Nucleotides (NT) are the building blocks of DNA and RNA and are normal components of the human diet and the non-protein-nitrogen fraction of milkReference Schlimme, Martin and Meisel1. Nucleosides (NS) and NT are not considered essential since cells possess biochemical pathways for their de novo synthesis and salvage. However, in certain circumstances, NT may become conditionally essential nutrients that modulate different biological processes, such as lipid metabolism and immune function, and have an important role in cell proliferation, maturation, and apoptosisReference Sánchez-Pozo and Gil2–Reference Evans, Jones and Ziegler4. Besides, dietary NT have been shown to enhance gut recovery after different kinds of intestinal injuryReference Nunez, Bueno, Ayudarte, Almendros, Rios, Suarez and Gil5–Reference Belo, Marchbank, Fitzgerald, Ghosh and Playford9. Because of the biochemical and trophochemical properties of dietary NT and NS, the European Commission has permitted some of them to be used as a supplement in the manufacture of infant and follow-on formulas.
Although NT deficiencies have not been related to any particular disease, various studies have assessed the effect of exogenous NT and NS on the intracellular pool of NT and on cell proliferation and differentiation. In hepatic cells, our group showed that exogenous NS were actively incorporated by hepatocytes and stimulated hepatocyte proliferation and expression of c-myc, h-ras genes and α-fetoproteinReference Arnaud, Fontana, Angulo, Gil and Lopez-Pedrosa10, Reference Saez-Lara, Manzano, Angulo, Suarez, Torres, Gomez-Llorente, Gil and Fontana11. Gil et al. Reference Gil, Gómez-León and Rueda12 recently showed that intestinal explants hydrolyse RNA to NT, and NT to NS that were efficiently incorporated into intestinal cells, except for cytidine. Reports on the effect of NT in intestinal cell proliferation and differentiation are contradictory. Sato et al. Reference Sato, Nakano, Kawakami and Idota13 reported that a 10:1:1:1:1 CMP–AMP–UMP–IMP–GMP mixture enhanced proliferation, sucrase and maltase activities in IEC-6 cells but not in Caco-2 cells. In the same line, addition of NT (AMP, CMP, UMP, IMP, GMP) to a glutamine-free medium stimulated intestinal cell proliferation and increased alkaline phosphatase and sucrose-isomaltase activities in differentiated cellsReference He, Chu and Walker14. However, Boza et al. Reference Boza, Moennoz, Bournot, Blum, Zbinden, Finot and Ballevre15 showed that the proliferative effect of NT on Caco-2 cells was associated with the presence of glutamine in cell culture medium. Moreover, Tanaka et al. Reference Tanaka, Lee, Martinez-Augustin, He, Sanderson and Walker16 and Chen et al. Reference Chen, Wang, Deng and Ran17 observed that AMP supplementation decreased cellular proliferation and increased apoptosis in organ cultures and IEC-6 cells, respectively.
The goal of the present study was to reconcile the conflicting data published on the effect of NS on intestinal cells. The aim was to test the hypothesis that exogenous NS are taken up and modify the concentrations of intracellular NT and the energy charge with effects on the morphological, functional and gene expression changes associated to cell differentiation. Overall, these data would determine whether selective NS uptake by intestinal cells alter the intracellular NT pool, thereby enhancing the differentiation on enterocytes. For this purpose, we used IEC-6 cells that undergo differentiation when grown on an extracellular protein matrix. IEC-6 cells were grown in the presence or absence of an inosine–guanosine–cytidine–uridine supplement at physiologic levels (30 μm each, a concentration selected to match NS concentrations in human milk, which range from 0·5 to 37 μm)Reference Duchen and Thorell18.
Materials and methods
Materials
Plastic culture ware was purchased from Nunc (Roskilde, Denmark). Cell culture medium (Dulbecco's modified Eagle's medium), trypsin, insulin, NS and NT were from Sigma Aldrich (St. Louis, MO, USA). SuperScript II, RNase Out, Trizol, l-glutamine, penicillin/streptomycin, fetal bovine serum and PBS were from Invitrogen (Carlsbad, CA, USA). [α-32P]dCTP, Matrigel and dispase were purchased from Beckton Dickinson (Bedford, MA, USA). DNase I FPLCpure and TaqGold polymerase were from Amersham Pharmacia Biotech (Piscataway, NJ, USA). NIH Scion Image Beta 4.0.2 software (Scion Corporation, MD, USA) was used to quantify band intensity.
Cell culture
IEC-6 cell line was obtained from the American Type Culture Collection (Rockville, MD, USA) and was used between passages 20 and 25 in most experiments. A stock culture of IEC-6 was maintained using Dulbecco's modified Eagle's medium with 4·5 g/l glucose, 10 % fetal bovine serum, bovine insulin (0·1 U/ml), penicillin/streptomycin and l-glutamine (0·6 g/l). The stock was passaged twice a week and fed three times a week.
In order to determine the effects of NS on cell proliferation and differentiation, IEC-6 cells (5 × 105) were plated on 35 mm dishes coated with a thin layer of Matrigel. Media were supplemented with or without NS (cytidine, uridine, guanosine, inosine; 30 μm each). The culture media were replaced with fresh NS every 12 h. Cells were recovered from Matrigel by using dispase according to manufacturer's instructions.
Transmission electron microscopy studies
IEC-6 cells were grown on 3·0 μm pore size Transwell-clear polyester membranes precoated with Matrigel, or on 3·0 μm Transwell-COL type I and III collagen-coated membranes (Corning, Cultek, Spain). At 12 and 24 h, monolayers were washed and fixed in 2·5 % glutaraldehyde in cacodylate buffer (0·1 m, pH 7·3) and post-fixed in 1 % osmium tetroxide. Monolayers were then dehydrated in ethanol and embedded in Epon 812 resin. Ultrathin sections (50 nm) were contrasted with uranyl acetate and lead citrate and examined under a Zeiss 902 transmission electron microscope.
HPLC analysis of nucleosides and nucleotides
NS present in culture media were separated and quantified by means of an ion-exchange HPLC method with some changesReference Gehrke and Kuo19. Briefly, media were recovered using a syringe and filtered through a 0·45 μm filter to eliminate cell debris. Cleared media (40 μl) were injected into the chromatograph. Two mobile phases were used: (1) 0·1 m-potassium acetate, pH 4·5, 2 mm-hexasulphonic acid and 1 % acetonitrile; and (2) 0·1 m-potassium acid, pH 4·5, 2 mm-hexasulphonic acid and 6 % acetonitrile. NS were identified and quantified at 254 nm and compared with HPLC standards.
NT intracellular pools were analysed by using a HPLC methodReference Perret, Holman, Cross and Joseph20. Briefly, cells were rinsed with PBS and frozen using liquid nitrogen to avoid NT degradation. Cells were then treated with percloric acid and incubated for 45 min to precipitate proteins. Supernatants were neutralized with potassium hydroxide, cooled, centrifuged and filtered. Cleared supernatants (60 μl) were injected into the chromatograph. Two mobile phases were used: (1) 0·2 m-ammonium phosphate, 0·1 m-triethanolamine, pH 6; and (2) 0·2 m-ammonium phosphate, 0·1 m-triethanolamine, pH 6, and 15 % acetonitrile. NT were identified and quantified at 254 nm and compared with HPLC standards.
Enzyme activities
Alkaline phosphatase (EC 3.1.3.1) activity was measured on crude cell homogenates using a spectrophotometric method based on the use of p-nitrophenylphosphate, which is transformed to phosphate and p-nitrophenol. The latter is a coloured compound that can be measured at 405 nmReference Goldstein, Klein, Freier and Menczel21. Units were expressed per milligram protein. The protein concentration was determined by the bicinchoninic acid method, using bovine albumin serum as standardReference Brown, Jarvis and Hyland22.
RNA extraction and reverse transcription-PCR
Total RNA was extracted from cells grown on 35 mm dishes using Trizol according to the manufacturer's instructions. In order to avoid contamination by DNA, total RNA was treated with DNase I. The reaction took place at 37°C for 30 min. Finally, RNA was extracted using phenol–chloroform and precipitated with sodium acetate and ethanol. The pellet was washed with 70 % ethanol and stored dry at − 80°C. First-strand complementary DNA (cDNA) was synthesized with 0·1 μg DNase-treated RNA and 0·5 μg oligo-dT16 in 20 μl of a solution containing 1 × first-strand buffer, 10 mmol/l dithiothreitol, 500 μmol/l each dNTP and 200 U Moloney murine leukaemia virus RT. PCR reactions were carried out with primers for sucrase-isomaltase cDNA amplification forward primer, 5′-CAGAACACCACTGGTCTGAG-3′; reverse primer, 5′-TGGTTGTCATCTGCAGCAAC-3′). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control of a ‘housekeeping’ gene which was amplified with a forward primer (5′-TAAAGGGCATCCTGGGCT-3′) and a reverse primer (5′-TTACTC CTTGGAGGCCATG-3′). PCR reactions were performed in the same set with an Applied Biosystems DNA thermal cycler model 9700 as follows: an initial denaturing for 5 min at 94°C; forty cycles: 94°C for 30 s, 55°C for 30 s and 72°C for 30 s; final extension at 72°C for 1 min. DNA synthesis was resolved by standard agarose gel electrophoresis.
Northern blot analysis
A total of 10 mg of denatured RNA was loaded per lane in a formaldehyde–1 % agarose gel, electrophoresed, transferred to nylon filters, and fixed with a UV crosslinker. The integrity of each sample was confirmed by staining the RNA with ethidium bromide. Filters were sequentially hybridized with a Rnd3 [α-32P]dCTP-labelled cDNA probe, stripped and hybridized with a [α-32P]dCTP-labelled rat glyceraldehyde-3-phosphate dehydrogenase cDNA probe to validate Northern blot results.
Statistical analysis
Results are means and their standard errors (n 3). Since data were not normally distributed, comparisons between different groups were assessed by non-parametric tests. Mean comparisons between independent groups were calculated using the Mann–Whitney U-test. Mean comparisons within a group for different times were done using a Wilcoxon test for paired samples. Differences with P < 0·05 were considered significant. Data were analysed using the Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA).
Results
Uptake of nucleosides by IEC-6 cells
The time course of exogenous NS clearance in medium was determined. The culture media were replaced with fresh NS (30 μm each) every 12 h. The present results show that uridine and purine NS were taken up by IEC-6 cells (Table 1). Purine NS were cleared at higher rates than pyrimidine NS. The clearance rate of uridine concentrations was similar in proliferating and differentiated cells, the values decreasing to 69 % of the initial concentration at 12 h and remaining at that level at 24 and 48 h. Guanosine was initially taken up by differentiated cells at a higher rate than proliferating cells although the contrary was observed at 48 h. Inosine was the NS with the highest clearance rate in IEC-6 cells. In proliferating cells, inosine was completely cleared from culture media at 24 and 48 h. At all time-points, cytidine concentrations were higher than the dose in culture media (30 μm) in proliferating and differentiated cells. No uric acid was detectable in culture media.
Table 1 Nucleoside concentrations in media of proliferating and differentiated IEC-6 cells†
(Mean values with their standard errors for three determinations)
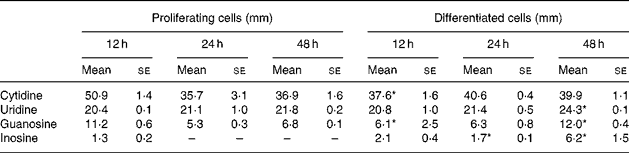
Mean values were significantly different from those of proliferating cells at the same time-point: *P < 0·05.
† IEC-6 cells were cultured in media supplemented with uridine, cytidine, guanosine and inosine (30 μm each).
Effects of nucleosides on the morphology of differentiated IEC-6 cells
Exogenous NS did not change the external morphology of proliferating or differentiated IEC-6 cells. Intestinal cells grown on Matrigel showed a polarized morphology, typical of differentiated intestinal cells, with presence of microvilli at the apical pole. Interestingly, the addition of NS had a positive effect on the maturation of intestinal cells. Differentiated IEC-6 cells supplemented with NS (D+N) showed more microvilli on their surface than did non-supplemented differentiated IEC-6 cells (D − N), and microvilli were still observed at 24 h in D+N but not D − N cells (Fig. 1). At 48 h, no difference between D+N and D − N cells was observed (data not shown).

Fig. 1 Transmission electron micrographs of differentiated IEC-6 cells grown with (A) or without (B) nucleosides. Cell media was supplemented with uridine, cytidine, guanosine and inosine (30 μm each). → , Microvilli.
Intracellular pool of nucleotides in IEC-6 cells
Intracellular TMP, GDP and UDP were not detectable by HPLC analysis in proliferating IEC-6 cells. Thymidine NT, GDP and uridine diphosphoglucose were not detectable in differentiated cells. Except for TTP, concentrations of intracellular NT decreased from 12 to 24 h in proliferating IEC-6 cells (P − N) and in proliferating cells supplemented with NS (P+N; Table 2). The differentiation state resulted in an increase in the pool of intracellular NT in comparison with the proliferating state.
Table 2 Intracellular nucleotide concentrations in proliferating and differentiated IEC-6 cells cultured in the presence or absence of a nucleoside supplement†
(Mean values with their standard errors for three determinations)
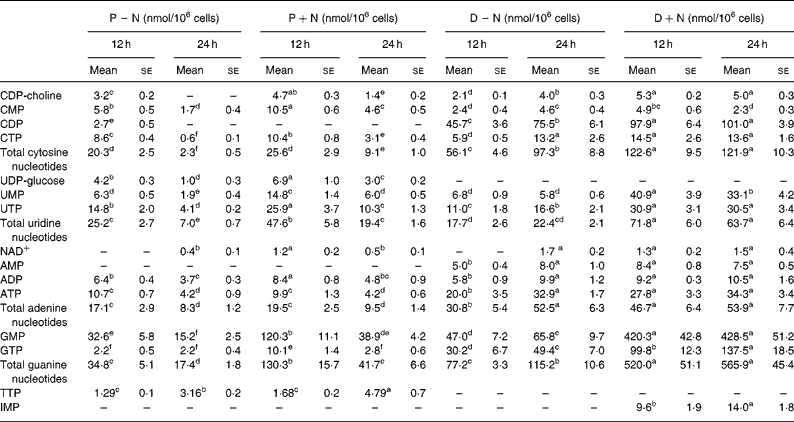
CDP-choline, cytidine diphosphocholine; D − N, differentiated cells; D+N, differentiated cells grown with nucleosides; P − N, proliferating cells; P+N, proliferating cells grown with nucleosides; UDP-glucose, uridine diphosphoglucose.
† Proliferating and differentiated IEC-6 cells were cultured in the presence or absence of a nucleoside supplement: uridine, cytidine, guanosine and inosine (30 μm each).
a–f Mean values within a row with unlike superscript letters were significantly different (P < 0.05).
Addition of NS significantly changed the concentrations of intracellular NT in proliferating and differentiated cells. At 12 and 24 h, concentrations of intracellular NT and total intracellular NT were significantly higher in D+N v. D − N cells (P < 0·05) with the exception of AMP and CMP. Intracellular IMP was detectable only in D+N cells. Interestingly, the pool of guanosine NT was 5·0-fold higher in D+N cells than in D − N cells and 2·4-fold higher in P+N v. P − N cells, and these differences were significant. Although intracellular ATP did not change, the GTP levels and energy charge were significantly higher in D+N v. D − N cells (Fig. 2). It is noteworthy that intracellular NT levels in D+N cells at 12 h were similar to or higher than levels in D − N cells at 24 h.
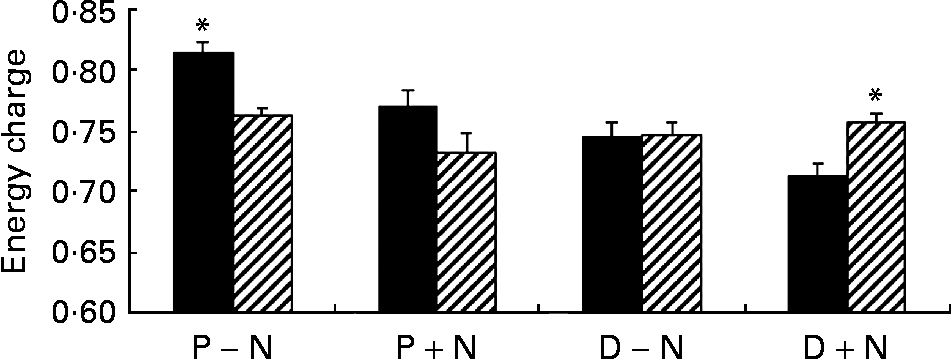
Fig. 2 Energy charge values in proliferating (P) and differentiated (D) IEC-6 cells cultured with (+N) or without nucleosides ( − N) at 12 h (■) and 24 h (▨). Cell media was supplemented with uridine, cytidine, guanosine and inosine (30 μm each). Values are means with their standard errors depicted by vertical bars for three calculations per group. * Significant effect of time, P < 0·05.
Alkaline phosphatase activity in IEC-6 cells
On the functional level, differentiated cells showed greater alkaline phosphatase activity, a marker of enterocyte differentiation, compared with proliferating cells. Interestingly, the activity of alkaline phosphatase was significantly higher in D+N v. D − N cells (Fig. 3).
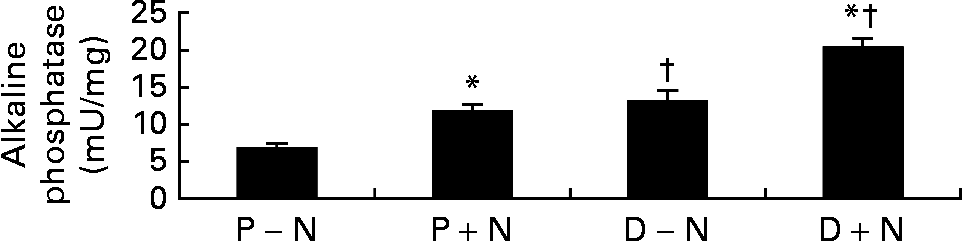
Fig. 3 Alkaline phosphatase activities in proliferating (P) and differentiated (D) IEC-6 cells cultured with (+N) or without nucleosides ( − N). Cell media was supplemented with uridine, cytidine, guanosine and inosine (30 μm each). Values are means with their standard errors depicted by vertical bars for three determinations per group. * Significant effect of nucleoside supplementation, P < 0·05. † Significant effect of cell state, P < 0·05.
Analysis of Rnd3 gene expression in differentiated IEC-6 cells
The differential display technique had been used to identify genes whose expression was regulated during enterocyte differentiation. Results obtained showed that Rnd3 mRNA levels were lower in differentiated than in proliferating IEC-6 cellsReference Vieites, Sanchez-Pozo, Gil and Suarez36. In the present study, addition of NS accelerated the reduction in Rnd3 mRNA levels in D+N v. D − N cells (Fig. 4).

Fig. 4 Effect of nucleoside supplementation on the levels of Rnd3 mRNA in differentiated IEC-6 cells. Cells were cultured with (○) or without (●) uridine, cytidine, guanosine and inosine (30 mm each). mRNA levels were analysed by Northern blot and normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase. Values are means with their standard errors depicted by vertical bars for three determinations per group. The mean of undifferentiated cells (time 0 h) was set to 1.
Discussion
Contradictory reports have been published on the effect of NT or NS on the proliferation and differentiation of intestinal cells, probably due to differences in experimental approach, including the use of different mixtures of nucleot(s)ides at varying concentrations, and to the use of distinct cancer cell lines (Caco-2, HT-29)Reference Sato, Nakano, Kawakami and Idota13–Reference Chen, Wang, Deng and Ran17. Cancer cell lines are known to have higher rates of de novo NS synthesis than normal cells. To avoid the interference of cell transformation, the effect of NS on cell differentiation was studied in IEC-6 cells because it is a non-transformed intestinal cell line that undergoes differentiation when grown on an extracellular protein matrix. The only published report on the effect of NT on IEC-6 cell differentiation showed that addition of NT enhanced proliferation but not differentiation, as assessed by alkaline phosphatase and sucrose-isomaltase activitiesReference He, Chu and Walker14. However, some aspects of the latter study can be questioned. First, NT rather than NS were used, and the high level of alkaline phosphatase activity in mature intestine is known to degrade NT to phosphate and NS that enter cells via specific transportersReference Aymerich, Pastor-Anglada and Casado23. Second, the possibility of a hormonal effect due to adenosine generation in the culture media cannot be ruled out. For these reasons, IEC-6 cells were grown in the presence or absence of an inosine–guanosine–cytidine–uridine supplement at physiologic levels (30 μm each, a concentration selected to match NS concentrations in human milk, which range from 0·5 to 37 μm)Reference Duchen and Thorell18. Finally, Sterling & CutroneoReference Sterling and Cutroneo24 detected no sucrase-isomaltase activity in differentiated IEC-6 cells, consistent with the present study, in which no expression of the sucrose-isomaltase gene was detected by reverse transcription-PCR in these cells.
According to the present findings, proliferating and differentiated IEC-6 cells control the selective uptake of NS (Table 1). Our group recently reported that hepatocytesReference Arnaud, Fontana, Angulo, Gil and Lopez-Pedrosa10, Reference Saez-Lara, Manzano, Angulo, Suarez, Torres, Gomez-Llorente, Gil and Fontana11 and intestinal explantsReference Gil, Gómez-León and Rueda12 are capable of selective NS uptake. The present results showed that the rate of uridine and purine NS clearance in IEC-6 cells was similar to that of adult hepatocytesReference Arnaud, Fontana, Angulo, Gil and Lopez-Pedrosa10 and of intestinal explantsReference Gil, Gómez-León and Rueda12. IEC-6 cells took up uridine, guanosine and inosine, up to 32, 63 and 100 % in proliferating cells, and 31, 80 and 94 % in differentiated cells, respectively. To date, three Na+-dependent concentrative and two Na+-independent equilibrative transporters have been identifiedReference Aymerich, Duflot, Fernandez-Veledo, Guillen-Gomez, Huber-Ruano, Casado and Pastor-Anglada25. In IEC-6 cells, Aymerich et al. Reference Aymerich, Foufelle, Ferre, Casado and Pastor-Anglada26 showed that these cells express high levels of equilibrative NS transporters (with low substrate selectivity) and of the concentrative NS transporter CNT2 (highly selective for purine NS and uridine). Differentiated IEC-6 cells mainly express CNT2 and, slightly, CNT1 (selective for pyrimidine NS and adenosine) with a decrease in the activity of equilibrative transporters. The apparent K m values for rat CNT2-mediated transport of uridine, guanosine and inosine are 24, 34 and 12 μm, respectivelyReference Larrayoz, Fernandez-Nistal, Garces, Gorraitz and Lostao27. We suggest that the predominance of CNT2 transporter activity would explain the preferential clearance of the former NS by proliferating and differentiated IEC-6 cells.
Concerning cytidine, Ohyanagi et al. Reference Ohyanagi, Nishimatsu, Kanbara, Usami and Saitoh28 and Arnaud et al. Reference Arnaud, Fontana, Angulo, Gil and Lopez-Pedrosa10 observed that hepatocytes hardly took up cytidine. In the present study and in intestinal explantsReference Gil, Gómez-León and Rueda12, there was an efflux of cytidine to the culture medium. The apparent K m for rat CNT2-mediated transport of cytidine is 505 μm, 16·8-fold higher than its initial concentration in culture media. Therefore, cytidine would be poorly transported by CNT2 into IEC-6 cells. The results suggest that de novo synthesis and salvage of uridine by uridine kinase is preferentially used to meet intracellular pyrimidine NT needs. Cells produce cytidine, in the form of CTP, from UTP by cytidine triphosphate synthetase, using ATP and glutamine. The increase of cytidine in culture media may be the result of cytidine export due to excessive biosynthesis from UTP.
The present study is the first report on the effect of exogenous NS on the intracellular NT pool in differentiated intestinal cells (Table 2). It is worth noting that the total intracellular NT pool was significantly higher in differentiated cells than in proliferating ones (D − N v. P − N). A similar result was observed in differentiated Caco-2 cellsReference He, Chu and Walker14, and it was concluded that differentiation may activate NT biosynthesis in intestinal cells. Analysis of the intracellular NT pool in IEC-6 cells showed that differentiated cells had no detectable intracellular thymidine NT compared to proliferating ones, probably related to the arrest of cell division.
Exogenous NS significantly altered the concentration and distribution of soluble intracellular NT. Incubation of IEC-6 cells with NS produced an increase in intracellular NT concentrations of proliferating (P+N v. P − N) and differentiated (D+N v. D − N) cells. This result agrees with the work of many authors in primary cells or cell linesReference Sato, Nakano, Kawakami and Idota13–Reference Chen, Wang, Deng and Ran17, Reference Mayer, Natsumeda, Ikegami, Faderan, Lui, Emrani, Reardon, Olah and Weber29–Reference Karle, Anderson and Cysyk32. We believe that salvage of NS contributed to de novo synthesis, thereby increasing the intracellular NT pool. Uptake and salvage of guanosine and inosine significantly increased the intracellular pool of guanosine NT rather than adenosine derivatives. Total guanosine NT, which play an important role in GTPase-mediated cell structure organization and signalling as well as in protein synthesisReference Hallett, Dagher and Atkinson33, Reference Hucul, Henshaw and Young34, increased 2·5-fold in P+N cells and 5-fold in D+N cells with respect to unsupplemented counterparts. The detection of intracellular IMP may indicate that the synthesis of purine NT was saturated in differentiated cells supplemented with exogenous NS (D+N). Indeed, the highest concentrations of intracellular purine NT were reached in these cells. The present data showed that intracellular ATP and NAD+ concentrations are tightly controlled in IEC-6 cells. Although exogenous NS modified NAD+ and adenosine NT values, the amount of total adenine NT and ATP levels were significantly different only at 12 h in D+N cells compared with D − N cells. These data contrasted with the energy charge values that were significantly higher in D+N than in D − N cells at 24 h (Fig. 2), and suggested that energy was more efficiently stored in NS-supplemented differentiated IEC-6 cells. It is noteworthy that uric acid was not detected in culture media although intestinal cells express xanthine oxidase. The result indicated that purine NS were incorporated into the intracellular NT pool rather than being catabolized to uric acid.
In parallel, we observed that exogenous NS enhanced the differentiation of IEC-6 cells. Morphologically, microvilli were more abundant in D+N cells and persisted longer than in D − N cells (Fig. 1). Moreover, at the enzymatic level, D+N cells had higher alkaline phosphatase activity D − N cells (Fig. 3). The present results agree with those of Sato et al. Reference Sato, Kawakami and Idota35, who observed that NS supplementation promoted the formation of tight junctions, the expression of microvilli, and the activities of maltase and sucrase-isomaltase. Concerning gene expression, we used the differential display technique to identify genes regulated during the initial stages of enterocyte differentiation. One of these differentiation-regulated genes was Rnd3 Reference Vieites, Sanchez-Pozo, Gil and Suarez36, a member of the Rho family of small GTPases involved in cytoskeleton arrangementsReference Hallett, Dagher and Atkinson33. Northern blot analyses showed that Rnd3 mRNA levels significantly diminished during IEC-6 differentiation. We are aware that, although statistically modest, the present results showed that NS supplementation accelerated the changes in Rnd3 mRNA levels during enterocyte differentiation (Fig. 4).
In conclusion, exogenous NS were selectively taken up by IEC-6 cells and increased the intracellular NT pool in proliferating and differentiated cells. Because de novo synthesis of NT is an energy-intensive process, cells fulfil their NT requirements by NS salvage that spares energy and intermediary metabolites. The increase in energy charge and intracellular GTP concentrations in differentiated cells may favour the turnover of proteins and nucleic acids required for the transition from the proliferative to differentiation state in IEC-6 cells. These changes associated with an enhanced maturation of intestinal cells, improving their differentiation at morphological, functional and gene expression levels. The present findings agree with the work of many authors in cells and animal models of injuryReference Sánchez-Pozo and Gil2–Reference Belo, Marchbank, Fitzgerald, Ghosh and Playford9, Reference Perez, Suarez, Gomez-Capilla, Sanchez-Medina and Gil37–Reference Grimble39, and provide new evidence of the beneficial effect of exogenous NS on the functionality of intestinal epithelial cells.
Acknowledgements
This work was financed by Spanish Science Ministry Research Project PM97-0171 and by ABBOTT research project 71 400. Raul de la Torre was the recipient of a Junta de Andalucia predoctoral fellowship.








