The gastrointestinal tract plays a key role in the control of the immune response, in addition to its primary function in the digestion and absorption of nutrients. First, it acts as a barrier against antigens from micro-organisms and food and, second, contains the gut-associated lymphoid tissues, which are the largest collection of lymphoid tissues in the body. The gut-associated lymphoid tissues are responsible for the main production and release of polymeric IgA in the body in order to defend mucosal surfaces from environmental microbes(Reference Forchielli and Walker1). The establishment of normal bacterial populations is very important to prevent overgrowth of potential pathogens as well as to contribute to the adequate maturation of the immune system, since misbalances in the microbiota composition have been consistently associated with several pathologies(Reference Asher, Montefort, Björkstén, Lai, Strachan, Weiland and Williams2). In consequence, the administration of probiotics, prebiotics or their association (symbiotics) has emerged as an interesting approach to modulate intestinal microbiota.
Probiotics are defined as living micro-organisms that upon ingestion in adequate amounts confer a health benefit to the host beyond inherent general nutrition(Reference Guarner and Schaafsma3). Although probiotic pharmaceutical formulations are nowadays a common way to administer probiotics, it would be better to incorporate them into the diet through dairy products or other fermented foods. Classically, it has been considered that the beneficial effects exerted by probiotics are achieved due to their ability to colonise the human gastrointestinal tract, thus inducing changes in the composition of the normal intestinal microflora and influencing the immune system. It is evident that most of the beneficial effects reported for probiotics are related to intestinal conditions, such as lactose (or food) intolerance, acute diarrhoea or inflammatory bowel disease(Reference Ouwehand, Salminen and Isolauri4, Reference Parvez, Malik, Ah Kang and Kim5). However, the use of probiotics against systemic immune conditions has also been reported. In fact, Olivares et al. have reported that dietary deprivation of fermented foods could induce a decrease in the innate immune response, which can be effectively counteracted by the ingestion of probiotics(Reference Olivares, MPaz Díaz-Ropero, Gómez, Sierra, Lara-Villoslada, Martín, Miquel Rodríguez and Xaus6). Moreover, it has been speculated, both in humans and in experimental models, that inflammation associated with rheumatoid arthritis may be modulated by the use of probiotics(Reference Malin, Verronen, Korhonen, Syväoja, Salminen, Mykkänen, Arvilommi, Eerola and Isolauri7–Reference Sheil, McCarthy, O'Mahony, Bennett, Ryan, Fitzgibbon, Kiely, Collins and Shanahan10). Furthermore, the effects of probiotics have been studied in the prevention and treatment of atopic disease(Reference Isolauri, Arvola, Sütas, Moilanen and Salminen11, Reference Rosenfeldt, Benfeldt, Nielsen, Michaelsen, Jeppesen, Valerius and Paerregaard12). The probiotic administration using a preventative protocol showed a higher efficacy than in a therapeutic approach, most probably due to probiotics exerting their beneficial effects by improving mucosal barrier function and enhancing both specific and non-specific immune responses(Reference Macfarlane and Cummings13, Reference Vaarala14). However, even though the results obtained after probiotic treatment in both human subjects and experimental animals are promising, it is clear that all probiotics are not equally beneficial, each bacteria may have particular mechanisms of action, and host characteristics may also determine which probiotic species and even strains may be optimal. Supporting this, several studies have shown that not all probiotics exert the same intestinal anti-inflammatory effects in experimental models of intestinal inflammation(Reference Shibolet, Karmeli, Eliakim, Swennen, Brigidi, Gionchetti, Campieri, Morgenstern and Rachmilewitz15, Reference Peran, Camuesco, Comalada, Bailon, Henriksson, Xaus, Zarzuelo and Galvez16). For this reason, it is necessary to test each new probiotic strain to better characterise its safety profile and potential use in the treatment of altered immune conditions.
Sepsis is a generalised and exacerbated and not properly regulated inflammatory response to bacterial translocation, which frequently appears as a secondary complication to a marked depression of the cell-mediated immune response, as it may occur in patients suffering from severe trauma(Reference Ayala, Lehman, Herdon and Chaudry17). The exact mechanisms involved in this immune dysregulation are not completely understood, although an enhanced generation of inflammatory mediators, including cytokines, chemokines, adhesion molecules, reactive oxygen and nitrogen species, from activated immune cells such as macrophages and polymorphonuclear cells, seems to play a key role(Reference Liu and Malik18). The release of lipopolysaccharide (LPS) from bacteria and its recognition by host cells are generally thought to be the initial events in the development of sepsis, thus triggering the inflammatory reaction that may subsequently result in multiple organ dysfunction syndrome associated with failure in different tissues, including lung, liver and brain, and frequently leading to death. Also, an increased permeability of the intestinal barrier has been reported in sepsis(Reference Jørgensen, Nielsen, Espersen and Perner19). There is controversy whether the increased permeability and the subsequent translocation of the gut bacteria are the cause or a consequence of the initial inflammatory response. Anyway, the translocated bacteria could boost the exacerbated inflammatory condition. The incidence of sepsis is still increasing, with high rates of mortality (up to 70 %) despite the use of life-support therapies(Reference Yende and Angus20). Therefore, more efficient strategies are required to modulate the inflammation and also to reduce the bacterial translocation occurring in sepsis, and, for this purpose, dietary manipulation through probiotic administration would be of great interest. Considering how probiotics exert their beneficial effect as well as the severity and the rapid onset of sepsis, preventative dietary administration of probiotic seems to be the most adequate protocol to perform these studies.
The aim of the present study was to evaluate the preventative effects of the probiotic Lactobacillus fermentum CECT5716 in a model of septic shock in mice induced by LPS, since, in previous studies, this probiotic has been shown to be able to modulate some of the potential mechanisms of sepsis. In this regard, immunomodulatory properties have been described for this probiotic, since it is able to enhance the Th1 response(Reference Díaz-Ropero, Martín, Sierra, Lara-Villoslada, Rodríguez, Xaus and Olivares21) and increase the IgA concentration in faeces helping the blockage and elimination of translocated bacteria(Reference Olivares, Díaz-Ropero, Sierra, Lara-Villoslada, Fonollá, Navas, Rodríguez and Xaus22). Furthermore, this strain has been described as displaying anti-infective properties against different pathogenic micro-organisms through different mechanisms, including inhibition of the adhesion to epithelial cells, secretion of anti-microbial compounds and improvement of the intestinal barrier function by inducing the expression of intestinal mucins(Reference Olivares, Díaz-Ropero, Martín, Rodríguez and Xaus23). Furthermore, several strains of this probiotic have been reported to display antioxidant properties that could account for their beneficial effects since oxidative stress is associated with inflammatory conditions, including sepsis or colonic inflammation(Reference Geier, Butler, Giffard and Howarth24–Reference Songisepp, Kals, Kullisaar, Mändar, Hütt, Zilmer and Mikelsaar28). All these data make L. fermentum CECT5716 an interesting candidate to evaluate its effects on altered immune response conditions, such as sepsis.
Materials and methods
Reagents
All chemicals, including LPS from Escherichia coli serotype 055:B5, were obtained from Sigma Chemical (Madrid, Spain), unless otherwise stated.
Preparation and administration of the probiotic
L. fermentum CECT5716 was provided by Puleva Biotech (Granada, Spain) and was normally grown in de Man, Rogosa and Sharpe (MRS) media at 37°C in anaerobic conditions using the Anaerogen system (Oxoid, Basingstoke, Hants, UK). For probiotic treatment, bacteria were suspended in a sterile PBS solution (109 colony-forming units/ml) and stored at − 80°C until usage.
Experimental design
Male BALB/c mice weighing 20–22 g were obtained from the Laboratory Animal Service of the University of Granada (Granada, Spain), housed in Makrolon cages and maintained in an air-conditioned atmosphere with a 12 h light–dark cycle, and provided with free access to tap water and food. All manipulations with animals were in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ as promulgated by the National Institute of Health. The mice were randomly assigned to three groups (n 10); two of them (healthy and control groups) received tap water and the other (treated group) received the probiotic suspended in drinking water at the final concentration of 108 colony-forming units/ml and daily prepared. Food and water intake was recorded daily for all groups. At 2 weeks after starting the experiment, endotoxic shock was induced in treated and control mice with an intraperitoneal injection of LPS (400 μg/mouse) to a final volume of 200 μl; healthy mice received sterile saline. Preliminary assays revealed that this dose of LPS did not induce the death of any mouse in the following 24 h. At 24 h after injection, mice were anaesthetised with halothane, blood samples were taken from the retro-orbital venous plexus, and then the mice were killed immediately. The following tissues were quickly removed and weighed: spleen, lungs, liver, kidneys and colon. Colonic specimens were frozen at − 80°C for myeloperoxidase (MPO) activity and inducible NO synthase (iNOS) expression. Liver samples were frozen in 1 ml TCA (50 g/l) for total glutathione content determination. One of the lungs was immediately processed for the measurement of TNF-α levels and the other was frozen at − 80°C for MPO activity and iNOS expression. All biochemical measurements were completed within 1 week from the time of sample collection and were performed in duplicate.
Biochemical determinations
MPO activity was measured in colonic tissue as previously described(Reference Sánchez de Medina, Gálvez, Romero and Zarzuelo29); the results were expressed as MPO units/g wet tissue; one unit of MPO activity was defined as that degrading 1 μmol H2O2/min at 25°C. Total glutathione content was quantified in liver with the recycling assay described by Anderson(Reference Anderson30), and the results were expressed as nmol/g wet tissue. Lung samples for TNF-α determination were immediately weighed, minced on an ice-cold plate and suspended in a tube with 10 mm-sodium phosphate buffer (pH 7·4) (1:5, w/v). The tubes were placed in a shaking water-bath (37°C) for 20 min and centrifuged at 9000 g for 30 s at 4°C; the supernatant fractions were frozen at − 80°C until assay. TNF-α was quantified by ELISA (Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK) and the results were expressed as pg/g wet tissue. Colonic and lung specimens were also used for protein extraction to evaluate iNOS expression by Western blotting, which was performed as described elsewhere(Reference Comalada, Camuesco, Sierra, Ballester, Xaus, Gálvez and Zarzuelo31). Blood samples were centrifuged (1500 rpm for 10 min), and plasma TNF-α was quantified by ELISA (Amersham Pharmacia Biotech) and the results were expressed as pg/ml.
Cytokine production in splenocytes
In order to obtain primary lymphocyte cultures, spleens were immediately disaggregated in Dulbecco's modified Eagle's medium plus 1 % penicillin/streptomycin after collection, centrifuged (1500 rpm; 5 min), and erythrocytes were lysed with a lysis buffer (NH4Cl (1·7 mol/l), KHCO3 (0·12 mol/l), ethylenediamine-tetra-acetic (9 mmol/l)) for 30 min at 4°C. Resting cells were counted using a haemocytometer and cultured to perform proliferation and stimulation assays in current culture medium (Dulbecco's modified Eagle's medium +10 % fetal bovine serum). Cells were incubated at 37°C in a humidified 5 % CO2 atmosphere.
Spleen-derived lymphocytes were cultured in six-well plates (10 × 106 cells/well) in 2 ml media and stimulated with concanavalin A (Con A; 5 μg/ml). Supernatant fractions were collected after 48 h and frozen until ELISA analysis. Cytokine production was measured with commercial murine ELISA kits (Cytosets™; Biosource International, Nivelles, Belgium) following the manufacturer's protocol. Total RNA was isolated using TRIzol© Reagent (Gibco-BRL, Carlsbad, CA, USA) following the manufacturer's protocol. cDNA was synthesised using a First-Strand cDNA Synthesis Kit (Amersham Biosciences). The primer sequences were: IL-2 (forward 5′-CTTCAAGCTCCACTTCAAGCT-3′, reverse 5′-CCATCTCCTCAGAAAGTCCACC-3′); IL-5 (forward 5′-TCAGGGAATAGGCACACTGG-3′, reverse 5′-CTCCGTCTTTCTTCTCCACAC-3′); IL-6 (forward 5′-TGATGGATGCTACCAAA-CTGG-3′, reverse 5′-AGGAGAGCATTGGAAATTGG-3′); IL-10 (forward 5′-TGCCTGCTCTTACTGACTGG, reverse 5′-TCATTTCCGATAAGGCTTGG); β-actin (forward 5′-AA-TCGTGCGTGACATCAAAG-3′, reverse 5′-ATGCCACAGGATTCCATACC-3′). The PCR was performed as described previously(Reference Comalada, Ballester, Bailón, Sierra, Xaus, Gálvez, de Medina and Zarzuelo32) but in this case the cycles used were: thirty-two cycles for IL-2, thirty-five cycles for IL-5 and IL-10, thirty cycles for IL-6 and twenty-five cycles for β-actin.
Statistics
All results are expressed as the mean values with their standard errors. Data were analysed by one-way ANOVA and post hoc least-significance tests. All statistical analyses were carried out with the Statgraphics 5.0 software package (StatPoint, Inc., Herndon, VA, USA) with differences considered significant set at P < 0·05.
Results
The preventative probiotic administration to mice for 2 weeks before septic shock induction did not affect body-weight gain compared with untreated groups (data not shown), and no sign of toxic effects was observed, similarly to that previously reported for rats and mice(Reference Díaz-Ropero, Martín, Sierra, Lara-Villoslada, Rodríguez, Xaus and Olivares21, Reference Peran, Camuesco, Comalada, Nieto, Concha, Adrio, Olivares, Xaus, Zarzuelo and Galvez27, Reference Peran, Sierra and Comalada33). After 2 weeks of probiotic consumption, mice received an intraperitoneal injection of saline or a sub-lethal dose of 400 μg LPS, and, 8 h after LPS administration, mice showed evident symptoms of endotoxic shock, similar to those described previously(Reference Leite, Pacheco, Gomes, Guedes, Castro-Faria-Neto, Bozza and Koatz34), including decreased motor activity, ruffled fur and ocular exudates. All mice survived 24 h after LPS injection, when they were killed after exsanguination. Macroscopic tissue modifications were observed as a consequence of the septic shock. Significant increases in colon weight:length ratio and in spleen and lung weights were observed in the control group when compared with healthy mice (P < 0·05; Table 1), whereas no significant modification was observed in the weights of liver and kidneys (Table 1). The probiotic treatment resulted in a significant improvement in these tissue modifications, showing similar values to those in the healthy group (Table 1).
Table 1 Effects of probiotic treatment on tissue weights in lipopolysaccharide-induced septic shock in mice
(Mean values with their standard errors for ten mice per group)
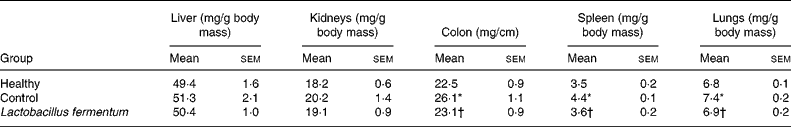
* Mean value was significantly different from that of the healthy group (P < 0·05).
† Mean value was significantly different from that of the control group (P < 0·05).
The beneficial effects exerted by the probiotic pre-treatment in this experimental model of septic shock were also confirmed biochemically. One of the key cytokines involved in sepsis is TNF-α, the systemic release of which induces increased vascular permeability and disseminated intravascular coagulation, responsible for the state of shock(Reference Annane, Bellissant and Cavaillon35). TNF-α levels were significantly increased 24 h after intraperitoneal administration of LPS to mice, both in the plasma and lungs, in comparison with normal mice. The administration of L. fermentum for 2 weeks before LPS injection resulted in a significantly lower TNF-α production when compared with untreated control mice (Fig. 1). In addition, sepsis promotes the activation and migration of leucocytes into various organs(Reference Annane, Bellissant and Cavaillon35), one of the most common events being the infiltration of activated neutrophils into lung tissue(Reference Welbourn and Young36). Thus a higher neutrophil influx was also observed in the lungs of control LPS-treated mice, evidenced by the 10-fold increase in lung MPO activity in comparison with healthy animals (P < 0·01) (Table 2). However, when this enzyme activity was evaluated in colonic tissue, no significant modification was observed among groups (Table 2), although macroscopic alteration was observed in this organ. The pre-treatment with the probiotic partially counteracted the increased lung MPO activity observed in control mice (P < 0·05; Table 2). Furthermore, sepsis is associated with an enhanced production of reactive oxygen metabolites, which cause oxidative damage in different organs, leading to multiple organ dysfunction(Reference Salvemini and Cuzzocrea37). In the present study, free radical overproduction was associated with a significant decrease in hepatic glutathione levels in septic mice (P < 0·01 v. healthy group; Table 2), which was significantly increased in the group of mice receiving the probiotic (P < 0·05 v. control group; Table 2), although probiotic pre-treatment was not able to restore the values obtained in healthy animals. Moreover, an increased iNOS expression after LPS administration was evidenced by Western blot both in the colon and lungs of septic control mice when compared with the healthy group, and this was down-regulated in those mice treated with L. fermentum (Fig. 2).

Fig. 1 Effect of Lactobacillus fermentum probiotic on TNF-α production in lipopolysaccharide (LPS)-induced septic shock in mice. Probiotic pre-treatment inhibits this cytokine production in the plasma (a) and lungs (b). The concentrations of the cytokine were analysed by ELISA. Values are means (n 10), with standard errors represented by vertical bars. ** Mean value was significantly different from that of the healthy group (P < 0·01). †† Mean value was significantly different from that of the control group (P < 0·01).
Table 2 Effects of probiotic treatment on lung myeloperosidase (MPO) activity and hepatic glutathione (GSH) content in lipopolysaccharide-induced septic shock in mice
(Mean values with their standard errors for ten mice per group)
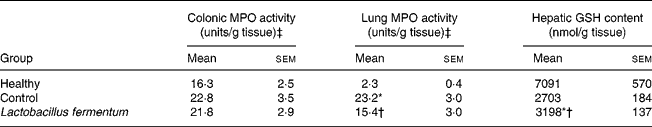
* Mean value was significantly different from that of the healthy group (P < 0·05).
† Mean value was significantly different from that of the control group (P < 0·05).
‡ One unit of MPO activity was defined as that degrading 1 μmol H2O2/min at 25°C.

Fig. 2 Effect of Lactobacillus fermentum probiotic on lung (a) and colonic (b) inducible NO synthase (iNOS) expression in lipopolysaccharide (LPS)-induced septic shock in mice. iNOS expression was analysed by Western blot using tissue homogenates as described in Materials and methods. In each lane 150 μg protein were loaded. β-Actin expression was used as the control for loading and transfer.
To further evaluate the effects of the probiotic treatment on the altered immunological response after LPS administration, the expression of different Th1, Th2 and Th3 cytokines (IL-2, IL-5, IL-6 and IL-10) in Con A-stimulated splenocytes was analysed. RT-PCR and/or ELISA analysis revealed that Con A-stimulated cytokine expression was altered in the splenocytes from septic mice (Fig. 3). In this sense, a clear increased expression of IL-2, IL-5 and IL-6 was observed in the control mice in comparison with healthy ones, while a decreased expression of the regulatory Th3 cytokine IL-10 was detected. Probiotic administration reduced the expression of the pro-inflammatory cytokines and restored the expression of IL-10 to those levels observed in Con A-stimulated lymphocytes obtained from healthy mice.

Fig. 3 (a) Effect of Lactobacillus fermentum probiotic on the expression of cytokines in concanavalin A (Con A)-activated T-lymphocytes from mice after lipopolysaccharide (LPS)-induced septic shock. Splenocytes were incubated with Con A during 48 h and the expressions of cytokines in cells were assessed by RT-PCR. (b) Effects of probiotic treatment on IL-2 and IL-10 secretion in Con A-stimulated splenocytes from mice with LPS-induced septic shock. (■), Healthy mice; (□), LPS control; (![]() ), LPS probiotic. Splenocytes were incubated with Con A during 48 h and the concentration of the cytokine in the supernatant fraction was analysed by ELISA. Values are means (n 10), with standard errors represented by vertical bars. * Mean value was significantly different from that of the healthy group (P < 0·05). † Mean value was significantly different from that of the control group (P < 0·05).
), LPS probiotic. Splenocytes were incubated with Con A during 48 h and the concentration of the cytokine in the supernatant fraction was analysed by ELISA. Values are means (n 10), with standard errors represented by vertical bars. * Mean value was significantly different from that of the healthy group (P < 0·05). † Mean value was significantly different from that of the control group (P < 0·05).
Discussion
Sepsis is a systemic inflammatory condition commonly caused by bacterial infections, which is associated with multiple organ failure and has a poor prognosis due to the lack of an effective treatment(Reference Patel, Gurka and Balk38). Bacterial pathogens and their products trigger the inflammatory response by transcriptional activation of inflammatory genes, leading to an excessive and uncontrolled release of a large number of inflammatory mediators, including cytokines, chemokines, adhesion molecules, reactive oxygen and nitrogen species. Recent studies have revealed that the incidence of sepsis is still increasing(Reference Yende and Angus20), and, in consequence, more efficient strategies to modulate the inflammation in sepsis are needed.
Although a general multiple organ failure has been observed in humans, mainly affecting the lungs, liver, kidneys and even the brain(Reference Hotchkiss and Karl39), in our experimental settings only the colon, lungs and spleen were enlarged due to inflammation or over-activation of the immune response, in the latter. Furthermore, an alteration of liver function was also evidenced since a significant depletion of the glutathione levels occurred. Other organs were not affected, maybe due to the fact that we evaluated the sepsis only 24 h after induction.
Several biochemical and immunological mechanisms have been proposed to be responsible for the alterations observed during sepsis. In this sense, a critical role of TNF-α in the development of LPS-mediated shock in mice has been consistently reported, since an excess of this cytokine promotes the major alterations observed during septic shock, including vasodilatation, impaired coagulation and fibrinolysis(Reference Annane, Bellissant and Cavaillon35). In addition, an altered expression of other Th1 and Th2 cytokines such as interferon γ, IL-2, IL-4 or IL-5 has also been described(Reference Hotchkiss and Karl39–Reference Hsu, Chiu, Yeh, Hou, Chou and Yeh41). IL-6, together with TNF-α, is also considered one of the most important mediators in the pathogenesis of sepsis and septic shock. It displays potent biological effects, including stimulation of B- and T-lymphocytes, and induction of the hepatic acute-phase response(Reference Barton42), thus promoting tissue injury, multiple organ failure and death(Reference Hack, De Groot, Felt-Bersma, Nuijens, Strack Van Schijndel, Eerenberg-Belmer, Thijs and Aarden43, Reference Watanabe, Hirasawa, Oda, Matsuda, Hatano and Tokuhisa44). Other mechanism involved in the pathogenesis of sepsis is the excessive production of NO, most probably derived from the increased expression of iNOS(Reference Cuzzocrea, Mazzon, EDi Paola, Esposito, Macarthur, Matuschak and Salvemini45). Furthermore, it is well reported that the generalised inflammatory response that occurs in sepsis is associated with an enhanced generation of reactive oxygen metabolites, mainly derived from neutrophil activity, that also contributes to multiple organ dysfunction(Reference Sener, Toklu, Kapucu, Ercan, Erkanli, Kaçmaz, Tilki and Yegen46).
As expected, LPS-administered mice showed an alteration of the cytokine profile production including increased levels of TNF-α, IL-2, IL-5 and IL-6 and a reduced expression of the regulatory cytokine IL-10. The involvement of NO and reactive oxygen metabolites was confirmed by an enhanced iNOS expression in the lungs and colon, and a glutathione depletion in the liver, respectively. Moreover, a neutrophil infiltration in the lungs was also evident, shown by augmented MPO activity.
The present study shows for the first time that feeding mice with the probiotic L. fermentum CECT5716 clearly prevented septic shock conditions by ameliorating the altered production of inflammatory mediators. Thus L. fermentum was able to down-regulate the increased levels of TNF-α both in the plasma and lungs. The ability of this probiotic to reduce TNF-α production in inflammatory conditions has been previously reported in an experimental model of rat colitis(Reference Peran, Camuesco, Comalada, Nieto, Concha, Adrio, Olivares, Xaus, Zarzuelo and Galvez27). The beneficial effects showed by L. fermentum can be derived from an improvement in the altered immune response induced by LPS, since there is a reduction in the expression of the pro-inflammatory cytokines IL-2, IL-5, IL-6 as well as an increase of IL-10 in Con A-stimulated splenocytes. Similarly to other probiotics, L. fermentum was more efficient at modulating IL-10 than other cytokines, including IL-2. This can be explained by a direct effect of the probiotic in inducing IL-10 expression in macrophages and lymphocytes as previously described(Reference Díaz-Ropero, Martín, Sierra, Lara-Villoslada, Rodríguez, Xaus and Olivares21), while the effect on IL-2 might be an indirect effect mediated both by the increased expression of IL-10 and the reduction on the inflammatory process exerted by the probiotic treatment.
The likely consequences of the immunomodulatory actions of L. fermentum in septic mice could be the amelioration of the tissue damage and inflammation induced by LPS. The latter was confirmed in the present study since MPO activity was partially reduced in the lungs from mice treated with the probiotic when compared with septic animals. Moreover, a reduction in the lung and colon iNOS expression was observed in probiotic-fed mice. The ability of this probiotic to inhibit iNOS expression in vivo has been previously reported to occur in the rat inflamed intestine after trinitrobenzenesulfonic acid instillation(Reference Peran, Sierra and Comalada33).
In addition to its immunomodulatory properties, other mechanisms could contribute to the beneficial effects displayed by L. fermentum to prevent mice from sepsis. In this regard, the probiotic also partially preserved the content of the antioxidant peptide glutathione in the liver, thus preventing the deleterious effects that reactive oxygen metabolites may induce once they are produced and released in this inflammatory condition. In fact, this lactobacilli strain has been reported to produce and release glutathione as well as its precursor, the dipeptide γ-Glu-Cys, displaying both compounds' free radical scavenger properties(Reference Peran, Camuesco, Comalada, Nieto, Concha, Adrio, Olivares, Xaus, Zarzuelo and Galvez27), which could counteract the intense oxidative stress situation in LPS-induced septic shock in mice. It is important to point out that the antioxidant effects exerted by L. fermentum could also collaborate in the inhibition of tissue neutrophil infiltration, as observed in the present study, since free radical generation causes a vicious circle that promotes additional infiltration of neutrophils into inflamed tissue, which in turn produce a large amount of free radicals that actively participate in the perpetuation of the inflammatory response(Reference Cuzzocrea, McDonald, Mazzon, Dugo, Lepore, Fonti, Ciccolo, Terranova, Caputi and Thiemermann47).
In conclusion, the dietary consumption of probiotics and the modulation of the intestinal microbiota could exert beneficial effects on potentially immune-related pathologies, as has been demonstrated in the present study with L. fermentum CECT5716 in the LPS model of septic shock in mice. Although the therapeutic use of probiotics could be of potential interest in the management of clinical sepsis, it is relevant to note that this process has a fast onset and a high severity, making their effective application difficult in many cases. However, probiotic administration, following a preventative approach, could be useful in those cases of programmed surgical interventions or other pathologies in where septicaemia frequently occurs as a secondary complication. Anyway, both efficacy and safety of probiotic treatment should be carefully tested in well-designed and conducted clinical trials.
Recently, some disappointing results have been reported after administration of a specific mixture of probiotics in the management of critically ill patients suffering from pancreatitis and some concerns have arisen about the clinical use of probiotics in those patients(Reference Besselink, van Santvoort and Buskens48). Nevertheless, some probiotic strains have been tested in immunocompromised patients suffering from HIV without major side effects(Reference Anukam, Osazuwa, Osadolor, Bruce and Reid49–Reference Bibiloni, Fedorak, Tannock, Madsen, Gionchetti, Campieri, De Simone and Sartor51). However, every probiotic strain is different, and for this reason they have to be individually checked, not only for their efficacy but also for safety reasons. We should not be disappointed about the above-mentioned undesirable effects, and carry on the research into the potential clinical uses of probiotics.
Acknowledgements
There is no conflict of interest to declare by any of the authors. All authors have contributed to the conception and design of the experiment, acquisition of the data and their subsequent analysis and interpretation. Similarly, all authors have been involved in the revising the paper critically and have approved the final version of the paper. J. G. supervised in all phases. The study was supported by the Spanish Ministry of Science and Technology (SAF2005-03 199), with funds from the European Union, and by Junta de Andalucia (CTS 164). M. C. is a recipient of the Ramon y Cajal Programme from the Spanish Ministry of Science and Technology; E. B. is a recipient from the Spanish Ministry of Education and Science. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD) is funded by the Instituto de Salud Carlos III.







