virgin olive oil consumption on the capacity of human monocyte-derived macrophages to release excess cholesterol
In the second part of the present study, we investigated the effect of EVOO consumption on the capacity of HMDM to release excess cholesterol, which is the second-limiting step in the RCT process. We used standard human apoA-I as the cholesterol acceptor. Monocytes were isolated from each volunteer at T0 and T12 and were transformed into macrophages (HMDM) as described in the Materials and methods section. HMDM were loaded with [3H]cholesterol and were incubated with 25 μg/ml of apoA-I to measure CE. The results revealed that 12 weeks of EVOO consumption increased the capacity of HMDM to transfer excess cholesterol to apoA-I by over 44 % (P< 0·001; Fig. 5).

Fig. 5 Extra-virgin olive oil (EVOO) consumption increases the capacity of human monocyte-derived macrophages (HMDM) to release excess cholesterol. Human monocytes were cultured in phorbol myristate acetate-Roswell Park Memorial Institute medium in the presence of autologous sera for 1 week to induce differentiation into macrophages (HMDM). HMDM obtained from the volunteers at baseline (T0) and after 12 weeks of EVOO consumption (T12) were loaded with [3H]cholesterol and were then incubated with 25 μg/ml of human apoA-I for 4 h. Cholesterol efflux was determined by liquid scintillation counting, and the percentage of radiolabelled cholesterol released (percentage of cholesterol efflux) was calculated using the following formula: (cpm in medium/cpm in cells+medium) × 100, where cpm is counts per minute. For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. *** Mean values were significantly different from those at T0 (P< 0·001).
In an attempt to explain the increase in cholesterol release from HMDM following EVOO consumption, we measured ABCA1, ABCG1 and SR-BI mRNA and protein expression in HMDM obtained from the volunteers at T0 and T12. As a result, we found that 12 weeks of EVOO consumption increased ABCA1 and ABCG1 mRNA expression by 27·51 % (P< 0·0001) and 26·48 % (P< 0·001), respectively (Fig. 6(a) and (b)). Interestingly, SR-BI mRNA expression was reduced by approximately 30 % (P< 0·0001; Fig. 6(c)). These results were confirmed by the protein expression measurements, which showed that there was a significant increase in ABCA1 (16·08 %, P< 0·001) and ABCG1 (35·79 %, P< 0·01) protein expression and a decrease in SR-BI protein expression (–2·51 %, P< 0·05) following 12 weeks of EVOO consumption (Fig. 6(d) and (e)).

Fig. 6 Extra-virgin olive oil (EVOO) increases ATP-binding cassette (ABC) A1 and ABCG1 mRNA and protein expression in human monocyte-derived macrophages (HMDM). Human monocytes were cultured and differentiated into macrophages in phorbol myristate acetate-Roswell Park Memorial Institute medium in the presence of autologous sera to induce differentiation into macrophages (HMDM). HMDM were lysed and their (a) ABCA1, (b) ABCG1 and (c) scavenger receptor class B type I (SR-BI) mRNA content was quantified by quantitative PCR (qPCR). The comparative threshold cycle (C t) method was used to quantify the transcript levels and to normalise β-actin expression. Western blotting was used to evaluate (d) ABCA1, ABCG1 and SR-BI protein levels in HMDM. (e) Protein bands were quantified by densitometry. The qPCR and Western blotting analyses were carried out at baseline (T0) and after 12 weeks of EVOO consumption (T12). For each subject, measurements were done in triplicate. A Wilcoxon matched-pairs signed-rank test was used to compare differences between groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from those at T0: * P< 0·05, ** P< 0. 01, *** P< 0·001.
To confirm the effect of EVOO consumption on cholesterol transporter protein levels, especially ABCA1 and ABCG1 in HMDM, we first incubated EVOO polyphenol extracts (0–320 μg/ml) with J774 macrophages and measured ABCA1 protein expression. Overnight incubation of J774 macrophages with EVOO polyphenol extracts significantly increased ABCA1 protein expression in a dose-dependent manner (Fig. 7(a)) as measured by Western blot analysis. In light of these results, we then investigated the effect of purified thyrosol and hydroxythyrosol (two major EVOO polyphenols) on ABCA1, ABCG1 and SR-BI protein expression in J774 macrophages. The results (Fig. 7) showed a significant increase in ABCA1 and ABCG1 protein expression in the presence of both tyrosol and hydroxytyrosol (5 and 10 μm; Fig. 7(b), (c), (e) and (f)), whereas no significant effect was observed with respect to SR-BI expression (Fig. 7(d) and (g)).

Fig. 7 Extra-virgin olive oil (EVOO) phenolic extracts (EVOO-PE) enhance ATP-binding cassette (ABC) A1 and ABCG1 protein expression in J774 macrophages. J774 macrophages were incubated with various concentrations of EVOO-PE, tyrosol or hydroxytyrosol (HT). After 16 h, the cells were lysed and the lysates were analysed by immunoblotting for ABCA1, ABCG1 and scavenger receptor class B type I (SR-BI) protein expression. (a) ABCA1 expression after incubating J774 macrophages with increasing concentrations of EVOO-PE. Cyclic AMP was used as a control for ABCA1 protein expression. (b) ABCA1, (c) ABCG1 and (d) SR-BI protein expression after incubating J774 macrophages with hydroxytyrosol. (e) ABCA1, (f) ABCG1 and (g) SR-BI protein expression after incubating J774 macrophages with tyrosol.
Discussion
Olive oil is the main source of fat in the Mediterranean diet. A large body of evidence shows that the Mediterranean diet, which is high in olive oil, is associated with a lower incidence of CHD(Reference Keys, Menotti and Karvonen40–Reference Estruch, Martinez-Gonzalez and Corella43). The beneficial effect of olive oil consumption is related to its high MUFA and phenol content, which have antioxidant and anti-inflammatory properties(Reference Covas, de la Torre and Farre-Albaladejo19, Reference Bogani, Galli and Villa44–Reference Sola, La Ville and Richard47). A diet rich in olive oil induces a change in lipid metabolism(Reference Chan, Demonty and Pelled48) and an increase in the resistance of LDL to lipid peroxidation(Reference Mata, Varela and Alonso49). Covas et al. (Reference Covas, de la Torre and Farre-Albaladejo19) showed that consuming EVOO increases the level of phenolic compounds in the serum and LDL, which may explain its protective effect against free radical-induced lipid peroxidation. However, no studies have investigated the effect of olive oil consumption by human subjects on the principal anti-atherogenic activity of HDL, which is their capacity to regulate or maintain cholesterol homeostasis.
HDL maintain cholesterol homeostasis by their role in RCT, facilitating the efflux of cholesterol from peripheral tissues and transporting it back to the liver to be eliminated in the bile and faeces(Reference Glomset3). This process involves the interaction of lipid-poor apoA-I and mature HDL with ABCA1/ABCG1 transporters and the SR-BI receptor, respectively(Reference Wang, Silver and Thiele50, Reference Kennedy, Barrera and Nakamura51); HDL-mediated cholesterol efflux is thus the natural rate-limiting step of RCT(Reference Fu52). CE is dependent on apoA-I and HDL concentrations and on the capacity of HDL to mediate the efflux. However, CE from macrophages is also dependent on the expression in the plasma membrane of cholesterol transporters, principally ABCA1 and ABCG1(Reference Wang, Collins and Ranalletta53). The goals of the present study were to investigate, in healthy volunteers, the effect of 12 weeks of EVOO consumption on the capacity of HDL to mediate CE, and the mechanisms by which EVOO consumption improves cholesterol homeostasis.
The measurements of the capacity of serum to mediate CE revealed that 12 weeks of EVOO consumption significantly stimulated CE from THP-1, J774 and Fu5AH cells (9·8, 23·3 and 15·2 %, respectively). This can be explained by the fact that serum is a heterogeneous liquid that contains pre-β and α-migrating HDL, which react with ABCA1/ABCG1 transporters and the SR-BI receptor, respectively. This could be related to physico-chemical changes that occur in HDL following long-term EVOO consumption, which contributes to the formation of smaller HDL particles and greater HDL phospholipidic layer fluidity, and an increase in the linoleic:linolenic acid ratio of HDL(Reference Sola, Motta and Maille27). Sola et al. (Reference Sola, Motta and Maille27, Reference Sola, La Ville and Richard47) reported that a MUFA-rich diet enhances HDL3-mediated CE from fibroblast cells, while Sakr et al. (Reference Sakr, Senault and Vacher54) demonstrated that oleic acid-rich fats increase the capacity of serum to promote CE from SR-BI-enriched Fu5AH cells and that this effect is correlated with an increase in phospholipid availability. Rosenblat et al. (Reference Rosenblat, Volkova and Coleman28) administered EVOO to apoE-deficient mice and obtained similar results. Efrat et al. (Reference Efrat, Rosenblat and Mahmood55) showed that EVOO consumption increases di-oleoylphosphatidylcholine (18 : 1) levels in HDL, which enhances their capacity to mediate CE from macrophages. The increase in phospholipidic layer fluidity is an indirect measure of a change in HDL lipid composition and confirmed that a change in the biochemical composition of HDL following the consumption of EVOO may, in part, contribute to the increase in their capacity to mediate CE. On the other hand, it is well known that the oxidation of HDL significantly affects their capacity to promote CE(Reference Berrougui, Isabelle and Cloutier35, Reference Bonnefont-Rousselot, Motta and Khalil56) and that dietary antioxidants increase the resistance of these lipoproteins to lipid peroxidation(Reference Sola, La Ville and Richard47).
While Sola et al. (Reference Sola, Fito and Estruch57) reported that virgin olive oil enrichment increases apoA-I concentrations in high-cardiovascular risk subjects. The present results indicated that EVOO consumption does not affect these parameters in healthy patients. Violante et al. (Reference Violante, Gerbaudo and Borretta58) also reported that 3 months of EVOO consumption by hypercholesterolaemic subjects increased serum apoA-I concentrations by about 9 %. However, given the conditions used in the present study, it was not surprising that there was no increase in apoA-I levels after 3 months of EVOO supplementation. On the one hand, all of our volunteers were healthy with no cardiovascular risk factors and, on the other hand, the apoA-I levels and lipid profiles of our volunteers were in normal ranges. In the present study, EVOO had no effect on circulating HDL levels or HDL subclass distribution, but did improve the capacity of HDL to modulate CE, clearly suggesting that EVOO consumption improves HDL functionality, which is more important than HDL concentrations in determining their atheroprotective capacity.
The use of J774, THP-1 and Fu5AH cells allowed us to demonstrate that EVOO consumption stimulates CE mediated by both pre-β-HDL and the mature fractions of HDL (HDL2 and HDL3). Pre-β-HDL are considered important extracellular acceptors of effluxed peripheral tissue cholesterol. They are formed following lipidation of apoA-I by ABCA1. The ABCA1 transporter mediates CE in a unidirectional manner to apoA-I and pre-β-HDL. Western blotting and PAGE analyses did not reveal any significant changes in pre-β-HDL levels or in HDL subclass (small, intermediate and large HDL particles) distribution between T0 and T12. Previous studies, including the present study, showed that oxidative modifications to apoA-I affect its capacity to interact with ABCA1 transporters and to mediate CE(Reference Berrougui, Isabelle and Cloutier35, Reference Shao, Tang and Heinecke37). While EVOO consumption did not have an effect on apoA-I levels that could explain the increased CE, it should be noted that olive oil contains several components (α-tocopherols, β-carotene, sterols, terpene, squalene and phenolic compounds(Reference Covas, de la Torre and Farre-Albaladejo19, Reference Konstantinidou, Covas and Munoz-Aguayo41, Reference Cabello-Moruno, Perona and Osada59)) with antioxidant activity that may protect apoA-I against oxidative damage, thus contributing to stronger apoA-I/and pre-β-HDL/ABCA1 interactions that mediate CE.
The efflux of cholesterol via ABCG1 and SR-BI is mediated by mature HDL, while oxidative damage to apoA-I and whole HDL particles is reduced by polyphenolic compounds, thus increasing CE. The increase in CE mediated by mature HDL also depends on their biochemical and physico-chemical properties, and the increase in the phospholipidic fluidity of HDL may in part explain the improvement in the capacity of HDL to mediate CE.
EVOO consumption increased the capacity of HMDM to release excess cholesterol to apoA-I by 44 % (P< 0·001), which is comparable to the 42 % increase in CE obtained by Rosenblat et al. (Reference Rosenblat, Volkova and Coleman28) with mouse peritoneal macrophages after 8 weeks of EVOO consumption. CE proteins on HMDM plasma membranes, especially ABCA1 and ABCG1 transporter proteins, were significantly up-regulated following EVOO consumption, as measured by mRNA and protein expression. The increase in protein expression may explain the significant increase in cholesterol release from HMDM. A recent study on healthy human subjects showed that consuming coffee, which has a high phenolic acid content, enhances HDL-mediated CE by increasing ABCG1 and SR-BI expression(Reference Uto-Kondo, Ayaori and Ogura60). While we cannot explain this discrepancy, it is surprising that polyphenols induced an increase in ABCG1 expression but had no effect on ABCA1 given that both proteins are regulated by the liver X receptor-α, which is activated by phenolic acids(Reference Sevov, Elfineh and Cavelier61), and that, in the present study, phenolic compounds purified from EVOO up-regulated both ABCA1 and ABCG1 transporters in J774 macrophages.
In contrast, SR-BI mRNA and protein expression was significantly down-regulated after 12 weeks of EVOO consumption. While we have not investigated the factors that may explain this down-regulation, Miles et al. (Reference Miles, Wallace and Calder62) showed that 12 weeks of olive oil consumption by mice caused a reduction in SR-BI protein expression. They interpreted this reduction as a beneficial effect of olive oil consumption in that it prevented the formation of foam cells, which they attributed to unsaturated fatty acids in the olive oil(Reference Miles, Wallace and Calder62). Wang et al. (Reference Wang, Collins and Ranalletta53) also showed that the SR-BI receptor does not promote macrophage RCT in vivo and that RCT occurs principally via ABCA1 and ABCG1 transporters. We did not observe a significant change in SR-BI expression following 24 h incubation with purified polyphenols.
It is noteworthy that, in addition to its beneficial effect on the capacity of serum and HDL to mediate CE, 12 weeks of EVOO consumption significantly reduced the total cholesterol:HDL and TAG:HDL ratios. These results are in agreement with those of Violante et al. (Reference Violante, Gerbaudo and Borretta58) and showed that EVOO consumption has a beneficial effect on other atherosclerosis markers.
In conclusion, the present results showed that 12 weeks of EVOO consumption significantly increased the capacity of HDL to mediate CE and of HMDM to release excess cholesterol. The increase in the capacity of HDL to mediate CE was independent of HDL concentration and was probably due to improvements in HDL functionality. The increase in the capacity of HMDM to release excess cholesterol can be explained by the increase in ABCA1 and ABCG1 mRNA and protein expression by these cells. The capacity of HDL to mediate CE and of macrophages to release excess cholesterol are two components of RCT, which suggests that EVOO could have a significant effect on the modulation, in vivo, of cholesterol homeostasis and significantly reduce cholesterol deposits in the arteries. Nevertheless, the present study presents some limitations: (1) the design of the study lacks a control group; (2) the diet of the participants was not controlled. Indeed, dietary changes, besides EVOO consumption, could promote an increase in HDL functionality (i.e. other polyphenols or antioxidants); (3) physical activity, which is another possible confounder variable that was not registered; and (4) the age of the participants was in the range of middle-aged/mature people, which limits the extrapolation of the obtained results to a young population. Therefore, due to these limitations, the present study should be considered as a pilot study. However, further studies, considering these limitations, are needed to investigate the effect of EVOO consumption on RCT.
Acknowledgements
This study was supported by a grant from the Canadian Institutes of Health Research (MOP-89912). The authors' contributions were as follows: A. K. designed the study and obtained funds from the Canadian Institutes of Health Research; O. H. carried out the experiments about the in vivo effect of olive oil; H. B. carried out the experiments on the in vitro effect of EVOO extracts; S. L. participated in the sample collections and the preparation of lipoproteins; O. H. and H. B drafted the manuscript; A. K. revised the manuscript and completed the discussion. All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.













